On-Line Screening, Isolation and Identification of Antioxidant Compounds of Helianthemum ruficomum
Abstract
:1. Introduction
2. Results and Discussion
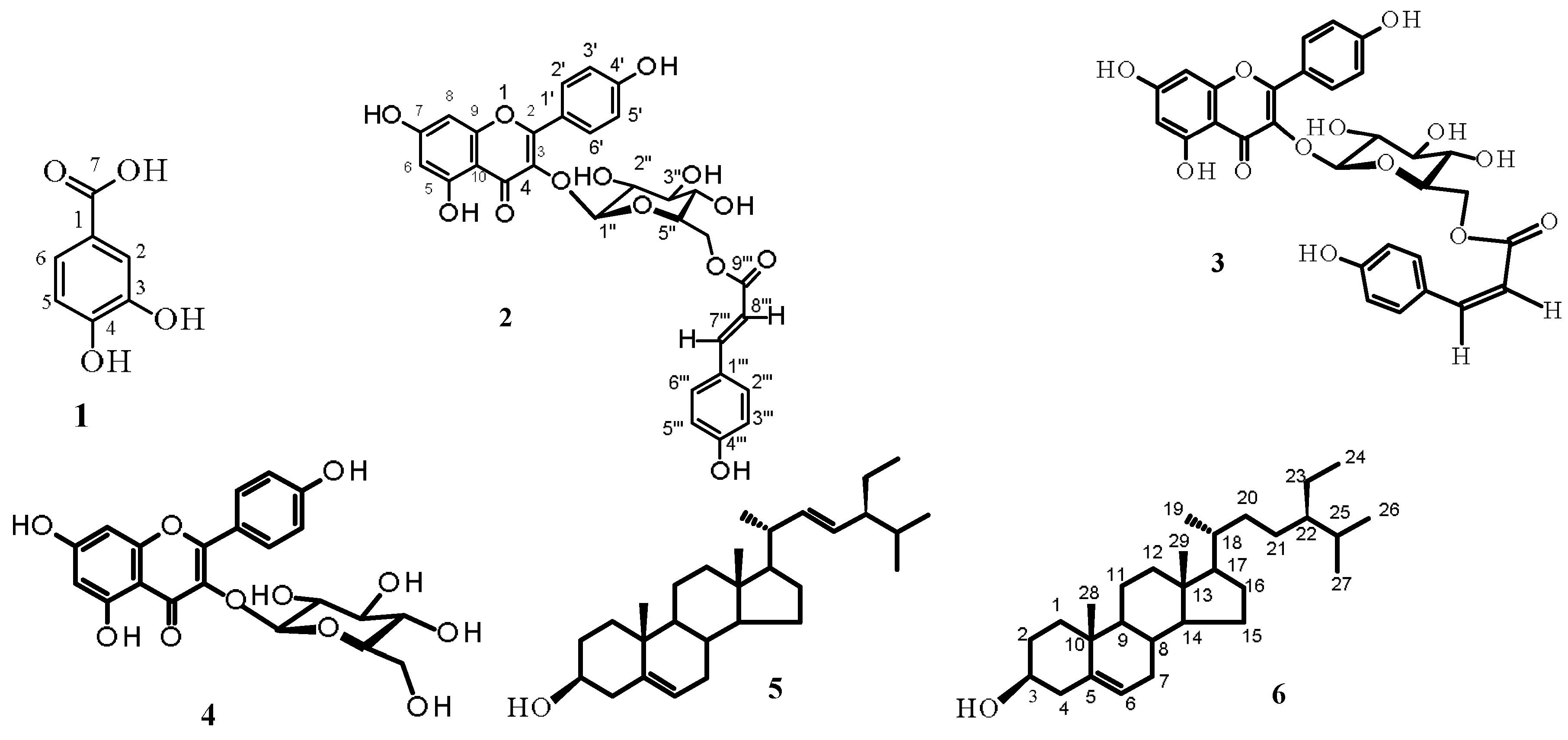
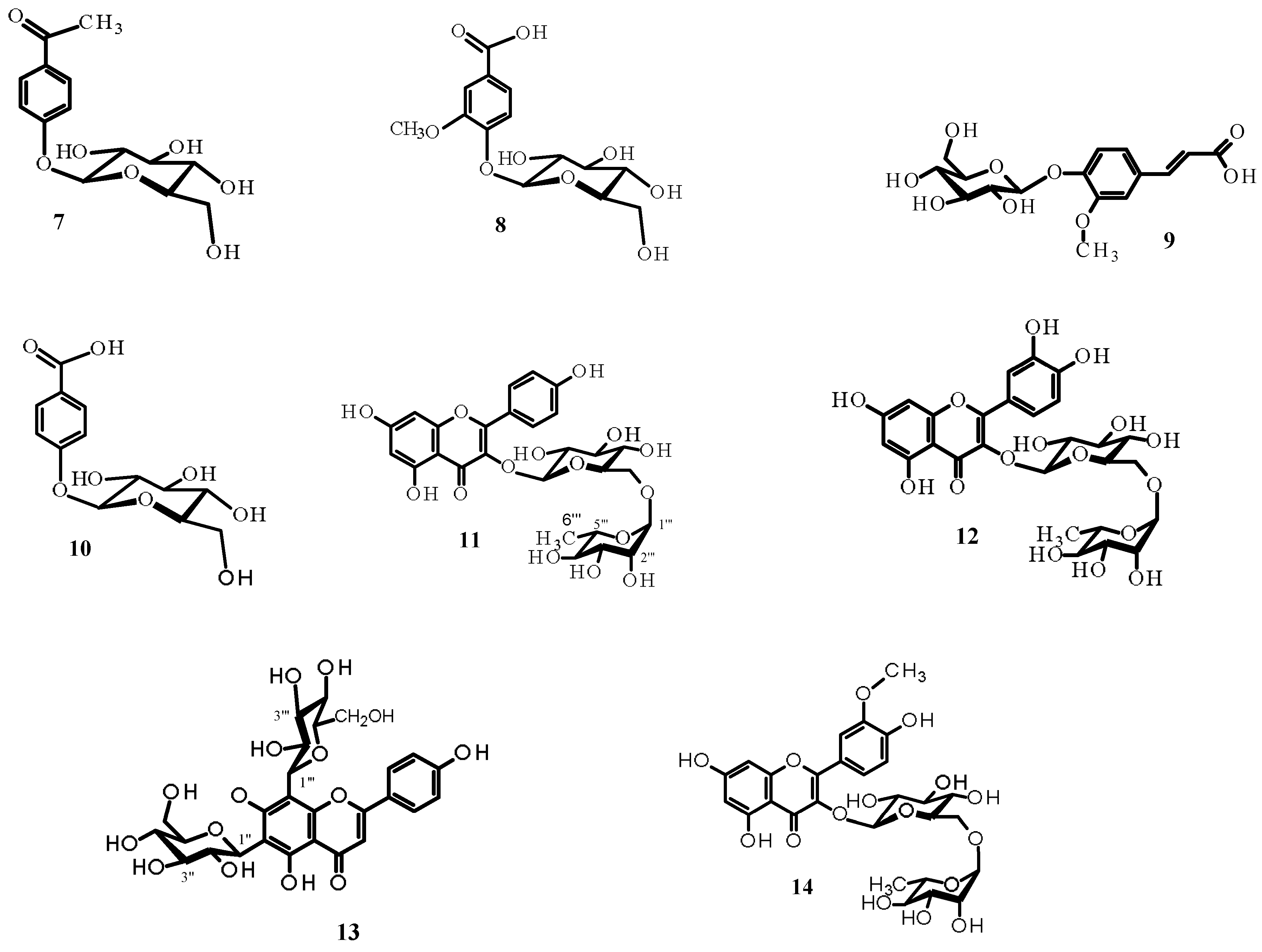
2.1. Isolation and Structure Elucidation of Compounds
2.2. Identification of Chromatographic Peaks and Antioxidant Activity of Plant Extracts and Pure Compounds
3. Materials and Methods
3.1. General Procedure
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Solvents and Chemicals
3.5. On-Line HPLC-ABTS•+ Assay
3.6. Oxygen Radical Absorbance Capacity (ORAC)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, R.; Singh, B.; Singh, S.; Kumar, N.; Kumar, S.; Arora, S. Umbelliferone—An antioxidant isolated from Acacia nilotica (L.) Willd. Ex. Del. Food. Chem. 2010, 120, 825–830. [Google Scholar] [CrossRef]
- Deshpande, S. Role of antioxidants in prevention of age-related macular degeneration. J. Med. Nutr. Nutraceut. 2012, 1, 83–86. [Google Scholar] [CrossRef]
- Neera, J.P.; Singh, S.; Singh, J. Role of free radicals and antioxidants in human health and disease. Int. J. Curr. Res. Rev. 2013, 5, 14–22. [Google Scholar]
- Agrawal, S.B.; Singh, S.; Agrawal, M. Ultraviolet-B induced changes in gene expression and antioxidants in plants. Adv. Bot. Res. 2009, 52, 47–86. [Google Scholar]
- Simova-Stoilova, L.; Vaseva, I.; Grigorova, B.; Demirevska, K.; Feller, U. Proteolytic activity and cysteine protease expression in wheat leaves under severe soil drought and recovery. Plant Physiol. Biochem. 2010, 48, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Zlatev, Z.S.; Lidon, F.C.; Ramalho, J.C.; Yordanov, I.T. Comparison of resistance to drought of three bean cultivars. Biol. Plant. 2006, 50, 389–394. [Google Scholar] [CrossRef]
