Exploring the Effects of Geographical Origin on the Chemical Composition and Quality Grading of Vitis vinifera L. cv. Chardonnay Grapes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Understanding Regional Effects on Grape Composition
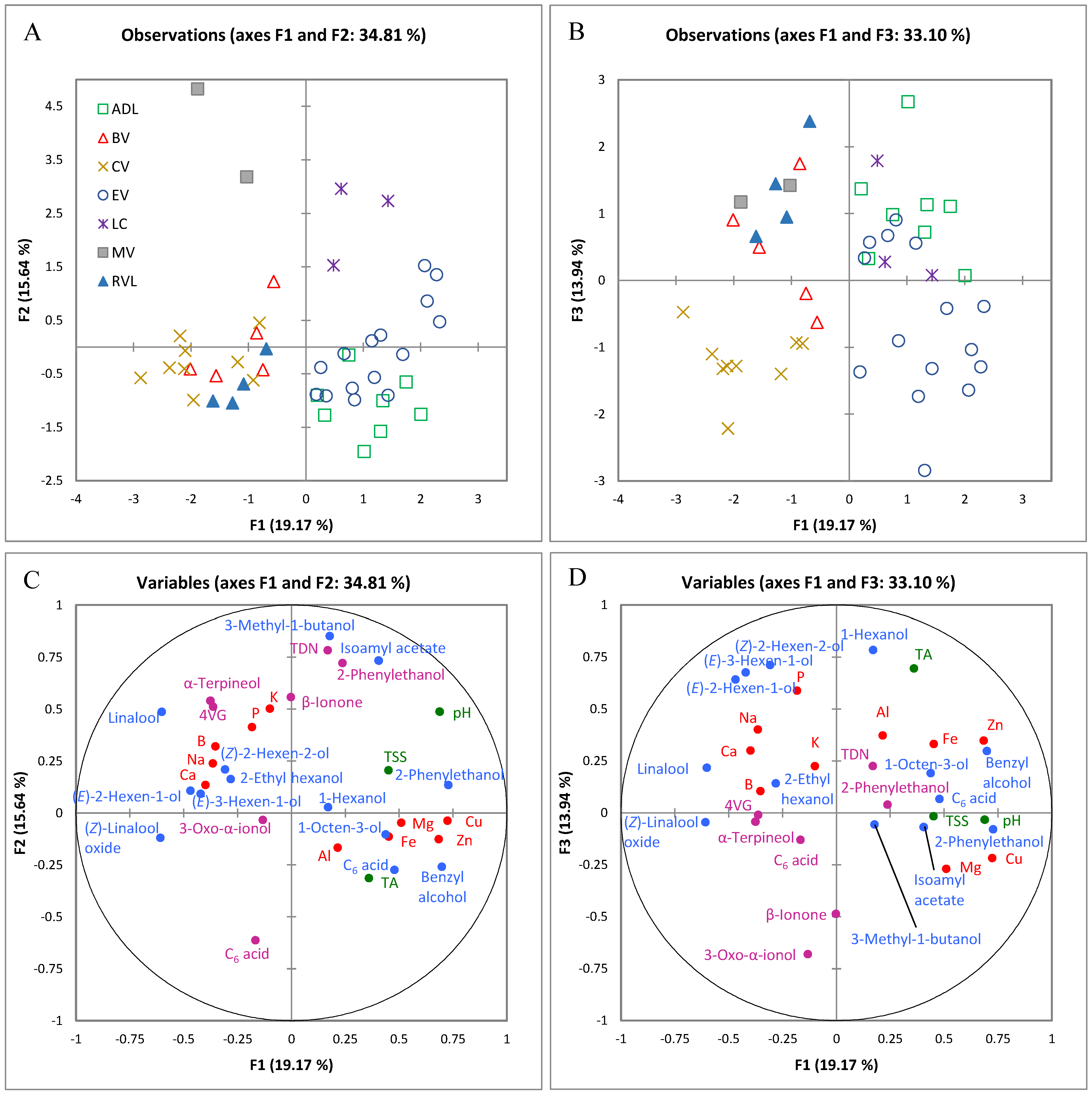
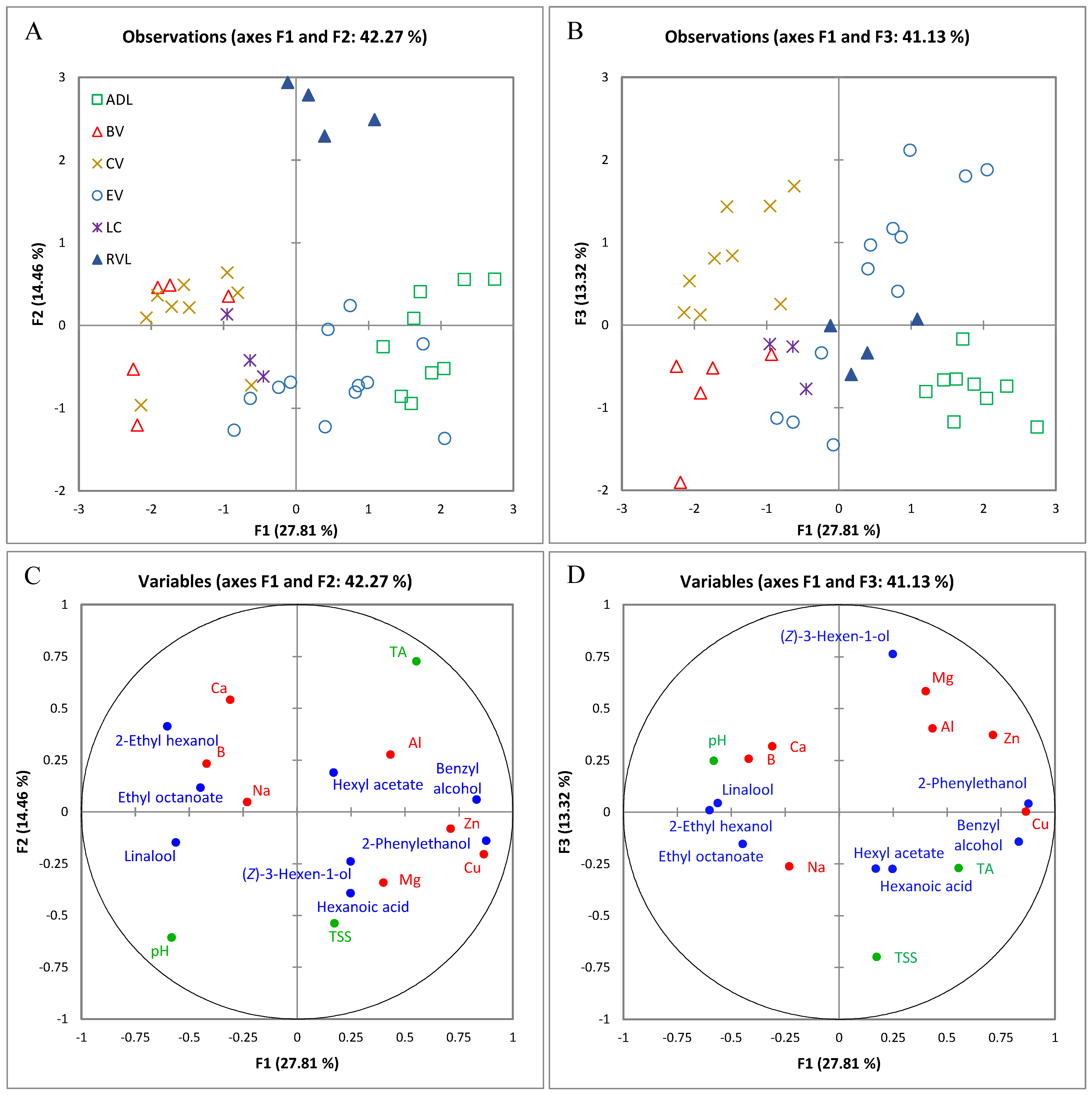
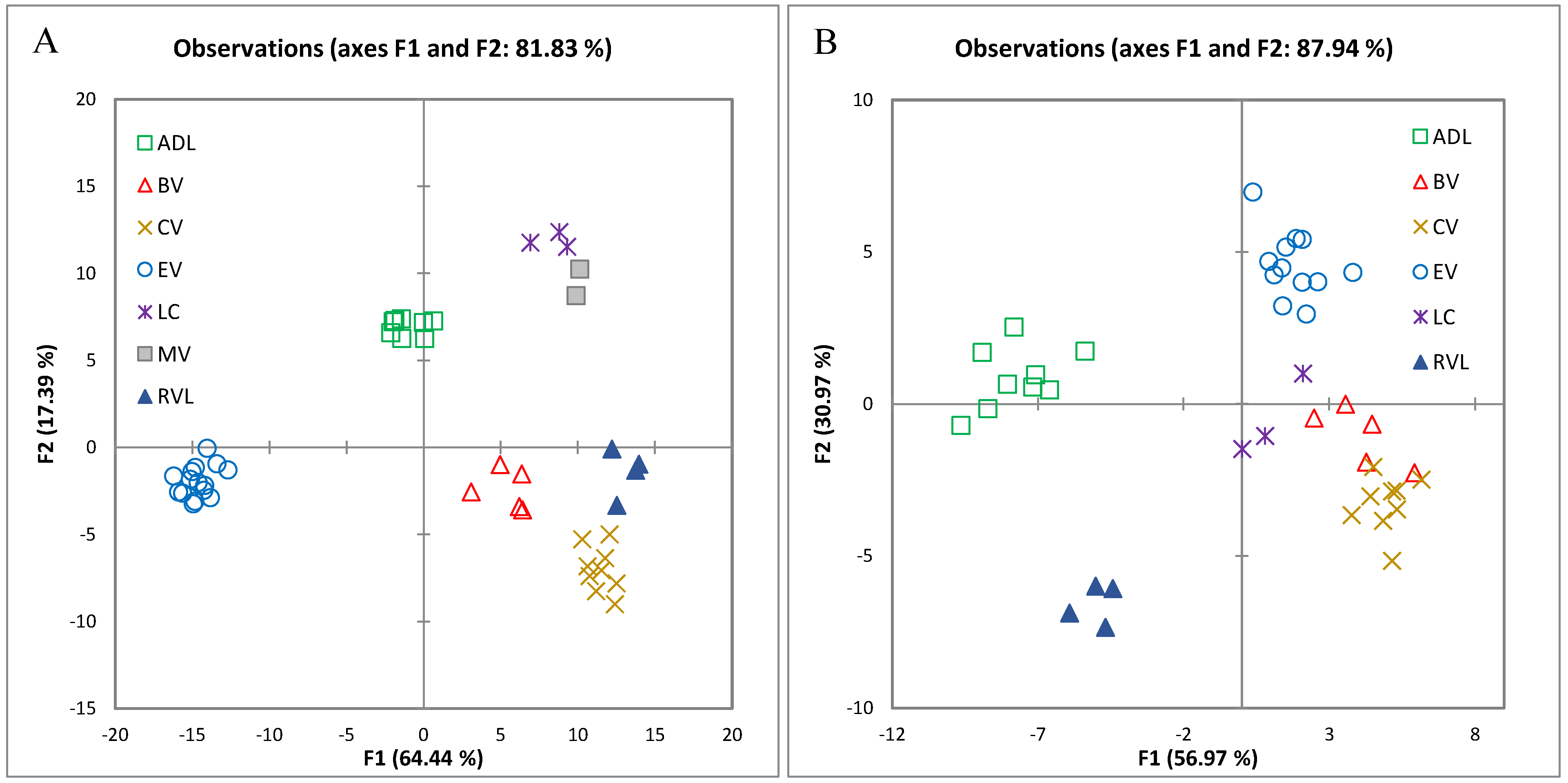
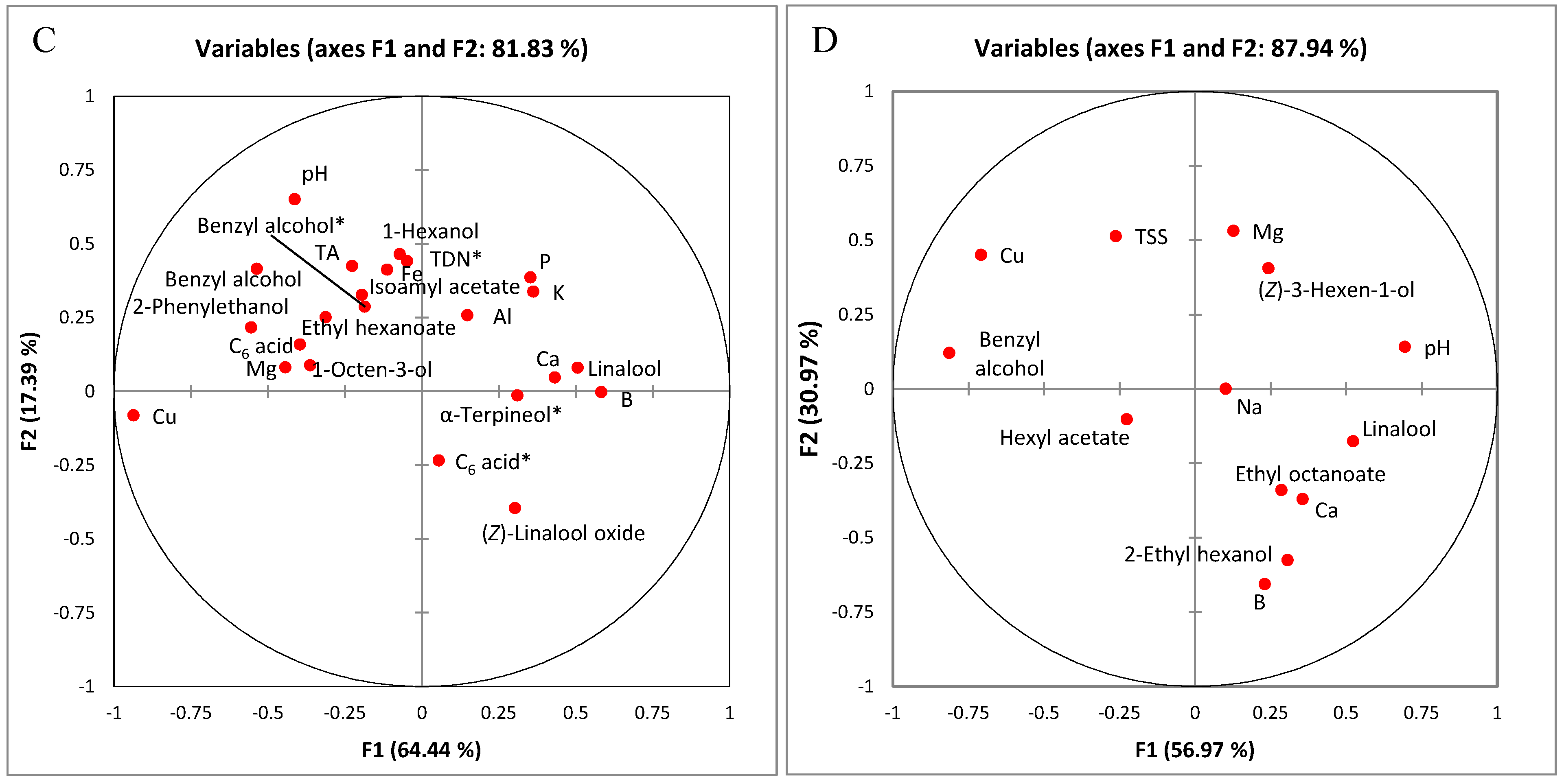
2.1.1. Classification According to Origin Using Multiple Factor Analysis of Analytical Variables
2.1.2. Differentiating Variables
2.1.3. Major and Trace Elements
2.1.4. Amino Acids
2.1.5. Glycosides
2.2. Prediction of Geographical Indication Based on Composition Variables
2.3. Correlating Grape Grading and Grape Composition
Impact of Climate on Berry Quality Grades
3. Materials and Methods
3.1. Chemicals
3.2. Grapes
3.3. Juice Basic Chemical Analysis
3.4. Headspace-Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS) of Free Volatiles from Grapes
3.5. Analysis of Hydrolysed Grape Glycosides by Gas Chromatography-Mass Spectrometry (GC-MS)
3.6. Element Analysis by Inductively Couple Plasma-Optical Emission Spectroscopy (ICP-OES)
3.7. Amino Acid Analysis by HPLC with Diode Array Detection
3.8. Climatic and Soil Data
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Trade Organization. World Trade Organization. WTO Analytical Index: TRIPS. Agreement on Trade-Related Aspects of Intellectual Property Rights. Available online: https://www.wto.org/english/res_e/booksp_e/analytic_index_e/trips_02_e.htm#article23 (accessed on 29 November 2016).
