Enhanced Performance of Magnetic Graphene Oxide-Immobilized Laccase and Its Application for the Decolorization of Dyes
Abstract
:1. Introduction
2. Results and Discussion
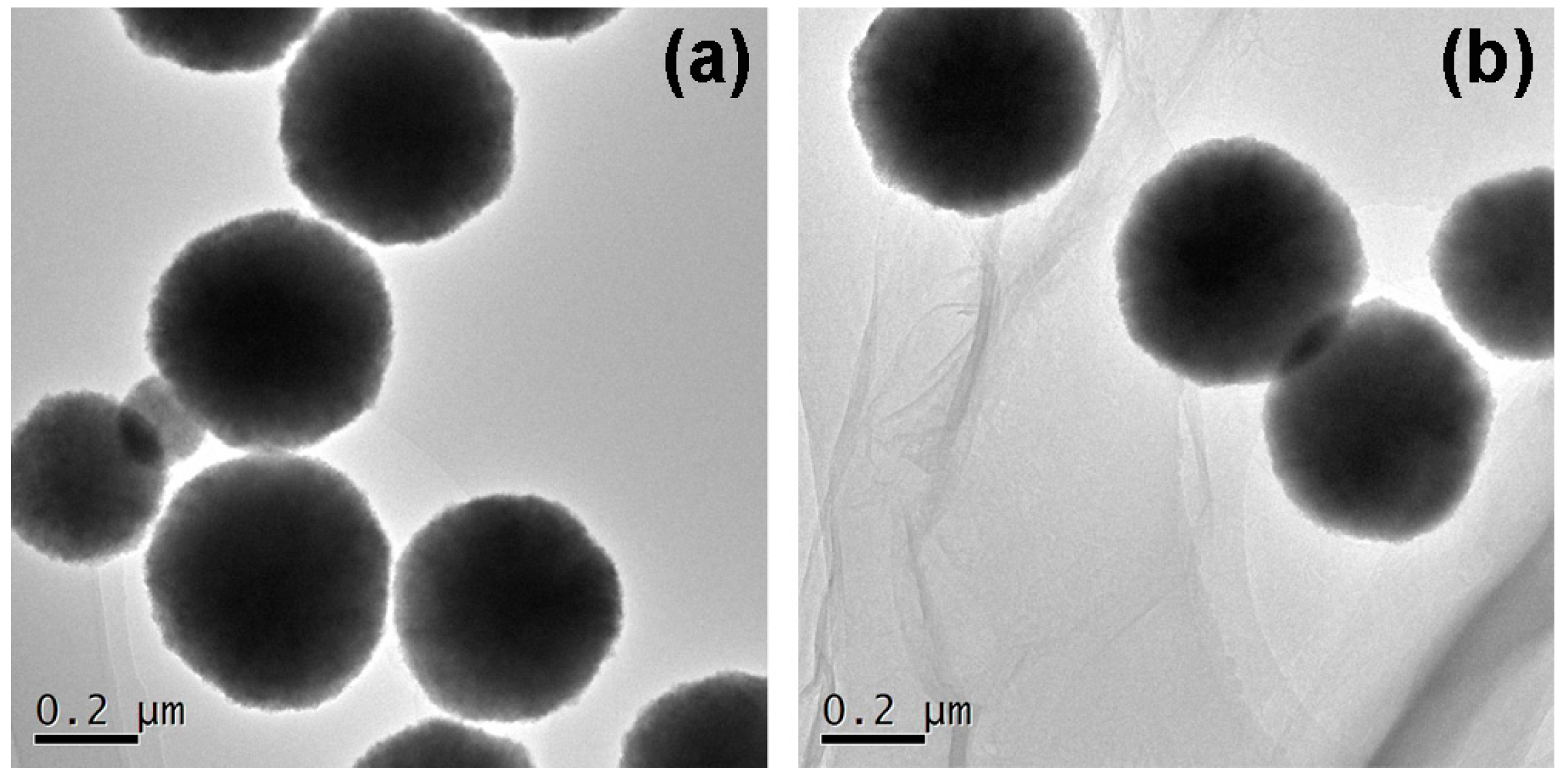
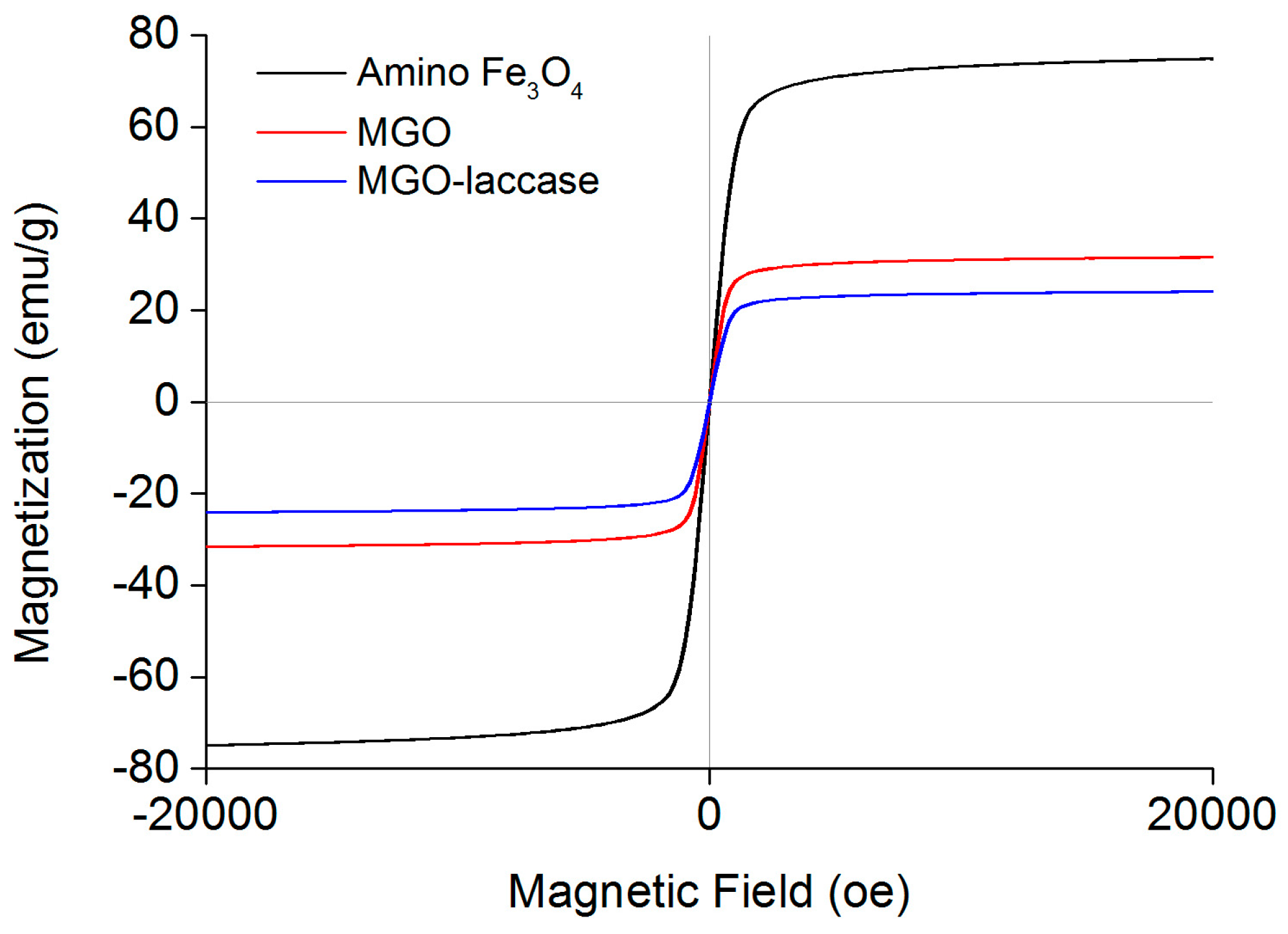
2.1. Characterizations of MGO-Laccase
2.2. Immobilization of Laccase on MGO-Laccase
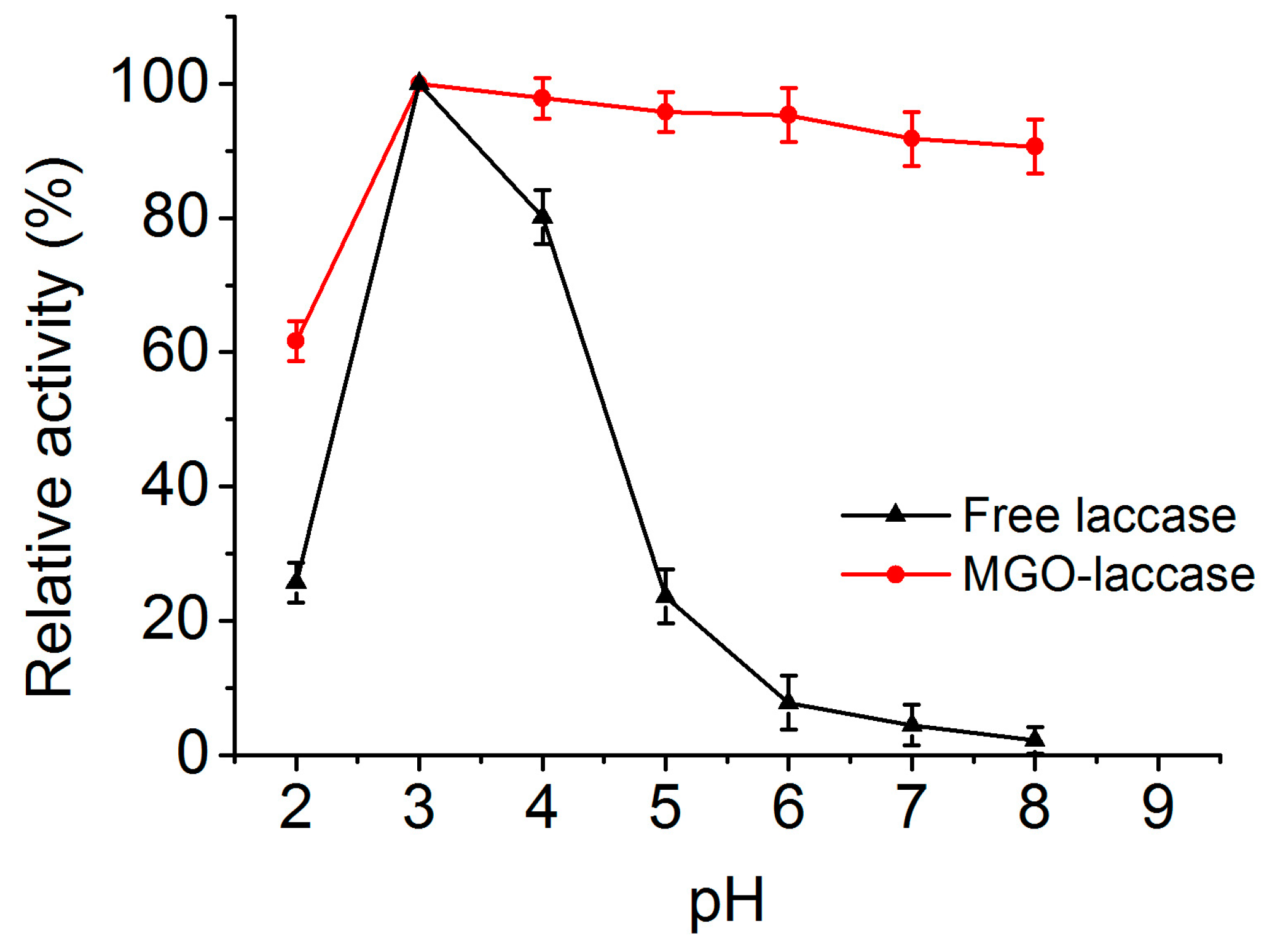
2.3. Effect of pH on the Activities of Free Laccase and MGO-Laccase
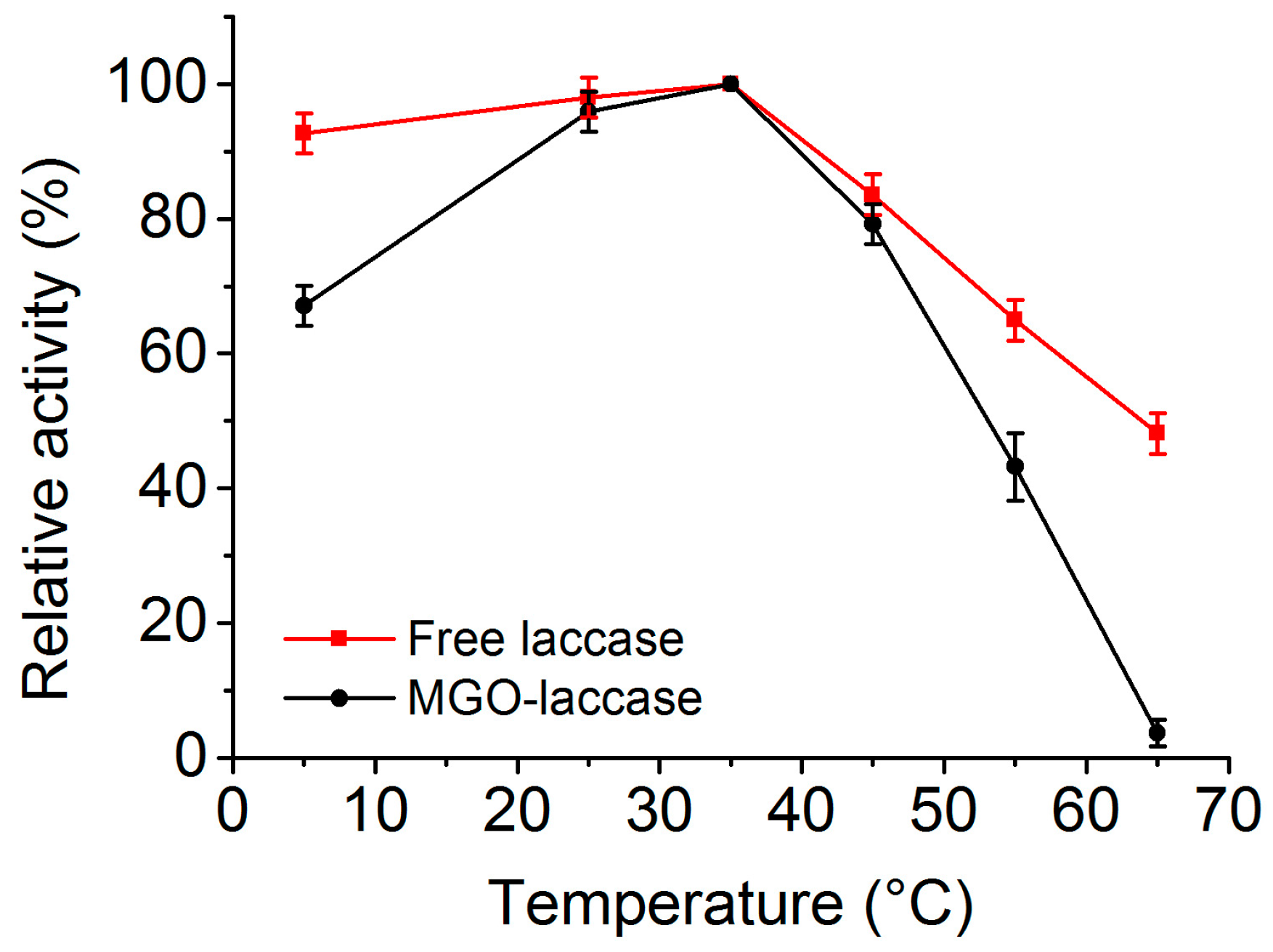
2.4. Effect of Tempertature on the Activities of Free Laccase and MGO-Laccase
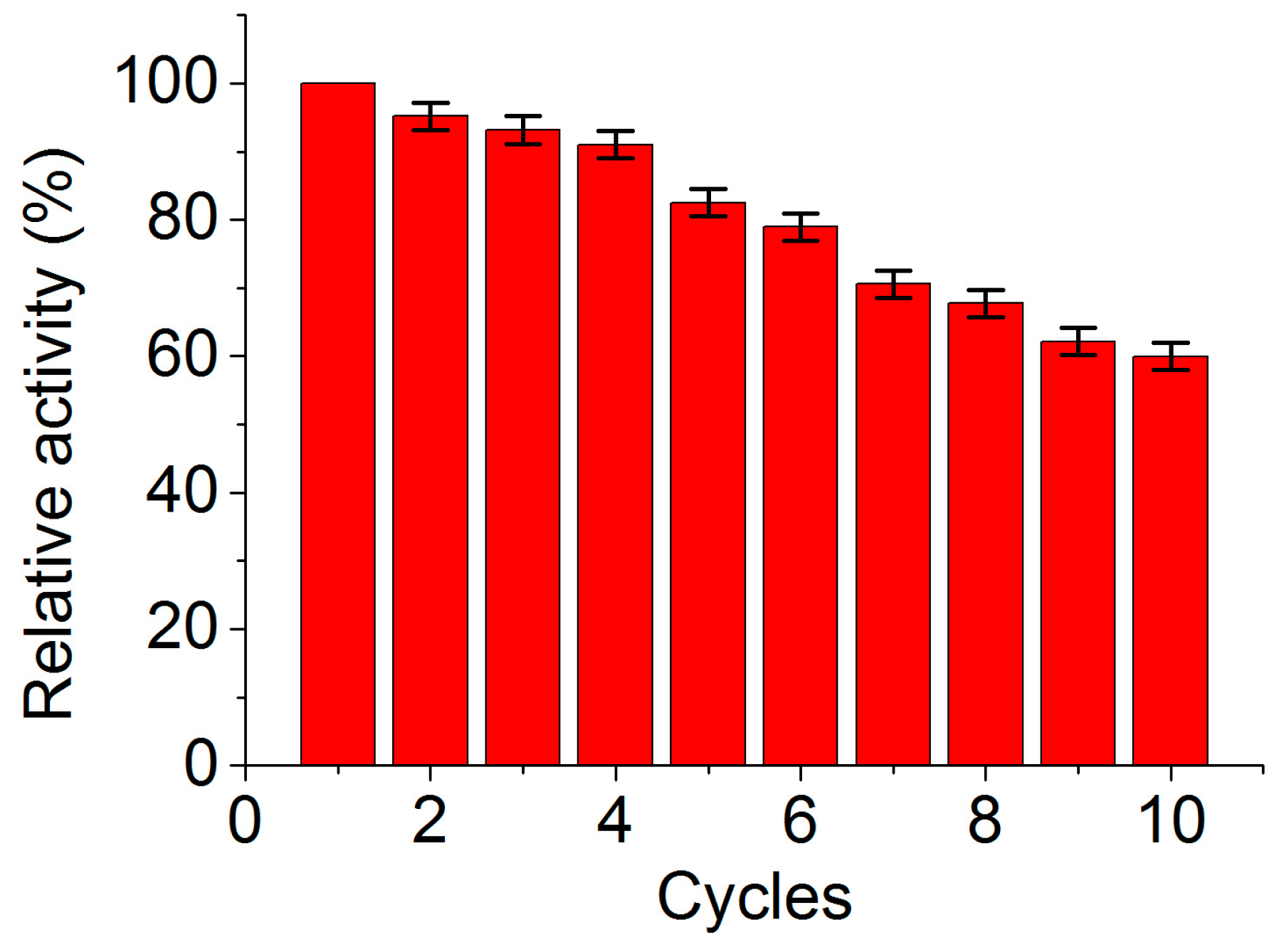
2.5. Reusability of MGO-Laccase
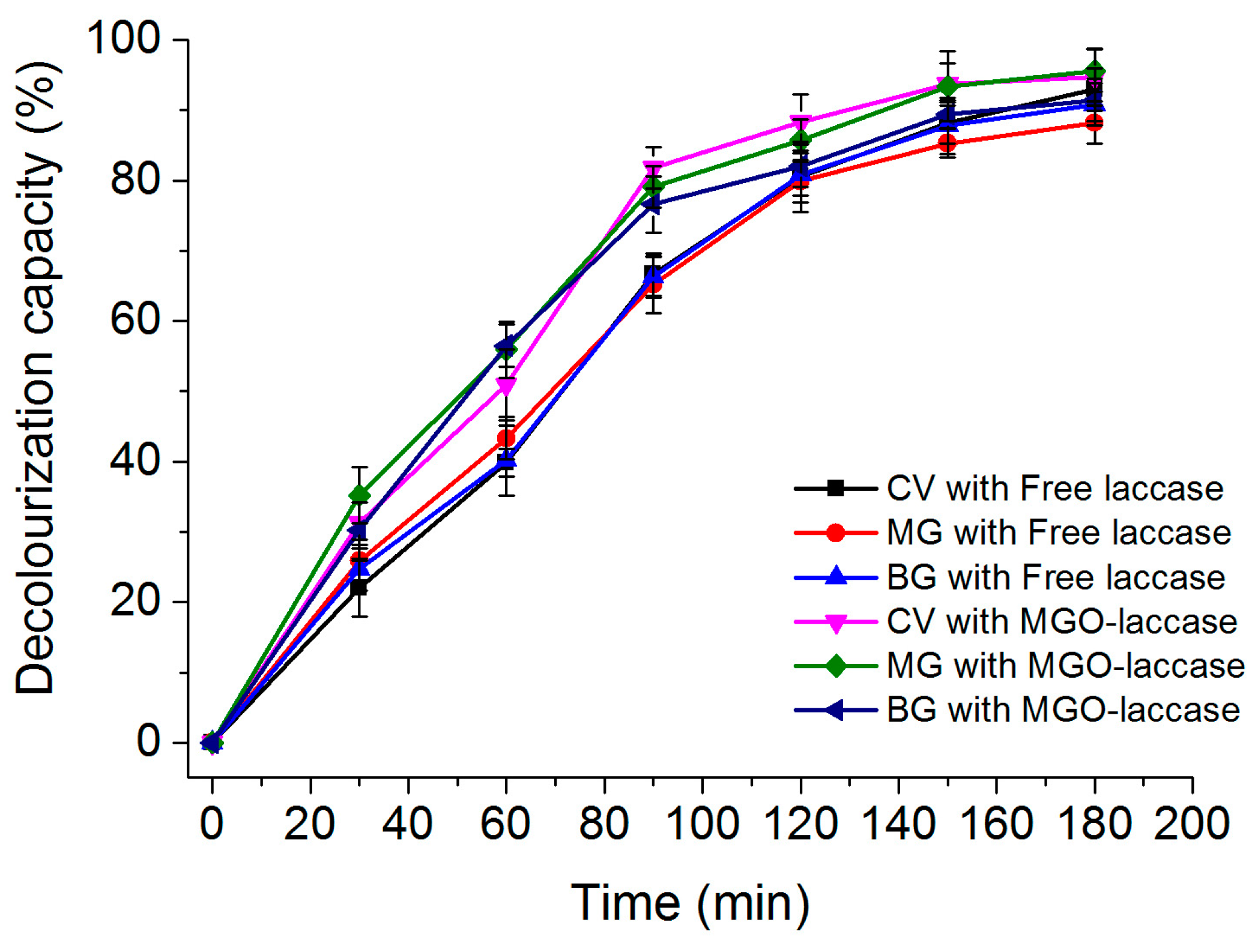
2.6. Decolorization of Dyes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Amino Fe3O4 Nanoparticles
3.3. Preparation of GO
3.4. Preparation of MGO-Laccase
3.5. Characterizations and Measurements
3.6. Laccase Activity Assay
3.7. Reusability of MGO-Laccase
3.8. Decolorization of Dyes with Free Laccase and MGO-Laccase
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jain, P.; Das, S.; Chakma, B.; Goswami, P. Aptamer-graphene oxide for highly sensitive dual electrochemical detection of Plasmodium lactate dehydrogenase. Anal. Biochem. 2016, 514, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Fang, A.J.; Wen, Y.Q.; Li, H.T.; Zhang, Y.Y.; Yao, S.Z. Rapid and highly-sensitive uric acid sensing based on enzymatic catalysis-induced upconversion inner filter effect. Biosens. Bioelectron. 