- Boussaha, S.; Bekhouche, K.; Boudjerda, A.; Leόn, F.; Koldaş, S.; Yaglioglu, A.S.; Demirtas, I.; Brouard, I.; Marchioni, E.; Zama, D.; et al. Chemical constituents, in vitro antioxidant and antiproliferative activities of Perralderia coronopifolia Coss. subsp. eu-coronopifolia M. var. typica M. extract. Rec. Nat. Prod. 2015, 9, 312–322. [Google Scholar]
- Mohamadi, S.; Zhao, M.; Amrani, A.; Marchioni, E.; Zama, D.; Benayache, F.; Benayache, S. On-line screening and identification of antioxidant phenolic compounds of Saccocalyx satureioides Coss. et Dur. Ind. Crop. Prod. 2015, 76, 910–919. [Google Scholar] [CrossRef]
- Bougandoura, A.; D’Abrosca, B.; Ameddah, S.; Scognamiglio, M.; Mekkiou, R.; Fiorentino, A.; Benayache, S.; Benayache, F. Chemical constituents and in vitro anti-inflammatory activity of Cistanche violacea Desf. (Orobanchaceae) extract. Fitoterapia 2016, 109, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Djebbari, R.; Chemam, Y.; Amrani, A.; Lassed, S.; Boubekri, N.; Zama, D.; Benayache, F.; Benayache, S. In vitro antioxidant activities of n-butanol extract of Helianthemum confertum. Int. J. Phytomed. 2015, 7, 119–122. [Google Scholar]
- Guzmán, B.; Vargas, P. Historical biogeography and character evolution of Cistaceae (Malvales) based on analysis of plastid rbcL and trnL-trnF sequences. Org. Divers. Evol. 2009, 9, 83–99. [Google Scholar] [CrossRef]
- Mabberly, D.J. The Plant Book; Cambridge University Pres: Cambridge, UK, 1997. [Google Scholar]
- Meckes, M.; Torres, J.; Calzada, F.; Rivera, J.; Camorlinga, M.; Lemus, H.; Rodríguez, G. Antibacterial properties of Helianthemum glomeratum, a plant used in Maya traditional medicine to treat diarrhea. Phytother. Res. 1997, 11, 128–131. [Google Scholar] [CrossRef]