- Bowers, S.A. Location, location, location: The case against extending Geographical Indication protection under the TRIPS agreement. AIPLA Q. J. 2003, 31, 129–164. [Google Scholar]
- Van Leeuwen, C.; Seguin, G. The concept of terroir in viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
- Versari, A.; Laurie, V.F.; Ricci, A.; Laghi, L.; Parpinello, G.P. Progress in authentication, typification and traceability of grapes and wines by chemometric approaches. Food Res. Int. 2014, 60, 2–18. [Google Scholar] [CrossRef]
- Son, H.S.; Hwang, G.S.; Kim, K.M.; Ahn, H.J.; Park, W.M.; van den Berg, F.; Hong, Y.S.; Lee, C.H. Metabolomic studies on geographical grapes and their wines using 1H NMR analysis coupled with multivariate statistics. J. Agric. Food Chem. 2009, 57, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Tarr, P.T.; Dreyer, M.L.; Athanas, M.; Shahgholi, M.; Saarlos, K.; Second, T.P. A metabolomics based approach for understanding the influence of terroir in Vitis vinifera L. Metabolomics 2013, 9, S170–S177. [Google Scholar] [CrossRef]
- Teixeira, A.; Martins, V.; Noronha, H.; Eiras-Dias, J.; Gerós, H. The first insight into the metabolite profiling of grapes from three Vitis vinifera L. cultivars of two controlled appellation (DOC) regions. Int. J. Mol. Sci. 2014, 15, 4237–4254. [Google Scholar] [PubMed]
- Schmidt, S.A.; Dillon, S.; Kolouchova, R.; Henschke, P.A.; Chambers, P.J. Impacts of variations in elemental nutrient concentration of Chardonnay musts on Saccharomyces cerevisiae fermentation kinetics and wine composition. Appl. Microbiol. Biotechnol. 2011, 91, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Minerals. In Understanding Wine Chemistry; John Wiley & Sons, Ltd.: West Sussex, UK, 2016; pp. 34–39. [Google Scholar]
- Cugnetto, A.; Santagostini, L.; Rolle, L.; Guidoni, S.; Gerbi, V.; Novello, V. Tracing the “terroirs” via the elemental composition of leaves, grapes and derived wines in cv Nebbiolo (Vitis vinifera L.). Sci. Hortic. 2014, 172, 101–108. [Google Scholar] [CrossRef]
- Protano, G.; Rossi, S. Relationship between soil geochemistry and grape composition in Tuscany (Italy). J. Plant Nutr. Soil Sci. 2014, 177, 500–508. [Google Scholar] [CrossRef]
- Cadot, Y.; Caillé, S.; Thiollet-Scholtus, M.; Samson, A.; Barbeau, G.; Cheynier, V. Characterisation of typicality for wines related to terroir by conceptual and by perceptual representations. An application to red wines from the Loire Valley. Food Qual. Pref. 2012, 24, 48–58. [Google Scholar] [CrossRef]
- Robinson, A.L.; Adams, D.O.; Boss, P.K.; Heymann, H.; Solomon, P.S.; Trengove, R.D. Influence of geographic origin on the sensory characteristics and wine composition of Vitis vinifera cv. Cabernet Sauvignon wines from Australia. Am. J. Enol. Vitic. 2012, 63, 467–476. [Google Scholar] [CrossRef]
- Fischer, U.; Roth, D.; Christmann, M. The impact of geographic origin, vintage and wine estate on sensory properties of Vitis vinifera cv. Riesling wines. Food Qual. Pref. 1999, 10, 281–288. [Google Scholar] [CrossRef]
- Lund, C.M.; Thompson, M.K.; Benkwitz, F.; Wohler, M.W.; Triggs, C.M.; Gardner, R.; Heymann, H.; Nicolau, L. New Zealand Sauvignon blanc distinct flavor characteristics: Sensory, chemical, and consumer aspects. Am. J. Enol. Vitic. 2009, 60, 1–12. [Google Scholar]
- Heymann, H.; Noble, A. Descriptive analysis of commercial Cabernet sauvignon wines from California. Am. J. Enol. Vitic. 1987, 38, 41–44. [Google Scholar]
- Goldner, M.C.; Zamora, M.C. Sensory characterization of Vitis vinifera cv. Malbec wines from seven viticulture regions of Argentina. J. Sens. Stud. 2007, 22, 520–532. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the aroma of grapes and wines: a review. S. Afr. J. Enol. Vitic. 1983, 4, 49–60. [Google Scholar]
