Oral Administration of the Japanese Traditional Medicine Keishibukuryogan-ka-yokuinin Decreases Reactive Oxygen Metabolites in Rat Plasma: Identification of Chemical Constituents Contributing to Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Amounts of Chemical Constituents in the Extracts of KBGY and Component Crude Drugs
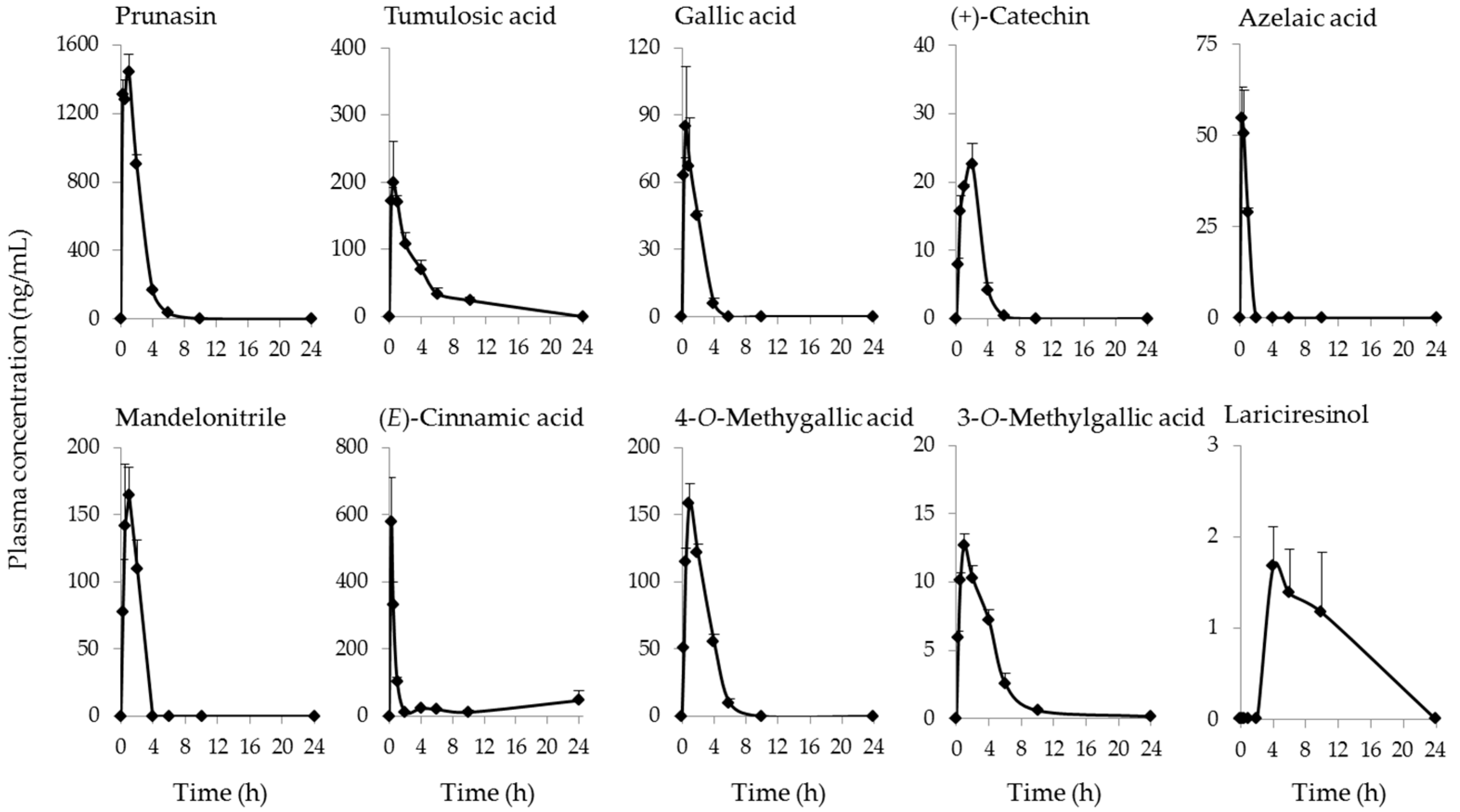
2.2. Identification and Quantification of Blood-Absorbed Constituents and Their Metabolites
2.3. Change in Oxidative Stress Parameter by KBGY Administration
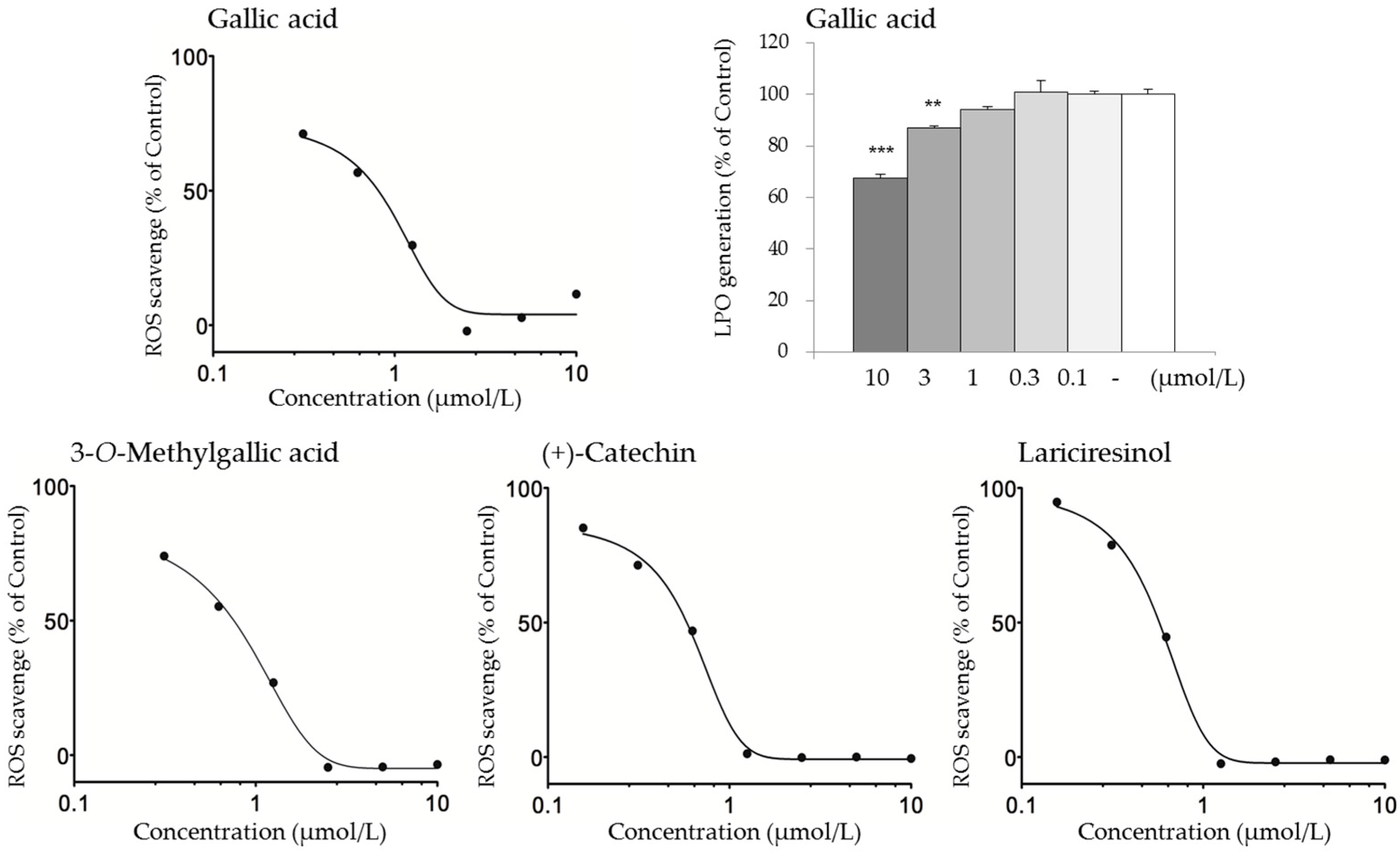
2.4. Active Constituents that May Contribute to the Antioxidant Activity of KBGY
3. Materials and Methods
3.1. Test Sample
3.2. Animals
3.3. Measurement of Constituents in KBGY and Its Component Crude Drugs
3.4. Pharmacokinetic Analysis of KBGY Constituents and Associated Metabolites
3.5. Measurement of d-ROMs in Plasma Samples
3.6. Lipid Hydroperoxide Generation Assays
3.7. ROS Scavenging Assays
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2675. [Google Scholar] [CrossRef] [PubMed]
- Dzialo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.; Carels, C.E.; Lundvig, D.M. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Sinha, A.A. Oxidative stress and autoimmune skin disease. Eur. J. Dermatol. 2013, 23, 5–13. [Google Scholar] [PubMed]
- Pastore, S.; Korkina, L. Redox imbalance in T cell-mediated skin diseases. Mediat. Inflamm. 2010, 2010, 861949. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Campanati, A.; Simonetti, O.; Liberati, G.; Offidani, A. Correlation between lipoprotein(a) and lipid peroxidation in psoriasis: Role of the enzyme paraoxonase-1. Br. J. Dermatol. 2012, 166, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Zilmer, K.; Leping, V.; Zilmer, M. Serum methylglyoxal level and its association with oxidative stress and disease severity in patients with psoriasis. Arch. Dermatol. Res. 2013, 305, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, H.; Shibata, R.; Ohshima, Y.; Todoroki, Y.; Sato, S.; Ohta, N.; Hiraoka, M.; Yoshida, A.; Nishima, S.; Mayumi, M. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci. 2003, 72, 2509–2516. [Google Scholar] [CrossRef]