- Rubio-Moraga, Á.; Argandoña, J.; Mota, B.; Pérez, J.; Verde, A.; Fajardo, J.; Gómez-Navarro, J.; Castillo-López, R.; Ahrazem, O.; Gómez-Gómez, L. Screening for polyphenols, antioxidant and antimicrobial activities of extracts from eleven Helianthemum taxa (Cistaceae) used in folk medicine in south-eastern Spain. J. Ethnopharmacol. 2013, 148, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Meckes, M.; Villarreal, M.L.; Tortoriello, J.; Berlin, B.; Berlin, E.A. A microbiological evaluation of medicinal plants used by the Maya people of Southern Mexico. Phytother. Res. 1995, 9, 244–250. [Google Scholar] [CrossRef]
- Calzada, F.; Alanís, A.D.; Meckes, M.; Tapia-Contreras, A.; Cedillo-Rivera, R. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to some medicinal plants used by the people of Southern Mexico. Phytother. Res. 1998, 12, 70–72. [Google Scholar] [CrossRef]
- Rigat, M.; Bonet, M.A.; Garcia, S.; Garnatje, T.; Vallès, J. Studies on pharmaceutical ethnobotany in the high river Tervalley (Pyrenees, Catalonia, Iberian Peninsula). J. Ethnopharmacol. 2007, 113, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Meckes, M.; Calzada, F.; Tapia-Contreras, A.; Cedillo-Rivera, R. Antiprotozoal propertiesof Helianthemum glomeratum. Phytother. Res. 1999, 13, 102–105. [Google Scholar] [CrossRef]
- Barbosa, E.; Calzada, F.; Campos, R. Antigiardial activity of methanolic extracts from Helianthemum glomeratum Lag. and Rubus coriifolius Focke in suckling mice CD-1. J. Ethnopharmacol. 2006, 108, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Bouzergoune, F.; Bitam, F.; Aberkane, M.C.; Mosset, P. Preliminary phytochemical and antimicrobial activity investigations on the aerial parts of Helianthemum kahiricum. Chem. Nat. Compd. 2013, 49, 751–752. [Google Scholar] [CrossRef]