- Hernandez-Orte, P.; Concejero, B.; Astrain, J.; Lacau, B.; Cacho, J.; Ferreira, V. Influence of viticulture practices on grape aroma precursors and their relation with wine aroma. J. Sci. Food Agric. 2015, 95, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Ferreira, V.; Fernández, P.; Cacho, J. A study of factors affecting wine volatile composition and its application in discriminant analysis. LWT--Food Sci. Technol. 1996, 29, 251–259. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; Boutegrabet, L.; Gougeon, R.D.; Schmitt-Kopplin, P. A grape and wine chemodiversity comparison of different appellations in Burgundy: Vintage vs terroir effects. Food Chem. 2013, 152, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Berna, A.Z.; Trowell, S.; Clifford, D.; Cynkar, W.; Cozzolino, D. Geographical origin of Sauvignon Blanc wines predicted by mass spectrometry and metal oxide based electronic nose. Anal. Chim. Acta 2009, 648, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Arrhenius, S.P.; McCloskey, L.P.; Sylvan, M. Chemical markers for aroma of Vitis vinifera var. Chardonnay regional wines. J. Agric. Food Chem. 1996, 44, 1085–1090. [Google Scholar] [CrossRef]
- Dunlevy, J.D.; Kalua, C.M.; Keyzers, R.A.; Boss, P.K. The production of flavour & aroma compounds in grape berries. In Grapevine Molecular Physiology & Biotechnology, 2nd ed.; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 293–340. [Google Scholar]
- Chassagne, D.; Vernizeau, S.; Nedjma, M.; Alexandre, H. Hydrolysis and sorption by Saccharomyces cerevisiae strains of Chardonnay grape must glycosides during fermentation. Enzyme Microb. Tech. 2005, 37, 212–217. [Google Scholar] [CrossRef]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Busto, O. Data fusion methodologies for food and beverage authentication and quality assessment – A review. Anal. Chim. Acta 2015, 891, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Williams, L.J.; Valentin, D. Multiple factor analysis: principal component analysis for multitable and multiblock data sets. WIREs Comput. Stat. 2013, 5, 149–179. [Google Scholar] [CrossRef]
- Tzouros, N.E.; Arvanitoyannis, I.S. Agricultural produces: synopsis of employed quality control methods for the authentication of foods and application of chemometrics for the classification of foods according to their variety or geographical origin. Crit. Rev. Food Sci. Nutr. 2001, 41, 287–319. [Google Scholar] [CrossRef] [PubMed]
- Saurina, J. Characterization of wines using compositional profiles and chemometrics. TrAC, Trends Anal. Chem. 2010, 29, 234–245. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: a basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Bramley, R.G.V.; Ouzman, J.; Boss, P.K. Variation in vine vigour, grape yield and vineyard soils and topography as indicators of variation in the chemical composition of grapes, wine and wine sensory attributes. Aust. J. Grape Wine Res. 2011, 17, 217–229. [Google Scholar] [CrossRef]
- Lee, J.E.; Hwang, G.S.; Van Den Berg, F.; Lee, C.H.; Hong, Y.S. Evidence of vintage effects on grape wines using 1H NMR-based metabolomic study. Anal. Chim. Acta 2009, 648, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.; Friant, P.; Choné, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of climate, soil, and cultivar on terroir. Am. J. Enol. Vitic. 2004, 55, 207–217. [Google Scholar]
- Alessandrini, M.; Gaiotti, F.; Belfiore, N.; Matarese, F.; D’onofrio, C.; Tomasi, D. Influence of vineyard altitude on Glera grape ripening (Vitis vinifera L.): effects on aroma evolution and wine sensory profile. J. Sci. Food Agric. 2016. [Google Scholar] [CrossRef] [PubMed]
- Baumes, R.; Wirth, J.; Bureau, S.; Gunata, Y.; Razungles, A. Biogeneration of C13-norisoprenoid compounds: Experiments supportive for an apo-carotenoid pathway in grapevines. Anal. Chim. Acta 2002, 458, 3–14. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Cozzolino, D.; Bastian, S.E.P.; Jeffery, D.W. Towards the creation of a wine quality prediction index: Correlation of Chardonnay juice and wine compositions from different regions and quality levels. Food Anal. Methods 2016, 9, 2842–2855. [Google Scholar] [CrossRef]
- Sabir, A.; Yazar, K.; Sabir, F.; Kara, Z.; Yazici, M.A.; Goksu, N. Vine growth, yield, berry quality attributes and leaf nutrient content of grapevines as influenced by seaweed extract (Ascophyllum nodosum) and nanosize fertilizer pulverizations. Sci. Hortic. 2014, 175, 1–8. [Google Scholar] [CrossRef]
- ASRIS. Australian Soil Resource Information System. Available online: http://www.asris.csiro.au (accessed on 13 November 2016).