2016, 86, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Homaei, A. Immobilization of Penaeus merguiensis alkaline phosphatase on gold nanorods for heavy metal detection. Ecotox. Environ. Saf. 2017, 136, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Hirakawa, H.; Suzuki, R.; Haga, T.; Iwata, F.; Nagamune, T. Immobilization of a Bacterial Cytochrome P450 Monooxygenase System on a Solid Support. Angew. Chem. Int. Edit. 2016, 55, 15002–15006. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.B.; Zhang, X.H. Gelatin-Immobilized Manganese Peroxidase with Novel Catalytic Characteristics and Its Industrial Exploitation for Fruit Juice Clarification Purposes. Catal. Lett. 2016, 146, 2221–2228. [Google Scholar] [CrossRef]
- Bavaro, T.; Cattaneo, G.; Serra, I.; Benucci, I.; Pregnolato, M.; Terreni, M. Immobilization of Neutral Protease from Bacillus subtilis for Regioselective Hydrolysis of Acetylated Nucleosides: Application to Capecitabine Synthesis. Molecules 2016, 21, 1621. [Google Scholar] [CrossRef] [PubMed]
- Escuin, P.C.; Garcia-Bennett, A.; Ros-Lis, J.V.; Foix, A.A.; Andres, A. Application of mesoporous silica materials for the immobilization of polyphenol oxidase. Food Chem. 2017, 217, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Hajar, M.; Vahabzadeh, F. Biolubricant production from castor oil in a magnetically stabilized fluidized bed reactor using lipase immobilized on Fe3O4 nanoparticles. Ind. Crop. Prod. 2016, 94, 544–556. [Google Scholar] [CrossRef]
- Liu, N.; Liang, G.; Dong, X.W.; Qi, X.L.; Kim, J.; Piao, Y. Stabilized magnetic enzyme aggregates on graphene oxide for high performance phenol and bisphenol A removal. Chem. Eng. J. 2016, 306, 1026–1034. [Google Scholar] [CrossRef]