- Alsabri, S.G.; Rmeli, N.B.; Zetrini, A.A.; Mohamed, S.B.; Meshri, M.I.; Aburas, K.M.; Bensaber, S.M.; Mrema, I.A.; Mosbah, A.A.; Allahresh, K.A.; et al. Phytochemical, anti-oxidant, anti-microbial, anti-inflammatory and anti-ulcer properties of Helianthemum lippii. J. Pharmacogn. Phytochem. 2013, 2, 86–96. [Google Scholar]
- Benabdelaziz, I.; Marcourt, L.; Benkhaled, M.; Wolfender, J.L.; Haba, H. Antioxidant and antibacterial activities and polyphenolic constituents of Helianthemum sessiliflorum Pers. Nat. Prod. Res. 2016, 15, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Alanis, A.D. Additional antiprotozoal flavonol glycosides of the aerial parts of Helianthemum glomeratum. Phytother. Res. 2007, 21, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.; Calzada, F.; Campos, R. In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. J. Ethnopharmacol. 2007, 109, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Benabdelaziz, I.; Haba, H.; Lavaud, C.; Harakat, D.; Benkhaled, M. Lignans and other constituents from Helianthemum sessiliflorum Pers. Rec. Nat. Prod. 2015, 9, 342–348. [Google Scholar]
- Calzada, F.; Lopez, R.; Meckes, M.; Cedillo-Rivera, R. Flavonoids of the aerial parts of Helianthemum glomeratum. Int. J. Pharmacogn. 1995, 33, 351–352. [Google Scholar] [CrossRef]
- Javidnia, K.; Nasiri, A.; Miri, R.; Jamalian, A. Composition of the essential oil of Helianthemum kahiricum Del. from Iran. J. Essent. Oil Res. 2007, 19, 52–53. [Google Scholar] [CrossRef]
- Quezel, P.; Santa, S. Nouvelle Flore de l’Algérie et des Régions Désertiques Méridionales; Editions du C.N.R.S: Paris, France, 1963; p. 710. [Google Scholar]
- Ozenda, P. Flore du Sahara Septentrional et Central; CNRS: Paris, France, 1958; p. 350. [Google Scholar]
- The Plant List (2010). Version 1. Available online: http://www.theplantlist.org/ (accessed on 1 January 2017).
