Pharmacokinetics Studies of 12 Alkaloids in Rat Plasma after Oral Administration of Zuojin and Fan-Zuojin Formulas
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Chromatographic and Mass Conditions
2.2. Sample Preparation
2.3. Method Validation
2.4. PK Studies
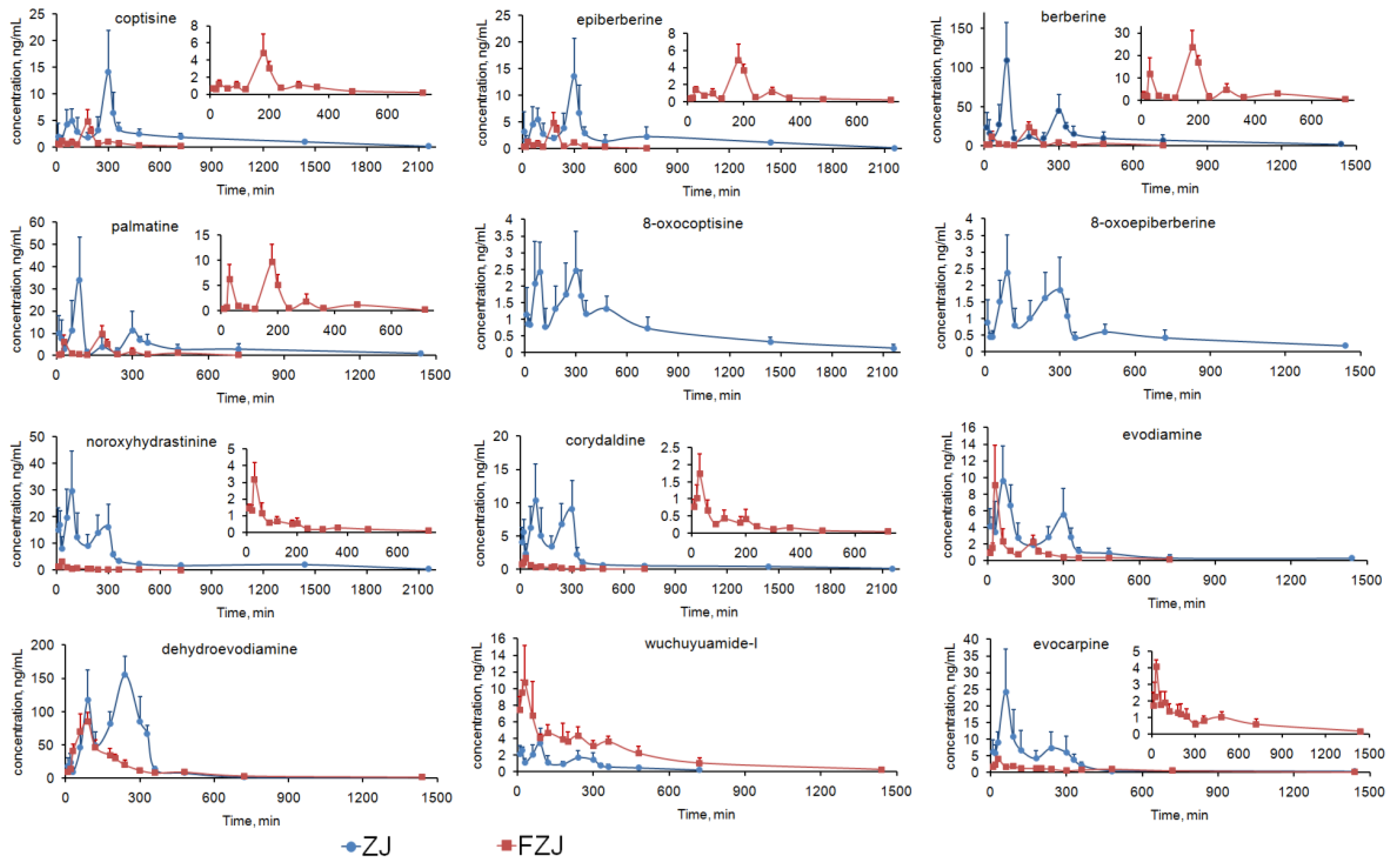
2.4.1. C–T Curves of the Alkaloids
- (1)
- For the alkaloids except QPAs, double peaks were visibly observed in the C–T curves after oral administration of ZJ extract, but the secondary peaks were attenuated and the C–T curves approximately matched the two-compartment model after administration of FZJ (calculated by two fitting methods using DAS 2.0 software, Table 2). Moreover, Tmax of the alkaloids after administration of ZJ extract were deferred from that of FZJ administration. Alkaloids of CR, especially berberine, have been reported to have inhibition effect on GI motility [45,46,47,48,49]. It probably play a major role in the double peak phenomenon, since the GI motility can affect the drug absorption. The content of berberine in ZJ extract were 20 times more than that in FZJ extract (Table S4), leading to more significant influence on the GI motility after oral administration. Thus, the double peak phenomenon and the delayed Tmax of the alkaloids after administration of ZJ extract could be attributed to the inhibition effect on GI motility caused by CR alkaloids, especially berberine.
- (2)
- In ZJ group, two plasma concentration peaks were observed at 90 and 300 min for all the four QPAs; but the primary plasma concentration peak was at 300 min for coptisine and epiberberine, and 90 min for palmatine and berberine (Table 1, significant differences between the plasma concentration values of the primary and secondary peaks were examined with t-test by Microsoft Office Excel 2007). Previous researches had suggested that the primary elimination route of berberine in vivo was renal excretion [50], and its C–T curve matched the two-compartment model following an intravenous administration [50,51]. Therefore, the enterohepatic circulation couldn’t be the major reason of the double peak phenomenon of QPAs, since the plasma concentration values of coptisine and epiberberine at 300 min were greater than that at 90 min. In addition, research findings of the main phase I metabolism of berberine [52,53,54] certified that metabolism couldn’t be the reason of the double peak phenomenon of QPAs. The absorption rate constant (Ka) of berberine and palmatine at jejunum had been reported to be greater than that at ileum and colon, which means the upper part of the intestine was their dominant absorption site [55,56,57]. Coptisine, in contrast, had a better absorption rate at colon compared with jejunum [57]. Therefore two absorption sites with different Ka could give a reasonable explanation about the double peak phenomenon of the four QPAs: plasma concentration peak at 90 min was mainly caused by the absorption at the upper part of the intestine, but mainly by the absorption at ileum and colon while that is at 300 min. Plasma concentration peaks of QPAs in FZJ group at 30 and 180 min were earlier than that of ZJ group, and peaks at 30 min caused by the absorption at the upper part of the intestine were attenuated. The possible reason was that the lower berberine content in FZJ weakened the inhibition effect on GI motility and subsequently led to the decreased residence time and absorption level of QPAs at upper part of the intestine.