- Ulu, A.; Koytepe, S.; Ates, B. Design of starch functionalized biodegradable P(MAA-co-MMA) as carrier matrix for l-asparaginase immobilization. Carbohyd. Polym. 2016, 153, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.K.; Ahmad, R.; Mousa, H.M.; Kim, I.G.; Kim, J.I.; Neupane, M.P.; Park, C.H.; Kim, C.S. High-performance glucose biosensor based on chitosan-glucose oxidase immobilized polypyrrole/Nafion/functionalized multi-walled carbon nanotubes bio-nanohybrid film. J. Colloid Interf. Sci. 2016, 482, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perrnan, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.X.; Yu, L.; Ding, X.X.; Li, P.W.; Dai, X.H.; Chen, X.M.; Zhou, H.Y.; Bai, Y.Z.; Ding, J. Magnetic solid-phase extraction based on graphene oxide for the determination of lignans in sesame oil. Food Chem. 2017, 217, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Huang, J.; Ding, Y.; Tang, H. Catalytic Oxidation of Phenol and 2,4-Dichlorophenol by Using Horseradish Peroxidase Immobilized on Graphene Oxide/Fe3O4. Molecules 2016, 21, 1044. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Husain, Q.; Naqvi, A.H. Graphene based magnetic nanocomposites as versatile carriers for high yield immobilization and stabilization of [small beta]-galactosidase. RSC Adv. 2016, 6, 53493–53503. [Google Scholar] [CrossRef]