- An, L.J.; Guan, S.; Shi, G.F.; Bao, Y.M.; Duan, Y.L.; Jiang, B. Protocatechuic acid from Alpinia oxyphylla against MPP+-induced neurotoxicity in PC12 cells. Food Chem. Toxicol. 2006, 44, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Ushiyama, M.; Furuya, T. Glycosylation of phenolic compounds by root culture of Panax ginseng. Phytochemistry 1989, 28, 3009–3013. [Google Scholar] [CrossRef]
- Cui, C.B.; Tezuka, Y.; Kikuchi, T.; Nakano, H.; Tamaoki, T.; Park, J.H. Constituents of a fern, Davallia mariesii Moore. IV.1) Isolation and structures of a novel norcarotane sesquiterpene glycoside, a chromone glucuronide and two epicatechin glycosides. Chem. Pharm. Bull. 1992, 40, 2035–2040. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Lamrini, M.; Klochkov, S.G. Lavandoside from Lavandula spica flowers. Chem. Nat. Compd. 2008, 44, 169–170. [Google Scholar] [CrossRef]
- Yang, F.; Chen, R.; Feng, L.; Li, H.D.; Zhang, H.; Liang, J.Y. Chemical constituents from the aerial part of Peganum nigellatrum. Chin. J. Nat. Med. 2010, 8, 199–201. [Google Scholar] [CrossRef]
- Strack, D.; Heilemann, J.; Wray, V.; Dirks, H. Structures and accumulation patterns of soluble and insoluble phenolics from Norway spruce needles. Phytochemistry 1989, 28, 2071–2078. [Google Scholar] [CrossRef]
- Mekhelfi, T.; Kerbab, K.; Guella, G.; Zaiter, L.; Benayache, S.; Benayache, F. Phytochemical constituents of Thymelaea microphylla Coss. et Dur. from Algeria. Pharm. Lett. 2014, 6, 152–156. [Google Scholar]
- Kumarasamy, Y.; Cox, P.J.; Jaspars, M.; Rashid, M.A.; Sarker, S.D. Bioactive flavonoid glycosides from the seeds of Rosa canina. Pharm. Biol. 2003, 41, 237–242. [Google Scholar] [CrossRef]
- Wei, Y.; Xie, Q.; Fisher, D.; Sutherland, I.A. Separation of patuletin-3-O-glucoside, astragalin, quercetin, kaempferol and isorhamnetin from Flaveria bidentis (L.) Kuntze by elution-pump-out high-performance counter-current chromatography. J. Chromatogr. A 2011, 1218, 6206–6211. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Tankhaeva, L.M.; Partilkhaev, V.V.; Rokhin, A.V. Chemical constituents of Caragana bungei shoots. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2012, 22, 490–496. [Google Scholar] [CrossRef]
- Xie, C.; Veitch, N.C.; Houghton, P.J.; Simmonds, M.S.J. Flavone C-glycosides from Viola yedoensis Makino. Chem. Pharm. Bull. 2003, 51, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedula, V.S.P.; Prakash, I. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J. 2012, 1, 239–242. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1995, 20, 933–956. [Google Scholar] [CrossRef]
- Pratt, D.E. Natural antioxidants of soybean oil seeds. In Autoxidation in Food and Biological Systems; Simic, M.G., Karel, M., Eds.; Plenum Press: New York, NY, USA, 1980; pp. 283–293. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Goto, T.; Teraminami, A.; Lee, J.Y.; Ohyama, K.; Funakoshi, K.; Kim, Y.I.; Hirai, S.; Uemura, T.; Yu, R.; Takahashi, N.; et al. Tiliroside, a glycosidic flavonoid, ameliorates obesity-induced metabolic disorders via activation of adiponectin signaling followed by enhancement of fatty acid oxidation in liver and skeletal muscle in obese-diabetic mice. J. Nutr. Biochem. 2012, 23, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Schinella, G.R.; Tournier, H.A.; Máñez, S.; De Buschiazzo, P.M.; Del Carmen Recio, M.; Ríos, J.L. Tiliroside and gnaphaliin inhibit human low density lipoprotein oxidation. Fitoterapia 2007, 78, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Protegente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Siddhuraju, P. The antioxidant activity and free radical-scavenging capacity of phenolics of raw and dry heated moth bean (Vigna aconitifolia) (Jacq.) Marechal seed extracts. Food Chem. 2006, 99, 149–157. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the flurescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Sample Availability: Samples of some compounds are available from the authors.