2.4.2. Systemic Exposure of the Alkaloids
- (1)
- The four QPAs from CR, especially berberine, had relatively high systemic exposure levels, but their AUC0→∞/D and Cmax/D values were extremely low. Previous studies suggested that absolute bioavailability of berberine was less than 1% [61,62], and it should be mainly attributed to its poor absorption. Transport experiments had confirmed that berberine, palmatine and coptisine had poor permeability across Caco-2 cell monolayer with apparent permeability coefficient (Papp) values between 0.1 and 1.0 × 10−6 cm/s [63] as poorly absorbed compounds [64]. TPAs and SIAs, in contrast, were components with low contents in CR but higher AUC0→∞/D and Cmax/D values indicating their better absorption properties. 3PRule suggested that the two TPAs and two SIAs had favorable physicochemical properties for Caco-2 permeability: PSA ≤ 60, MW ≤ 400 and LogD > −1. However, compounds must be dissolved in water before penetrating the intestinal epithelial cells, and the suitable values of solubility (LogS) ranged from 0 to −4 [65]. Therefore, systemic exposure levels of two TPAs, especially 8-oxocoptisine, appeared to be limited by their lower solubility than SIAs.
- (2)
- Evodiamine and dehydroevodiamine were two major IQAs in EF, but the results suggested that dehydroevodiamine had a higher systemic exposure level regardless of the dose modification. Our previous study demonstrated that both evodiamine and dehydroevodiamine had high permeability across Caco-2 cell monolayer with Papp values of 2.32 × 10−5 and 1.26 × 10−5 cm/s, respectively [66], which were close to the values predicted by 3PRule; but it was also found that the feeding concentration of evodiamine was limited due to its poor solubility in the transport experiment. Thus, its solubility should be the major obstacle to a higher systemic exposure level for evodiamine (predicted LogS < −5), and the poor solubility can be attributed to its flat, rigid, and unsaturated structure. In contrast, the solubilities of dehydroevodiamine and wuchuyuamide-I were improved because of the inner salt structure or the broken ring structure.
- (3)
- Effects on the absorption and elimination of the alkaloids from CR and EF were illustrated by comparing the AUC0→∞/D, Cmax/D and t1/2 of the alkaloids after the administrations of ZJ and FZJ extract. Since the t1/2 values of some alkaloids, such as evodiamine, were not accurately calculated because of the multi-peak phenomenon, MRT0→∞ would be more reliable in the comparison. MRT0→∞ and t1/2 values of the four QPAs halved of FZJ group compared with that of ZJ group, but Cmax/D and AUC0→∞/D increased 4–9 times and 3–4 times, respectively. The comparisons indicated that absorptions of QPAs were increased, but their eliminations were accelerated as a result of increased EF intake. The QPAs like coptisine, palmatine and berberine were P-gp substrates [63,67], and their efflux ratio could be reduced by EF on the Caco-2 transport model [68], so EF may possibly promote the absorption of QPAs by inhibiting P-gp. On the other hand, EF could also induce hepatic UDP-glucuronosyltransferase 1A1 and then accelerate the elimination of QPAs [69]. In the present study, QPAs’ systemic exposure (AUC0→∞/D) were finally increased when the proportion of EF increased, suggesting that EF had a greater influence on the absorption than on the elimination of QPAs after the compatibility of CR and EF.
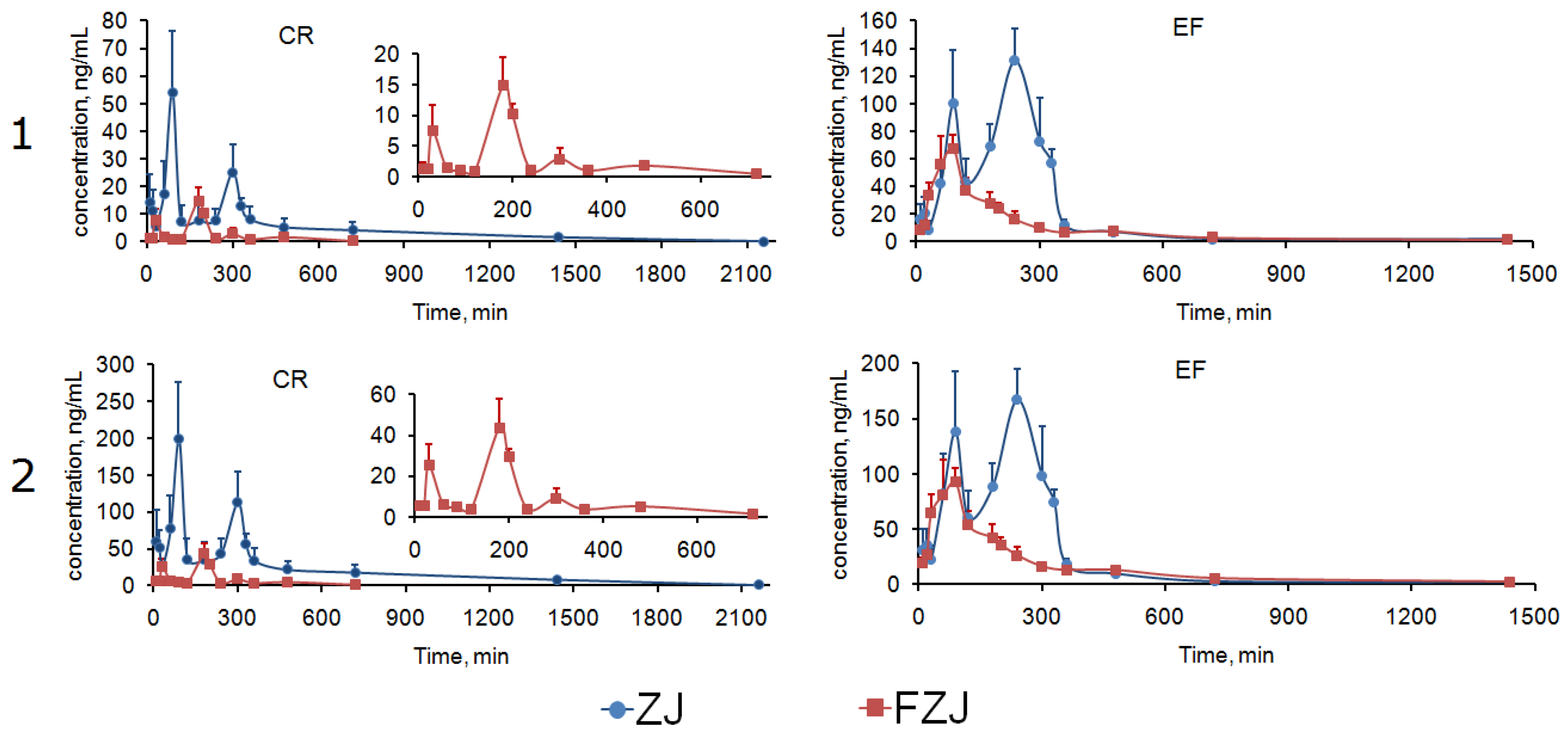
2.4.3. Integrated PK Analysis
3. Experimental Section
3.1. Chemicals and Materials
3.2. Animals
3.3. Apparatus and Analytical Conditions
3.4. Preparations of ZJ and FZJ Extracts
3.5. Preparation of Standard Solutions, Calibration Standards and Quality-Control Samples
3.6. Sample Preparation
3.7. Method Validation
3.8. Pharmacokinetic Study
3.9. Integrated PK Analysis
3.9.1. AUC-based Weighting Method
3.9.2. Total Drug Concentration Method
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kong, W.J.; Zhao, Y.L.; Shan, L.M.; Xiao, X.H.; Liu, J.; Guo, W.Y. Progress of Zuojinwan. Chin. J. Exp. Tradit. Med. Form. 2008, 14, 73–77. [Google Scholar]
- Chao, D.C.; Lin, L.J.; Kao, S.T.; Huang, H.C.; Chang, C.S.; Liang, J.A.; Wu, S.L.; Hsiang, C.Y.; Ho, T.Y. Inhibitory effects of Zuo-Jin-Wan and its alkaloidal ingredients on activator protein 1, nuclear factor-κB, and cellular transformation in HepG2 cells. Fitoterapia 2011, 82, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.X.; Yang, D.J.; Shi, J.; Cai, H.B.; Mo, Z.X. Proportion of Coptidis rhizoma and Evodiae fructus in the compound preparation: Its effect in inducing apoptosis of SGC-7901 cells. J. South Med. Univ. 2011, 31, 226–229. [Google Scholar]
- Wen, B.; Xiong, M.L.; Dai, W.Y.; Liang, Q.H.; Liu, L.; Chen, W.W. Effects of Zuojin Pill and Fanzuojin Pill on the expression of methyltransferase in rats with experimental colorectal carcinoma at different stages. World Chin. J. Digestol. 2009, 17, 2074–2078. [Google Scholar]
- Wen, B.; Huang, Q.L.; Gong, Y.Q.; Chen, W.W. In vitro and in vivo anti-colorectal carcinoma activities of Zuojin Pill and its major constituents. World Chin. J. Digestol. 2009, 17, 1936–1941. [Google Scholar] [CrossRef]