- Amirbandeh, M.; Taheri-Kafrani, A. Immobilization of glucoamylase on triazine-functionalized Fe3O4/graphene oxide nanocomposite: Improved stability and reusability. Int. J. Biol. Macromol. 2016, 93, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, X.; Shi, J.; Wang, X.; Zhang, S.; Han, P.; Jiang, Z. In situ synthesized rGO–Fe3O4 nanocomposites as enzyme immobilization support for achieving high activity recovery and easy recycling. Biochem. Eng. J. 2016, 105, 273–280. [Google Scholar] [CrossRef]
- Hou, C.; Zhou, L.; Zhu, H.; Wang, X.; Hu, N.; Zeng, F.; Wang, L.; Yin, H. Mussel-inspired surface modification of magnetic@graphite nanosheets composite for efficient Candida rugosa lipase immobilization. J. Ind. Microbiol. Biot. 2015, 42, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.; Al-Lohedan, H.; Tawfik, A.; Ezzat, A. Application of Super-Amphiphilic Silica-Nanogel Composites for Fast Removal of Water Pollutants. Molecules 2016, 21, 1392. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Shih, M.C.; Chiu, H.C.; Huang, K.S. Magnetic Pycnoporus sanguineus-Loaded Alginate Composite Beads for Removing Dye from Aqueous Solutions. Molecules 2014, 19, 8276–8288. [Google Scholar] [CrossRef] [PubMed]
- Saber-Samandari, S.; Saber-Samandari, S.; Joneidi-Yekta, H.; Mohseni, M. Adsorption of anionic and cationic dyes from aqueous solution using gelatin-based magnetic nanocomposite beads comprising carboxylic acid functionalized carbon nanotube. Chem. Eng. J. 2017, 308, 1133–1144. [Google Scholar] [CrossRef]