- Walker, G.M. Metals in yeast fermentation processes. Adv. Appl. Microbiol. 2004, 54, 197–229. [Google Scholar] [PubMed]
- Haynes, R.J. Effects of liming on phosphate availability in acid soils. Plant Soil 1982, 68, 289–308. [Google Scholar] [CrossRef]
- Walker, G.M. Yeast nutrition. In Yeast Physiology and Biotechnology; John Wiley & Sons: West Sussex, UK, 2000; pp. 88–90. [Google Scholar]
- Dombek, K.M.; Ingram, L.O. Magnesium limitation and its role in apparent toxicity of ethanol during yeast fermentation. Appl. Environ. Microbiol 1986, 52, 975–981. [Google Scholar] [PubMed]
- Sommer, S.; Wehgmann-Herr, P.; Wacker, M.; Fischer, U. Rationale for a stronger disposition of Chardonnay wines for stuck and sluggish fermentation. S. Afr. J. Enol. Vitic. 2015, 36, 181–190. [Google Scholar] [CrossRef]
- Bell, S.-J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine R. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Guitart, A.; Cacho, J. Changes in the concentration of amino acids during the ripening of Vitis vinifera Tempranillo variety from the Denomination d'Origine Somontano (Spain). Am. J. Enol. Vitic. 1999, 50, 144–154. [Google Scholar]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Nykänen, L. Formation and occurrence of flavor compounds in wine and distilled alcoholic beverages. Am. J. Enol. Vitic. 1986, 37, 84–96. [Google Scholar]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of Chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef] [PubMed]
- Stines, A.P.; Grubb, J.; Gockowiak, P.A.; Henschke, P.A.; Høj, P.B.; van Heeswick, R. Proline and arginine accumulation in developing berries of Vitis vinifera L. in Australian vineyards: Influence of vine cultivar, berry maturity and tissue type. Aust. J. Grape Wine Res. 2000, 6, 150–158. [Google Scholar] [CrossRef]
- Amerine, M.A.; Ough, C.S. Methods for Analysis of Musts and Wines; John Wiley and Sons: New York, NY, USA, 1980. [Google Scholar]
- Marais, J.; Van Wyk, C.J.; Rapp, A. Carotenoid levels in maturing grapes as affected by climatic regions, sunlight and shade. S. Afr. J. Enol. Vitic. 1991, 12, 64–69. [Google Scholar]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Isoprenoids. In Understanding Wine Chemistry; John Wiley & Sons: West Sussex, UK, 2016; pp. 68–76. [Google Scholar]
- Reynolds, A.G.; Wardle, D.A. Influence of fruit microclimate on monoterpene levels of Gewürztraminer. Am. J. Enol. Vitic. 1989, 40, 149–154. [Google Scholar]
- Constantini, V.; Bellincontro, A.; de Santis, D.; Botondi, R.; Mencarelli, F. Metabolic changes of Malvasia grapes for wine production during postharvest drying. J. Agric. Food Chem. 2006, 54, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Romieu, C.; Tesniere, C.; Than-Ham, L.; Flanzy, C.; Robin, J.-P. An examination of the importance of anaerobiosis and ethanol in causing injury to grape mitochondria. Am. J. Enol. Vitic. 1992, 43, 129–133. [Google Scholar]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-vine grape drying effect on volatile compounds and aromatic series in must from Pedro Ximenez grape variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality—A review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Qian, M.C.; Fang, Y.; Shellie, K. Volatile composition of Merlot wine from different vine water status. J. Agric. Food Chem. 2009, 57, 7459–7463. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, J.M.; Bastian, S.E.P.; Jeffery, D.W. Snapshot of Australian production practices for Chardonnay wines. Wine Vitic. J. 2016, 31, 27–32. [Google Scholar]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.; Tavares, R.; Sousa, M.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical changes throughout grape berry development and fruit and wine quality. Food 2007, 1, 1–22. [Google Scholar]