- Deng, Y.T.; Liao, Q.F.; Li, S.H.; Bi, K.S.; Pan, B.Y.; Xie, Z.Y. Simultaneous determination of berberine, palmatine and jatrorrhizine by liquid chromatography-tandem mass spectrometry in rat plasma and its application in a pharmacokinetic study after oral administration of coptis-evodia herb couple. J. Chromatogr. B 2008, 863, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.; Yang, X.W. Five new alkaloids from Coptidis Rhizoma-Euodiae Fructus couple and their cytotoxic activities against gastrointestinal cancer cells. Fitoterapia 2014, 93, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.; Jin, H.W.; Yang, X.W. New limonoids from Coptidis Rhizoma-Euodiae Fructus couple. J. Asian Nat. Prod. Res. 2014, 16, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.; Yang, X.W. Alkaloids from Zuojin Formula and their cytotoxicities against proliferation of cancer cells. Chin. Tradit. Herb. Drugs 2014, 45, 8–15. [Google Scholar]
- Geng, Z.P.; Zheng, H.J.; Zhang, Y.; Luo, W.Z.; Qu, X.Y. Simultaneous determination of six alkaloids in Coptis chinensis of different regions by RP-HPLC. China J. Chin. Mater. Med. 2010, 35, 2576–2580. [Google Scholar]
- Chen, C.; Yu, Z.; Li, Y.; Fichna, J.; Storr, M. Effects of berberine in the gastrointestinal tract—A review of actions and therapeutic implications. Am. J. Chin. Med. 2014, 42, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Ng, L.T.; Hsu, F.F.; Shieh, D.E.; Chiang, L.C. Cytotoxic effects of Coptis chinensis and Epimedium sagittatum extracts and their major constituents (berberine, coptisine and icariin) on hepatoma and leukaemia cell growth. Clin. Exp. Pharmacol. Physiol. 2004, 31, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Xun, K.L.; Wang, Y.T.; Chen, X.P. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anticancer Drugs 2009, 20, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.R.; Zhao, Y.Y.; Dong, F.X.; Yan, Z.Y.; Zheng, W.J.; Fan, J.P.; Sun, G.L. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, S.B.; Yuan, T.Y.; Wu, Y.J.; Yan, Y.; Li, L.; Xu, X.N.; Gong, L.L.; Qin, H.L.; Fang, L.H.; et al. Coptisine protects rat heart against myocardial ischemia/reperfusion injury by suppressing myocardial apoptosis and inflammation. Atherosclerosis 2013, 231, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.W.; Yao, X.Q.; Chen, Z.Y.; Ko, W.H.; Huang, Y. Cardiovascular actions of berberine. Cardiovasc. Drug Rev. 2001, 19, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Min, B.S.; Yokozawa, T.; Lee, J.H.; Kim, Y.S.; Choi, J.S. Anti-Alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol. Pharm. Bull. 2009, 32, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.K.; Dhir, A. Berberine: A plant alkaloid with therapeutic potential for central nervous system disorders. Phytother. Res. 2010, 24, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Hirano, H.; Osawa, E.; Yamaoka, Y.; Yokoi, T. Gastric-mucous membrane protection activity of coptisine derivatives. Biol. Pharm. Bull. 2001, 24, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.D.; Yang, M.C.; Lee, K.H.; Kim, K.R.; Choi, S.U.; Lee, K.R. Protoberberine alkaloids and their reversal activity of P-gp expressed multidrug resistance (MDR) from the rhizome of Coptis japonica Makino. Arch. Pharm. Res. 2006, 29, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.C.; Wang, Y.H.; Liou, K.T.; Chen, C.M.; Chen, C.H.; Wang, W.Y.; Chang, S.; Hou, Y.C.; Chen, K.T.; Chen, C.F.; et al. Anti-inflammatory effects and mechanisms of the ethanol extract of Evodia rutaecarpa and its bioactive components on neutrophils and microglial cells. Eur. J. Pharmacol. 2007, 555, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.L.; Hu, C.P. Evodiamine: A novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules 2009, 14, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Lee, Y.B.; Kim, H.S. Effects of dehydroevodiamine exposure on glutamate release and uptake in the cultured cerebellar cells. Neurochem. Res. 2004, 29, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Chou, C.J.; Liao, J.F.; Chen, C.F. Dehydroevodiamine attenuates beta-amyloid peptide-induced amnesia in mice. Eur. J. Pharmacol. 2001, 413, 221–225. [Google Scholar] [CrossRef]
- Chiou, W.F.; Liao, J.F.; Chen, C.F. Comparative study of the vasodilatory effects of three quinazoline alkaloids isolated from Evodia rutaecarpa. J. Nat. Prod. 1996, 59, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, N.; Ishii, E.; Tominaga, K.; Tezuka, Y.; Nagaoka, T.; Kadota, S.; Kuroki, T.; Yano, I. Highly selective antibacterial activity of novel alkyl quinolone alkaloids from a Chinese herbal medicine, Gosyuyu (Wu-Chu-Yu), against Helicobacter pylori in vitro. Microbiol. Immunol. 2000, 44, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.Z.; Lee, J.H.; Lee, D.; Lee, H.S.; Hong, Y.S.; Kim, Y.H.; Lee, J.J. Quinolone alkaloids with inhibitory activity against nuclear factor of activated T cells from the fruits of Evodia rutaecarpa. Biol. Pharm. Bull. 2004, 27, 926–928. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, W.; Yang, X.W. New cytotoxic quinolone alkaloids from fruits of Evodia rutaecarpa. Fitoterapia 2012, 83, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Q.; Yang, X.W. Simultaneous determination of seven alkaloids and two flavonoid glycosides in Wuzhuyu decoction by RP-HPLC-DAD. J. Chin. Pharm. Sci. 2012, 21, 338–344. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Stenberg, P.; Bergström, C.A.; Luthman, K.; Artursson, P. Theoretical predictions of drug absorption in drug discovery and development. Clin. Pharmacokinet. 2002, 41, 877–899. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Hao, H.P.; Wang, G.J.; Sun, J.G.; Liang, Y.; Xie, L.; Zhang, Y.T.; Yan, B. Integrated pharmacokinetic study of multiple effective components contained in total Panax notoginsenosides. Chin. J. Nat. Med. 2008, 6, 377–381. [Google Scholar] [CrossRef]
- Xie, Y.; Hao, H.P.; Kang, A.; Liang, Y.; Xie, T.; Sun, S.Q.; Dai, C.; Zheng, X.; Xie, L.; Li, J.; et al. Integral pharmacokinetics of multiple lignan components in normal, CCl4-induced hepatic injury and hepatoprotective agents pretreated rats and correlations with hepatic injury biomarkers. J. Ethnopharmacol. 2010, 131, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.X.; Qian, Z.L.; Li, H.; Guo, L.W.; Pan, L.M.; Zhang, Q.C.; Tang, Y.P. Integrated pharmacokinetics of major bioactive components in MCAO rats after oral administration of Huang-Lian-Jie-Du-Tang. J. Ethnopharmacol. 2012, 141, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Okusanya, O.; Forrest, A.; DiFrancesco, R.; Bilic, S.; Rosenkranz, S.; Para, M.F.; Adams, E.; Yarasheski, K.E.; Reichman, R.C.; Morse, G.D.; et al. Compartmental pharmacokinetic analysis of oral amprenavir with secondary peaks. Antimicrob. Agents Chemother. 2007, 51, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Peris-Ribera, J.E.; Torres-Molina, F.; Garcia-Carbonell, M.C.; Aristorena, J.C.; Pla-Delfina, J.M. Pharmacokinetics and bioavailability of diclofenac in the rat. J. Pharmacokinet. Biopharm. 1991, 19, 647–665. [Google Scholar] [CrossRef] [PubMed]
- Lennernäs, H.; Regårdh, C.G. Regional gastrointestinal absorption of the beta-blocker pafenolol in the rat and intestinal transit rate determined by movement of 14C-polyethylene glycol (PEG) 4000. Pharm. Res. 1993, 10, 130–135. [Google Scholar] [CrossRef]
- Piyapolrungroj, N.; Zhou, Y.S.; Li, C.; Liu, G.; Zimmermann, E.; Fleisher, D. Cimetidine absorption and elimination in rat small intestine. Drug Metab. Dispos. 2000, 28, 65–72. [Google Scholar] [PubMed]
- Plusquellec, Y.; Campistron, G.; Staveris, S.; Barre, J.; Jung, L.; Tillement, J.P.; Houin, G. A double-peak phenomenon in the pharmacokinetics of veralipride after oral administration: A double-site model for drug absorption. J. Pharmacokinet. Biopharm. 1987, 15, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.K.; Shojaei, A.H. Effect of a lipoidic excipient on the absorption profile of compound UK 81252 in dogs after oral administration. J. Pharm. Pharm. Sci. 2004, 7, 8–12. [Google Scholar] [PubMed]
- Lipka, E.; Lee, I.D.; Langguth, P.; Spahn-Langguth, H.; Mutschler, E.; Amidon, G.L. Celiprolol double-peak occurrence and gastric motility: Nonlinear mixed effects modeling of bioavailability data obtained in dogs. J. Pharmacokinet. Biopharm. 1995, 23, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Metsugi, Y.; Miyaji, Y.; Ogawara, K.; Higaki, K.; Kimura, T. Appearance of double peaks in plasma concentration-time profile after oral administration depends on gastric emptying profile and weight function. Pharm. Res. 2008, 25, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, T.; Kasutani, M.; Tanaka, H.; Iwaki, M.; Tanino, T. Pharmacokinetics of epinastine and a possible mechanism for double peaks in oral plasma concentration profiles. Biol. Pharm. Bull. 2001, 24, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Eaker, E.Y.; Sninsky, C.A. Effect of berberine on myoelectric activity and transit of the small intestine in rats. Gastroenterology 1989, 96, 1506–1513. [Google Scholar] [CrossRef]
- Feng, Y.J.; Li, Y.Y.; Chen, C.Q.; Lin, X.H.; Yang, Y.H.; Cai, H.D.; Lv, Z.W.; Cao, M.H.; Li, K.; Xu, J.; et al. Inhibiting roles of berberine in gut movement of rodents are related to activation of the endogenous opioid system. Phytother. Res. 2013, 27, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.H.; Li, H.Y. Relaxant action of berberine, palmatine and total alkaloids from Rhizoma Coptidis on rat gastric fundus. Pharmacol. Clin. Chin. Mater. Med. 2006, 22, 48–50. [Google Scholar]
- Tsai, C.S.; Ochillo, R.F. Pharmacological effects of berberine on the longitudinal muscle of the guinea-pig isolated ileum. Arch. Int. Pharmacodyn. Ther. 1991, 310, 116–131. [Google Scholar] [PubMed]
- Yuan, J.; Shen, X.Z.; Zhu, X.S. Effect of berberine on transit time of human small intestine. Chin. J. Integr. Tradit. West. Med. 1994, 14, 718–720. [Google Scholar]
- Li, Z.J.; Wei, Y.; Chu, T.W. Radioiodination, biodistribution and pharmacokinetics of berberine in mice. J. Radioanal. Nucl. Chem. 2005, 265, 355–359. [Google Scholar] [CrossRef]