- Navarro, P.; Gabaldon, J.A.; Gomez-Lopez, V.M. Degradation of an azo dye by a fast and innovative pulsed light/H2O2 advanced oxidation process. Dyes Pigm. 2017, 136, 887–892. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Fu, C.C.; Juang, R.S. Effective removal of sulfur dyes from water by biosorption and subsequent immobilized laccase degradation on crosslinked chitosan beads. Chem. Eng. J. 2016, 304, 313–324. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Dosseto, A.; Richardson, C.; Price, W.E.; Nghiem, L.D. Continuous adsorption and biotransformation of micropollutants by granular activated carbon-bound laccase in a packed-bed enzyme reactor. Bioresource Technol. 2016, 210, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, Y.H.; Yang, X.D.; Ng, T.B.; Ye, X.Y.; Lin, J. Degradation of tetracycline by immobilized laccase and the proposed transformation pathway. J. Hazard. Mater. 2017, 322, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Zhao, H.; Liu, C.; Huang, Q.; Cao, H. Transformation and products of captopril with humic constituents during laccase-catalyzed oxidation: role of reactive intermediates. Water Res. 2016, 106, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, T.; Park, H.S.; Mo, A.Y.; Choi, M.S.; Kim, D.H.; Park, S.M. Detoxification of furanic and phenolic lignocellulose derived inhibitors of yeast using laccase immobilized on bacterial cellulosic nanofibers. J. Mol. Catal. B-Enzym. 2016, 134, 196–205. [Google Scholar] [CrossRef]