- Smith, P. Recent advances in objective chemical measures of wine quality. In ASVO and WISA Seminar; Petrie, P.R., Ed.; Australian Society of Viticulture and Oenology: Mildura, Victoria, 2013; pp. 44–49. [Google Scholar]
- Smith, P.A. Assessment of relationships between grape chemical composition and grape allocation grade for Cabernet Sauvignon, Shiraz and Chardonnay. Aust. N. Z. Wine Ind. J. 2015, 2015, 30–32. [Google Scholar]
- Sweetman, C.; Sadras, V.O.; Hancock, R.D.; Soole, K.; Ford, C.M. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. J. Exp. Bot. 2014, 65, 5975–5988. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.H.; Weedon, M.M. The impact of high temperatures on Vitis vinifera cv. Semillon grapevine performance and berry ripening. Front. Plant Sci. 2013, 4, 491. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.H.; Rogiers, S.Y.; Steel, C.C. Susceptibility of Chardonnay grapes to sunburn. Vitis 2006, 45, 147–148. [Google Scholar]
- Deloire, A. New method to determine optimal ripeness for white wine styles. Pract. Winery Vineyard 2013, Winter, 75–79. [Google Scholar]
- Scafidi, P.; Pisciotta, A.; Patti, D.; Tamborra, P.; Di Lorenzo, R.; Barbagallo, M.G. Effect of artificial shading on the tannin accumulation and aromatic composition of the Grillo cultivar (Vitis vinifera L.). BMC Plant Biol. 2013, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.B.; Whetton, P.H.; Barlow, E.W.R. Climate change and winegrape quality in Australia. Clim. Res. 2008, 36, 99–111. [Google Scholar] [CrossRef]
- Boss, P.K.; Pearce, A.D.; Zhao, Y.; Nicholson, E.L.; Dennis, E.G.; Jeffery, D.W. Potential grape-derived contributions to volatile ester concentrations in wine. Molecules 2015, 20, 7845–7873. [Google Scholar] [CrossRef] [PubMed]
- Iland, P.G.; Bruer, N.; Edwards, G.; Weeks, S.; Wilkes, E. Chemical Analysis of Grapes and Wine: Techniques and Concepts; Patrick Iland Wine Promotions: Campbelltown, Australia, 2004. [Google Scholar]
- Wheal, M.S.; Fowles, T.O.; Palmer, L.T. A cost-effective acid digestion method using closed polypropylene tubes for inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of plant essential elements. Anal. Methods 2011, 3, 2854–2863. [Google Scholar]
- Australian Government Bureau of Meteorology. Climate data online. Available online: www.bom.gov.au (accessed on 6 October 2016).
- Jolliffe, I.T. Principal component analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Olarte Mantilla, S.M.; Collins, C.; Iland, P.; Kidman, C.; Ristic, R.; Hasted, A.; Jordans, C.; Bastian, S. Relationships between grape and wine sensory attributes and compositional measures of cv. Shiraz. Am. J. Enol. Vitic. 2015, 66, 177–186. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available.

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambetta, J.M.; Cozzolino, D.; Bastian, S.E.P.; Jeffery, D.W. Exploring the Effects of Geographical Origin on the Chemical Composition and Quality Grading of Vitis vinifera L. cv. Chardonnay Grapes. Molecules 2017, 22, 218. https://doi.org/10.3390/molecules22020218
Gambetta JM, Cozzolino D, Bastian SEP, Jeffery DW. Exploring the Effects of Geographical Origin on the Chemical Composition and Quality Grading of Vitis vinifera L. cv. Chardonnay Grapes. Molecules. 2017; 22(2):218. https://doi.org/10.3390/molecules22020218
Chicago/Turabian StyleGambetta, Joanna M., Daniel Cozzolino, Susan E.P. Bastian, and David W. Jeffery. 2017. "Exploring the Effects of Geographical Origin on the Chemical Composition and Quality Grading of Vitis vinifera L. cv. Chardonnay Grapes" Molecules 22, no. 2: 218. https://doi.org/10.3390/molecules22020218