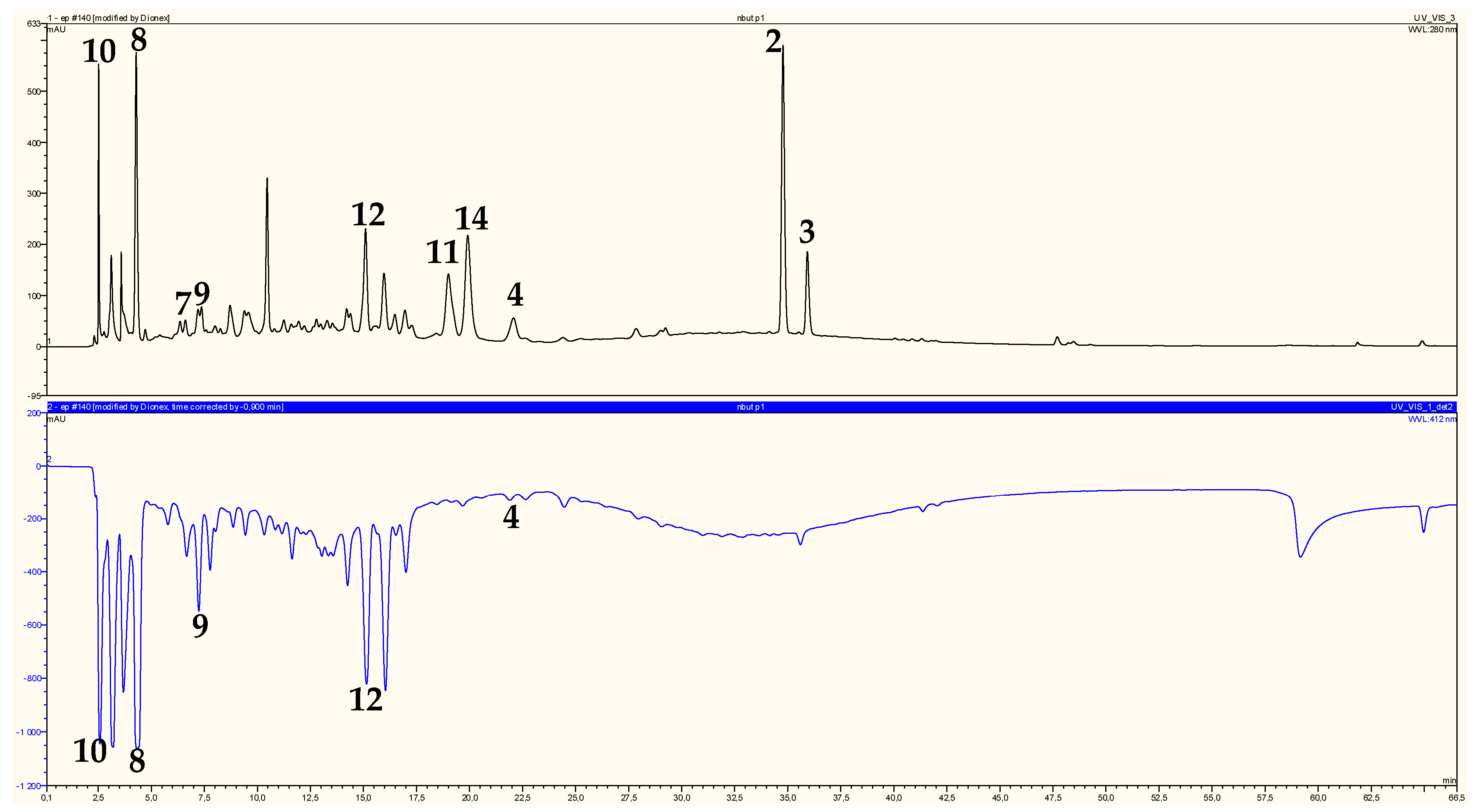
| Peaks | Compounds | ABTS (µgTE/mL) | Radical-Scavenging Activity (mAU) | Scavenging Activity Percent (%) |
|---|---|---|---|---|
| A | Not identified | 131.83 | 546 | 17.40 |
| B | Not identified | 137.47 | 569 | 16.86 |
| 1 | Protocatechuic acid | 81.56 | 341 | 8.37 |
| C | Not identified | 63.96 | 269 | 5.48 |
| D | Not identified | 42.57 | 181 | 3.97 |
| E | Not identified | 118.05 | 490 | 18.50 |
| 4 | Astragalin | 11.48 | 54 | 1.97 |
| Peaks | Compounds | ABTS (µgTE/mL) | Radical-Scavenging Activity (mAU) | Scavenging Activity Percent (%) |
|---|---|---|---|---|
| 8 | Vanillic acid 4-O-β-d-glucopyranoside | 201.91 | 833 | 35.27 |
| 9 | Lavandoside | 81.66 | 341 | 9.37 |
| 10 | 4-hydroxybenzoic acid 4-O-β-d-glucopyranoside | 199.50 | 823 | 20.31 |
| 12 | Rutin | 143.59 | 594 | 23.20 |
| Peaks | Pure Compounds or Extracts | ABTS (µgTE/mL) | Radical-Scavenging Activity (mAU) | ORAC (µMolTE/mg) | TEAC (µMolTE/mg) |
|---|---|---|---|---|---|
| EtOAc extract | - | - | 18.43 ± 12.58 | 432.06 ± 15.84 | |
| n-BuOH extract | - | - | 5.64 ± 0.21 | 430.66 ± 80.25 | |
| 1 | Protocatechuic acid | 47.59 | 202 | 690.40 ± 45.67 | 469.48 ± 15.71 |