- Dai, Y.R.; Yao, J.; Song, Y.H.; Liu, X.L.; Wang, S.Y.; Yuan, Y. Enhanced performance of immobilized laccase in electrospun fibrous membranes by carbon nanotubes modification and its application for bisphenol A removal from water. J. Hazard. Mater. 2016, 317, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Wang, Y.; Bai, Y.; Li, R.; Gao, R. Site-Specific, Covalent Immobilization of Dehalogenase ST2570 Catalyzed by Formylglycine-Generating Enzymes and Its Application in Batch and Semi-Continuous Flow Reactors. Molecules 2016, 21, 895. [Google Scholar] [CrossRef] [PubMed]
- Kahar, U.; Sani, M.; Chan, K.G.; Goh, K. Immobilization of α-Amylase from Anoxybacillus sp. SK3-4 on ReliZyme and Immobead Supports. Molecules 2016, 21, 1196. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, X.; Deng, C.; Yang, P.; Zhang, X. Immobilization of Trypsin on Superparamagnetic Nanoparticles for Rapid and Effective Proteolysis. J. Proteome Res. 2007, 6, 3849–3855. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Li, X.; Jia, W.; Zhao, Y.; Ren, S. Recyclable magnetic graphene oxide for rapid and efficient demulsification of crude oil-in-water emulsion. Fuel 2017, 189, 79–87. [Google Scholar] [CrossRef]
- Waifalkar, P.P.; Parit, S.B.; Chougale, A.D.; Sahoo, S.C.; Patil, P.S.; Patil, P.B. Immobilization of invertase on chitosan coated γ-Fe2O3 magnetic nanoparticles to facilitate magnetic separation. J. Colloid Interf. Sci. 2016, 482, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, G.; Li, Y.; Zhu, H.; Peng, X.; Gao, X. One-step fabrication of functionalized magnetic adsorbents with large surface area and their adsorption for dye and heavy metal ions. Dalton Trans. 2014, 43, 11637–11645. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.C.; Dwivedi, A.D.; Le, T.T.; Seo, S.H.; Kim, E.J.; Chang, Y.S. Magnetite graphene oxide encapsulated in alginate beads for enhanced adsorption of Cr(VI) and As(V) from aqueous solutions: Role of crosslinking metal cations in pH control. Chem. Eng. J. 2017, 307, 220–229. [Google Scholar] [CrossRef]
- Wang, F.; Guo, C.; Yang, L.R.; Liu, C.Z. Magnetic mesoporous silica nanoparticles: Fabrication and their laccase immobilization performance. Bioresource Technol. 2010, 101, 8931–8935. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Wang, Q.; Fu, S. Laccase stabilization by covalent binding immobilization on activated polyvinyl alcohol carrier. Lett. Appl. Microbiol. 2002, 35, 451–456. [Google Scholar]
- Song, X.; Wu, H.; Shi, J.; Wang, X.; Zhang, W.; Ai, Q.; Jiang, Z. Facile fabrication of organic–inorganic composite beads by gelatin induced biomimetic mineralization for yeast alcohol dehydrogenase encapsulation. J. Mol. Catal. B Enzym. 2014, 100, 49–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Li, L.; Li, J.; Jiang, Z.; Jiang, Y.; Chen, Y. Enzymatic conversion of Baicalin into Baicalein by β-glucuronidase encapsulated in biomimetic core-shell structured hybrid capsules. J. Mol. Catal. B Enzym. 2009, 57, 130–135. [Google Scholar] [CrossRef]
- Homaei, A.; Etemadipour, R. Improving the activity and stability of actinidin by immobilization on gold nanorods. Int. J. Biol. Macromol. 