- Chen, C.M.; Chang, H.C. Determination of berberine in plasma, urine and bile by high-performance liquid chromatography. J. Chromatogr. B 1995, 665, 117–123. [Google Scholar] [CrossRef]
- Chen, H.X.; Huang, J.L.; Li, J. Structural elucidation of in vivo and in vitro metabolites of epiberberine in rat and its liver and intestines by liquid chromatography-tandem mass spectrometry. Anal. Lett. 2010, 43, 2505–2517. [Google Scholar] [CrossRef]
- Li, Y.; Ren, G.; Wang, Y.X.; Kong, W.J.; Yang, P.; Wang, Y.M.; Li, Y.H.; Yi, H.; Li, Z.R.; Song, D.Q.; et al. Bioactivities of berberine metabolites after transformation through CYP450 isoenzymes. J. Transl. Med. 2011, 9, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.M.; Han, F.M.; Chen, H.X.; Peng, Z.H.; Chen, Y. Identification of palmatine and its metabolites in rat urine by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, Y.F.; Yang, Q.; Li, Y.J.; Bao, T.D.; Weng, X.G.; Pan, G.F.; Zhu, X.X. Absorption of extractive Rhizoma Coptidis in rat everted gut scas. China J. Chin. Mater. Med. 2008, 33, 1056–1060. [Google Scholar]
- Tan, X.M.; Guo, Y.L.; Zhong, Y.F. In situ intestinal absorption kinetics of berberine and jatrorrhizine from extractive Rhizoma Coptidis in rats. China J. Chin. Mater. Med. 2010, 35, 755–758. [Google Scholar]
- Zhang, H.; An, R.; Xu, R.C.; Zhang, Y.Z.; Zhang, B.S.; Wang, X.H. Absorption of alkaloids in Gegen Qinlian decoction and different compatibilities in rat everted gut scas model. Chin. Tradit. Pat. Med. 2012, 34, 620–625. [Google Scholar]
- Deng, Y.T.; Liao, Q.F.; Bi, K.S.; Yao, M.C.; Jiang, X.F.; Xie, Z.Y. Studies on the solubility rules of components in Coptis-Evodia herb couple. Chin. Tradit. Pat. Med. 2008, 30, 900–903. [Google Scholar]
- Peng, M.X.; Wu, Y.J.; Cheng, Y.Y. Study on the solubility changes of Rhizoma Coptidis’ main chemical constituents influenced by the proportion of Rhizoma Coptidis and Fructus Evodiae. China J. Chin. Mater. Med. 2003, 28, 629–632. [Google Scholar]
- Hai, P.T.; Gonzalez-Alvarez, I.; Bermejo, M.; Garrigues, T.; Huong, L.T.T.; Cabrera-Perez, M.A. The use of rule-based and QSPR approaches in ADME profiling: A case study on Caco-2 permeability. Mol. Inform. 2013, 32, 459–479. [Google Scholar]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Hao, H.P.; Xie, H.G.; Lai, L.; Wang, Q.; Liu, C.X.; Wang, G.J. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Qiu, F.R.; Jiang, J.; Gao, C.L.; Tan, Y.Z. Intestinal absorption mechanisms of berberine, palmatine, jateorhizine, and coptisine: Involvement of P-glycoprotein. Xenobiotica 2011, 41, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Yee, S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Merz, K.M., Jr. Prediction of aqueous solubility of a diverse set of compounds using quantitative structure-property relationships. J. Med. Chem. 2003, 46, 3572–3580. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Teng, J.; Wang, Y.; Xu, W. The permeability and the efflux of alkaloids of the Evodiae fructus in the Caco-2 model. Phytother. Res. 2009, 23, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Maeng, H.J.; Yoo, H.J.; Kim, I.W.; Song, I.S.; Chung, S.J.; Shim, C.K. P-glycoprotein-mediated transport of berberine across Caco-2 cell monolayers. J. Pharm. Sci. 2002, 91, 2614–2621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Qiu, F.R.; Jiang, J.; Gao, C.L. Comparative study of transport character of jatrorrhizine, coptisine, palmatine and berberine in Zuojin Wan and its similar formulae in Caco-2 cells. China J. Tradit. Chin. Med. Pharm. 2011, 26, 999–1003. [Google Scholar]
- Ma, B.L.; Yao, M.K.; Han, X.H.; Ma, Y.M.; Wu, J.S.; Wang, C.H. Influences of Fructus Evodiae pretreatment on the pharmacokinetics of Rhizoma coptidis alkaloids. J. Ethnopharmacol. 2011, 137, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Metsugi, Y.; Ogawara, K.; Higaki, K.; Kimura, T. Evaluation of in vivo dissolution behavior and GI transit of griseofulvin, a BCS class II drug. Int. J. Pharm. 2008, 352, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.H.; Kim, J.O.; Chung, H.K.; Choi, S.M.; Kim, J.H.; Kwon, J.W.; Yoo, M.; Lee, J.H.; Lee, M.G. Pharmacokinetics of oral amlodipine orotate in vagotomized dogs. Biopharm. Drug Dispos. 2006, 27, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Marathe, P.H.; Wen, Y.; Norton, J.; Greene, D.S.; Barbhaiya, R.H.; Wilding, I.R. Effect of altered gastric emptying and gastrointestinal motility on metformin absorption. Br. J. Clin. Pharmacol. 2000, 50, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Teng, J. Chemical constituents of the unripe fruits of Evodia rutaecarpa. J. Chin. Pharm. Sci. 2007, 16, 20–23. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2015; Volume I, pp. 763–764. [Google Scholar]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research; Center for Veterinary Medicine. Guidance for Industry, Bioanalytical Method Validation. USA. 2013. Available online: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm368107.pdf (accessed on 26 January 2017).