- Tsuboi, H.; Kouda, K.; Takeuchi, H.; Takigawa, M.; Masamoto, Y.; Takeuchi, M.; Ochi, H. 8-hydroxydeoxyguanosine in urine as an index of oxidative damage to DNA in the evaluation of atopic dermatitis. Br. J. Dermatol. 1998, 138, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Li, C.; Kao, R.L.; Stone, W.L. Lipid hydroperoxides inhibit nitric oxide production in RAW264.7 macrophages. Free Radic. Biol. Med. 1999, 26, 526–537. [Google Scholar] [CrossRef]
- Fujita, K.; Yamamoto, T.; Kamezaki, T.; Matsumura, A. Efficacy of keishibukuryogan, a traditional Japanese herbal medicine, in treating cold sensation and numbness after stroke: Clinical improvement and skin temperature norMalization in 22 stroke patients. Neurol. Med. Chir. 2010, 50, 1–5. [Google Scholar] [CrossRef]
- Ishikawa, S.; Kubo, T.; Sunagawa, M.; Tawaratsumita, Y.; Sato, T.; Ishino, S.; Hisamitsu, T. Influence of Chinese herbal medicine on reactive oxygen and blood fluidity in rats. Kampo Med. 2011, 62, 337–346. [Google Scholar] [CrossRef]
- Hikiami, H.; Goto, H.; Sekiya, N.; Hattori, N.; Sakakibara, I.; Shimada, Y.; Terasawa, K. Comparative efficacy of Keishi-bukuryo-gan and pentoxifylline on RBC deformability in patients with “oketsu” syndRome. Phytomedicine 2003, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, K.; Hikiami, H.; Goto, H.; Nakagawa, T.; Shibahara, N.; Shimada, Y. Keishibukuryogan (gui-zhi-fu-ling-wan), a Kampo formula, decreases disease activity and soluble vascular adhesion molecule-1 in patients with rheumatoid arthritis. Evid. Based Complement. Altern. Med. 2006, 3, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Kuo, Y.H.; Lin, F.Y.; Lin, Y.L.; Chiang, W. Effect of Adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) Testa and its phenolic components on Cu2+-treated low-density lipoprotein (LDL) oxidation and lipopolysaccharide (LPS)-induced inflammation in RAW 264.7 macrophages. J. Agric. Food Chem. 2009, 57, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Wu, D.G.; Chen, Y.W. Chemical constituents and bioactivities of plants from the genus Paeonia. Chem. Biodivers. 