2015, 72, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Antony, N.; Balachandran, S.; Mohanan, P.V. Immobilization of diastase α-amylase on nano zinc oxide. Food Chem. 2016, 211, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, S.; Xu, Z.; Qiu, Y.; Li, S.; Xu, H. Modified nanoporous titanium dioxide as a novel carrier for enzyme immobilization. Biosens. Bioelectron. 2016, 80, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Xia, T.T.; Liu, C.Z.; Hu, J.H.; Guo, C. Improved performance of immobilized laccase on amine-functioned magnetic Fe3O4 nanoparticles modified with polyethylenimine. Chem. Eng. J. 2016, 295, 201–206. [Google Scholar] [CrossRef]
- Hermanova, S.; Zarevucka, M.; Bousa, D.; Pumera, M.; Sofer, Z. Graphene oxide immobilized enzymes show high thermal and solvent stability. Nanoscale 2015, 7, 5852–5858. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Leng, J.; Yang, X.; Liao, L.; Liu, L.; Xiao, A. Enhanced Performance of Magnetic Graphene Oxide-Immobilized Laccase and Its Application for the Decolorization of Dyes. Molecules 2017, 22, 221. https://doi.org/10.3390/molecules22020221
Chen J, Leng J, Yang X, Liao L, Liu L, Xiao A. Enhanced Performance of Magnetic Graphene Oxide-Immobilized Laccase and Its Application for the Decolorization of Dyes. Molecules. 2017; 22(2):221. https://doi.org/10.3390/molecules22020221
Chicago/Turabian StyleChen, Jing, Juan Leng, Xiai Yang, Liping Liao, Liangliang Liu, and Aiping Xiao. 2017. "Enhanced Performance of Magnetic Graphene Oxide-Immobilized Laccase and Its Application for the Decolorization of Dyes" Molecules 22, no. 2: 221. https://doi.org/10.3390/molecules22020221