| 4 | Astragalin | 1.48 | 13 | 300.006 ± 82.71 | 144.69 ± 15.36 |
| 8 | Vanillic acid 4-O-β-d-glucopyranoside | 16.49 | 75 | 650.91 ± 185.18 | 105.56 ± 8.95 |
| 9 | Lavandoside | 91.49 | 381 | 37.44 ± 24.74 | 12.14 ± 7.34 |
| 10 | 4-Hydroxybenzoic acid 4-O-β-d-glucopyranoside | 60.45 | 254 | 434.86 ± 36.43 | 407.94 ± 32.73 |
| 12 | Rutin | 155.66 | 644 | 612.77 ± 165.45 | 555.66 ± 20.79 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chemam, Y.; Benayache, S.; Marchioni, E.; Zhao, M.; Mosset, P.; Benayache, F. On-Line Screening, Isolation and Identification of Antioxidant Compounds of Helianthemum ruficomum. Molecules 2017, 22, 239. https://doi.org/10.3390/molecules22020239
Chemam Y, Benayache S, Marchioni E, Zhao M, Mosset P, Benayache F. On-Line Screening, Isolation and Identification of Antioxidant Compounds of Helianthemum ruficomum. Molecules. 2017; 22(2):239. https://doi.org/10.3390/molecules22020239
Chicago/Turabian StyleChemam, Yasmine, Samir Benayache, Eric Marchioni, Minjie Zhao, Paul Mosset, and Fadila Benayache. 2017. "On-Line Screening, Isolation and Identification of Antioxidant Compounds of Helianthemum ruficomum" Molecules 22, no. 2: 239. https://doi.org/10.3390/molecules22020239