- Wang, S.; Hu, Y.; Tan, W.; Wu, X.; Chen, R.; Cao, J.; Chen, M.; Wang, Y. Compatibility art of traditional Chinese medicine: From the perspective of herb pairs. J. Ethnopharmacol. 2012, 143, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
| Analytes | Group | AUC0→t (ng·h/mL) | AUC0→∞ (ng·h/mL) | MRT0→t (min) | MRT0→∞ (min) | t1/2 (min) | Tmax (min) | Cmax (ng/mL) | Tsec (min) | Csec (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| coptisine | ZJ | 68.73 ± 10.82 | 71.59 ± 10.72 | 641.65 ± 62.14 | 735.50 ± 91.37 | 480.47 ± 115.11 | 300 | 14.13 ± 7.75 | 90 | 4.96 ± 2.29 * |
| FZJ | 10.99 ± 1.64 | 11.85 ± 1.25 | 250.52 ± 27.45 | 312.29 ± 83.18 | 176.05 ± 73.47 | 180 | 4.78 ± 2.31 | - | - | |
| epiberberine | ZJ | 68.46 ± 15.94 | 69.61 ± 15.46 | 645.43 ± 62.71 | 696.78 ± 87.57 | 366.42 ± 101.94 | 300 | 13.65 ± 7.05 | 90 | 5.51 ± 2.17 * |
| FZJ | 10.13 ± 1.09 | 11.40 ± 1.27 | 233.44 ± 25.91 | 315.37 ± 52.51 | 235.17 ± 101.63 | 180 | 4.82 ± 1.97 | - | - | |
| palmatine | ZJ | 88.34 ± 24.92 | 96.63 ± 33.22 | 404.26 ± 105.70 | 525.01 ± 192.01 | 339.21 ± 144.61 | 90 | 34.07 ± 19.25 | 300 | 11.18 ± 8.71 * |
| FZJ | 18.19 ± 4.98 | 18.90 ± 5.50 | 231.35 ± 21.68 | 269.46 ± 38.99 | 150.78 ± 103.54 | 180 | 9.74 ± 3.53 | 30 | 6.25 ± 2.89 * | |
| berberine | ZJ | 277.48 ± 50.15 | 299.84 ± 55.27 | 387.56 ± 67.86 | 514.76 ± 164.64 | 378.28 ± 194.53 | 90 | 109.40 ± 48.27 | 300 | 44.68 ± 21.28 * |
| FZJ | 48.07 ± 9.60 | 50.24 ± 10.74 | 244.37 ± 17.40 | 280.76 ± 37.56 | 133.26 ± 50.10 | 180 | 23.67 ± 7.42 | 30 | 11.64 ± 7.28 * | |
| 8-oxocoptisine | ZJ | 24.74 ± 3.90 | 27.02 ± 2.23 | 607.27 ± 59.56 | 809.87 ± 254.49 | 535.67 ± 158.71 | 300 | 2.46 ± 1.17 | 90 | 2.42 ± 0.89 |
| FZJ | - | - | - | - | - | - | - | - | - | |
| 8-oxoepiberberine | ZJ | 14.30 ± 2.32 | 16.63 ± 3.22 | 450.77 ± 59.37 | 740.15 ± 138.92 | 592.65 ± 274.26 | 90 | 2.38 ± 1.14 | 300 | 1.85 ± 0.99 |
| FZJ | - | - | - | - | - | - | - | - | - | |
| noroxyhydrastinine | ZJ | 133.19 ± 18.49 | 140.06 ± 17.83 | 529.35 ± 75.19 | 662.05 ± 153.25 | 644.66 ± 169.16 | 90 | 29.64 ± 15.15 | 300 | 16.29 ± 8.38 |
| FZJ | 4.95 ± 0.65 | 5.37 ± 0.73 | 181.73 ± 16.42 | 314.79 ± 172.07 | 243.81 ± 175.97 | 30 | 3.17 ± 1.00 | - | - | |
| corydaldine | ZJ | 45.14 ± 7.46 | 47.27 ± 7.36 | 402.95 ± 56.32 | 513.14 ± 109.81 | 595.01 ± 123.69 | 90 | 10.34 ± 5.45 | 300 | 9.03 ± 4.31 |
| FZJ | 2.86 ± 0.35 | 3.10 ± 0.46 | 170.94 ± 11.39 | 239.52 ± 47.43 | 233.20 ± 77.36 | 30 | 1.73 ± 0.58 | - | - | |
| dehydroevodiamine | ZJ | 532.34 ± 57.78 | 537.43 ± 54.97 | 260.43 ± 15.40 | 282.90 ± 34.14 | 192.57 ± 135.94 | 240 | 155.16 ± 27.92 | 90 | 117.29 ± 45.45 |
| FZJ | 274.77 ± 23.19 | 285.60 ± 24.08 | 279.59 ± 56.33 | 357.86 ± 114.71 | 301.96 ± 172.11 | 90 | 85.27 ± 13.37 | - | - | |
| evodiamine | ZJ | 31.71 ± 2.94 | 31.84 ± 3.11 | 315.19 ± 44.82 | 359.80 ± 45.66 | 141.31 ± 71.63 | 60 | 9.59 ± 4.22 | 300 | 5.54 ± 3.14 |
| FZJ | 10.83 ± 1.77 | 11.72 ± 2.06 | 178.31 ± 22.86 | 254.67 ± 42.11 | 227.88 ± 62.98 | 30 | 9.10 ± 4.79 | 180 | 2.21 ± 0.85 | |
| wuchuyuamide-I | ZJ | 11.54 ± 1.40 | 12.77 ± 1.43 | 231.61 ± 27.74 | 313.20 ± 68.30 | 218.14 ± 89.78 | 90 | 3.45 ± 1.73 | 20 | 2.55 ± 0.38 |
| FZJ | 48.25 ± 8.86 | 50.11 ± 8.81 | 346.41 ± 46.61 | 413.10 ± 94.85 | 295.01 ± 109.89 | 30 | 10.73 ± 4.43 | - | - | |
| evocarpine | ZJ | 55.65 ± 12.40 | 59.02 ± 13.34 | 251.37 ± 29.26 | 390.99 ± 119.47 | 539.67 ± 383.48 | 60 | 24.20 ± 12.95 | 240 | 7.36 ± 4.89 |
| FZJ | 17.84 ± 3.99 | 19.57 ± 4.79 | 422.27 ± 69.79 | 557.88 ± 117.71 | 414.88 ± 60.07 | 30 | 4.07 ± 0.44 | - | - |
| Analytes | ZJ | FZJ | ||
|---|---|---|---|---|
| r2 | AIC | r2 | AIC | |
| noroxyhydrastinine | 0.313 ± 0.350 | 118.137 ± 17.623 | 0.802 ± 0.128 | 16.505 ± 12.486 |
| corydaldine | 0.243 ± 0.267 | 85.431 ± 9.718 | 0.805 ± 0.066 | 3.947 ± 8.187 |
| dehydroevodiamine | 0.324 ± 0.280 | 154.46 ± 11.416 | 0.886 ± 0.055 | 107.718 ± 6.140 |
| evodiamine | 0.428 ± 0.169 | 71.547 ± 6.327 | 0.853 ± 0.232 | 30.067 ± 6.112 |
| wuchuyuamide-I | 0.544 ± 0.140 | 35.057 ± 11.851 | 0.519 ± 0.407 | 66.844 ± 7.076 |
| evocarpine | 0.734 ± 0.201 | 78.232 ± 11.393 | 0.740 ± 0.167 | 27.61 ± 10.527 |
| Analytes | ZJ | FZJ | |||||
|---|---|---|---|---|---|---|---|
| AUC0→∞/D | Cmax/D | Wi * | AUC0→∞/D | Cmax/D | Wi * | ||
| CR alkaloids | coptisine | 0.40 | 0.08 | 0.09 | 1.80 | 0.72 | 0.12 |
| epiberberine | 0.62 | 0.12 | 0.09 | 1.91 | 0.81 | 0.11 | |
| palmatine | 0.77 | 0.27 | 0.13 | 3.03 | 1.56 | 0.19 | |
| berberine | 0.84 | 0.31 | 0.39 | 2.56 | 1.21 | 0.50 | |
| 8-oxocoptisine | 44.44 | 3.98 | 0.04 | - | - | - | |
| 8-oxoepiberberine | 97.25 | 13.92 | 0.02 | - | - | - | |
| noroxyhydrastinine | 1687.47 | 357.11 | 0.18 | 268.50 | 158.50 | 0.05 | |
| corydaldine | 1688.21 | 369.29 | 0.06 | 310.00 | 173.00 | 0.03 | |
| EF alkaloids | dehydroevodiamine | 39.69 | 11.46 | 0.84 | 12.15 | 3.63 | 0.78 |
| evodiamine | 8.55 | 2.57 | 0.05 | 3.73 | 2.89 | 0.03 | |
| wuchuyuamide-I | 172.57 | 46.62 | 0.02 | 278.39 | 59.61 | 0.14 | |
| evocarpine | 35.17 | 14.42 | 0.09 | 13.16 | 2.74 | 0.05 | |
| PK Parameters | Integrated Method | ZJ | FZJ | ||
|---|---|---|---|---|---|
| CR Alkaloids | EF Alkaloids | CR Alkaloids | EF Alkaloids | ||
| AUC0→t (ng·h/mL) | A | 165.85 ± 25.24 | 454.02 ± 49.50 | 31.01 ± 4.38 | 222.33 ± 18.87 |
| B | 740.58 ± 96.29 | 632.55 ± 69.91 | 97.24 ± 12.03 | 352.74 ± 31.92 | |
| AUC0→∞ (ng·h/mL) | A | 174.90 ± 23.60 | 458.50 ± 46.98 | 36.92 ± 8.61 | 230.95 ± 19.29 |
| B | 790.50 ± 129.01 * | 640.61 ± 64.83 * | 113.78 ± 19.69 * | 368.20 ± 33.08 * | |
| MRT0→t (min) | A | 465.48 ± 62.56 | 260.53 ± 15.54 | 251.24 ± 19.62 | 282.29 ± 55.06 |
| B | 498.47 ± 54.24 | 262.85 ± 17.19 | 243.73 ± 17.92 | 295.12 ± 49.32 | |
| MRT0→∞ (min) | A | 464.99 ± 65.15 | 283.73 ± 34.85 | 254.95 ± 60.94 | 359.90 ± 111.27 |
| B | 513.57 ± 53.75 | 293.62 ± 40.77 | 272.09 ± 72.64 | 373.48 ± 90.75 | |
| t1/2 (min) | A | 537.02 ± 408.47 | 195.02 ± 136.85 | 141.62 ± 31.75 | 303.14 ± 162.88 |
| B | 573.75 ± 447.62 | 218.36 ± 137.70 | 186.77 ± 91.91 | 334.42 ± 104.19 | |
| Tmax (min) | A | 90 | 240 | 180 | 90 |
| B | 90 | 240 | 180 | 90 | |
| Cmax (ng/mL) | A | 54.14 ± 22.14 | 131.18 ± 23.45 | 14.82 ± 4.70 | 67.02 ± 10.36 |
| B | 198.70 ± 76.91 * | 167.10 ± 28.40 * | 43.79 ± 14.12 * | 92.367 ± 12.51 * | |
| Tsec (min) | A | 300 | 90 | 30 | - |
| B | 300 | 90 | 30 | - | |
| Csec (ng/mL) | A | 24.99 ± 10.08 | 99.90 ± 38.75 | 7.51 ± 4.19 | - |
| B | 113.27 ± 40.70 | 138.18 ± 54.42 | 25.35 ± 10.26 | - | |
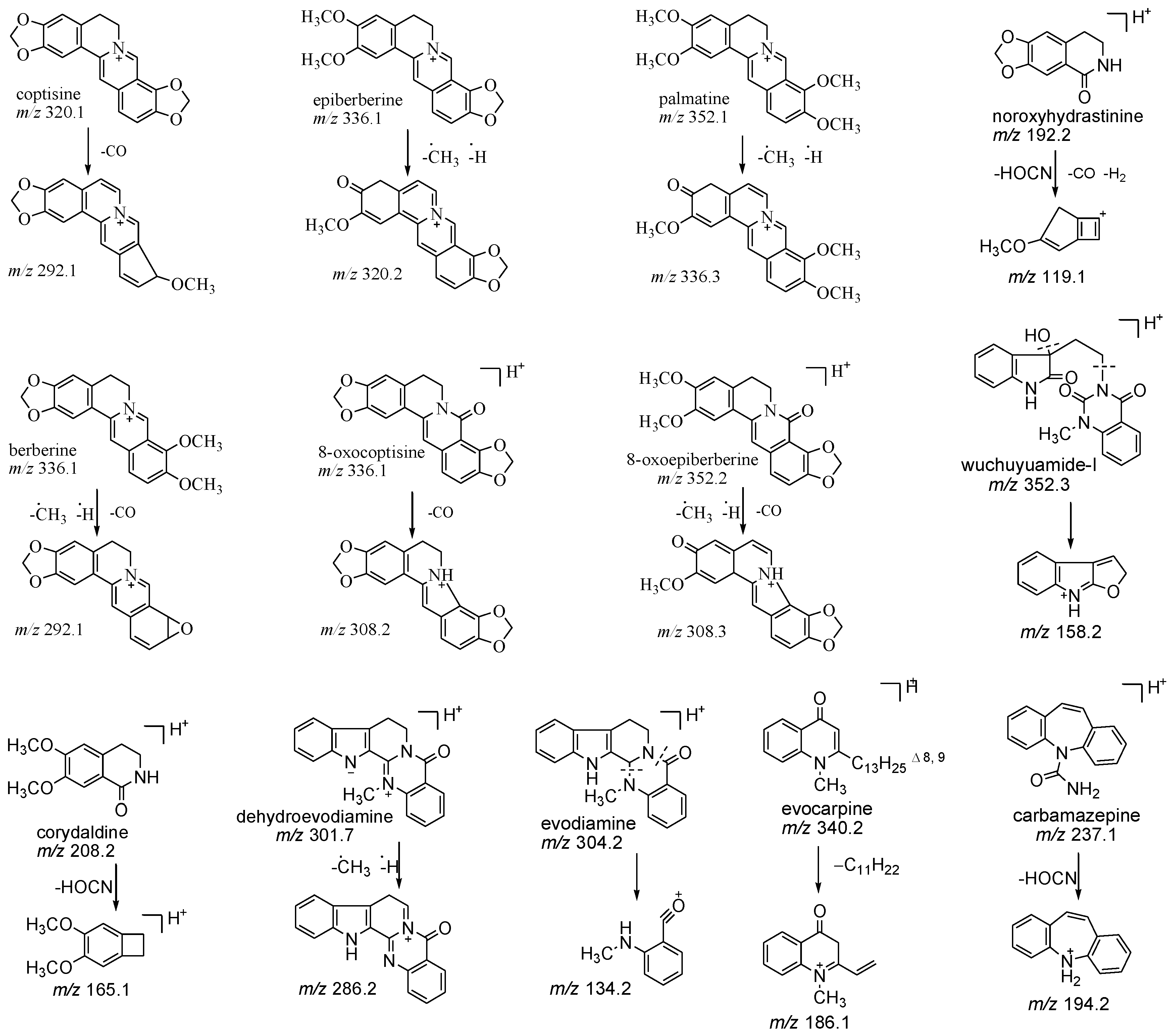
| Analytes | Q1 (Da) | Q3 (Da) | DP (V) | CE (eV) |
|---|---|---|---|---|
| coptisine | 320.1 | 292.1 | 110.1 | 42.3 |
| epiberberine | 336.1 | 320.2 | 101.0 | 46.2 |
| palmatine | 352.1 | 336.3 | 86.9 | 42.7 |
| berberine | 336.1 | 292.1 | 85.7 | 46.8 |
| 8-oxocoptisine | 336.1 | 308.2 | 102.6 | 38.1 |
| 8-oxoepiberberine | 352.2 | 308.3 | 105.5 | 48.2 |
| noroxyhydrastinine | 192.2 | 119.1 | 74.6 | 33.8 |
| corydaldine | 208.2 | 165.1 | 68.3 | 26.3 |
| dehydroevodiamine | 301.7 | 286.2 | 99.6 | 53.0 |
| evodiamine | 304.2 | 134.2 | 91.8 | 36.4 |
| wuchuyuamide-I | 352.3 | 158.2 | 55.8 | 31.7 |
| evocarpine | 340.2 | 186.1 | 136.5 | 54.5 |
| carbamazepine (IS) | 237.1 | 194.2 | 76.9 | 25.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, P.; Zhang, Y.-B.; Yang, Y.-F.; Xu, W.; Yang, X.-W. Pharmacokinetics Studies of 12 Alkaloids in Rat Plasma after Oral Administration of Zuojin and Fan-Zuojin Formulas. Molecules 2017, 22, 214. https://doi.org/10.3390/molecules22020214
Qian P, Zhang Y-B, Yang Y-F, Xu W, Yang X-W. Pharmacokinetics Studies of 12 Alkaloids in Rat Plasma after Oral Administration of Zuojin and Fan-Zuojin Formulas. Molecules. 2017; 22(2):214. https://doi.org/10.3390/molecules22020214
Chicago/Turabian StyleQian, Ping, You-Bo Zhang, Yan-Fang Yang, Wei Xu, and Xiu-Wei Yang. 2017. "Pharmacokinetics Studies of 12 Alkaloids in Rat Plasma after Oral Administration of Zuojin and Fan-Zuojin Formulas" Molecules 22, no. 2: 214. https://doi.org/10.3390/molecules22020214
APA StyleQian, P., Zhang, Y.-B., Yang, Y.-F., Xu, W., & Yang, X.-W. (2017). Pharmacokinetics Studies of 12 Alkaloids in Rat Plasma after Oral Administration of Zuojin and Fan-Zuojin Formulas. Molecules, 22(2), 214. https://doi.org/10.3390/molecules22020214