2010, 7, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.K.; Kim, J.M.; Koo, J.Y.; Kang, S.S.; Bae, K.; Kim, Y.S.; Chung, J.H.; Yun-Choi, H.S. Platelet anti-aggregatory and blood anti-coagulant effects of compounds isolated from Paeonia lactiflora and Paeonia suffruticosa. Die Pharm. 2010, 65, 624–628. [Google Scholar]
- Kim, H.K.; Yun, Y.K.; Ahn, Y.J. Fumigant toxicity of cassia bark and cassia and cinnamon oil compounds to Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). Exp. Appl. Acarol. 2008, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liao, Q.; Xu, X.; Yao, M.; Zhou, Y.; Lin, M.; Zhang, P.; Xie, Z. Analysis of essential oils from cassia bark and cassia twig samples by GC-MS combined with multivariate data analysis. Food Anal. Methods 2014, 7, 1840–1847. [Google Scholar] [CrossRef]
- Buckingham, J. Dictionary of Natural Products; Chapman & Hall: London, UK, 1994; Volume 3, pp. 2479–3725. [Google Scholar]
- Fukuda, T.; Ito, H.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Yoshida, T. Anti-tumor promoting effect of glycosides from Prunus persica seeds. Biol. Pharm. Bull. 2003, 26, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Webster, G. Combination azelaic acid therapy for acne vulgaris. J. Am. Acad. Dermatol. 2000, 43, S47–S50. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E.; Wagstaff, A.J. Azelaic acid 15% gel: In the treatment of papulopustular rosacea. Am. J. Clin. Dermatol. 2004, 5, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.X.; Jin, X.L.; Gu, S.Y.; Peng, Y.; Zhang, K.R.; Ou-Yang, B.C.; Wang, Y.; Xiao, W.; Wang, Z.Z.; Aa, J.Y.; et al. Integrated identification, qualification and quantification strategy for pharmacokinetic profile study of Guizhi Fuling capsule in healthy volunteers. Sci. Rep. 2016, 6, 31364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xiong, Z.; Sui, Y.; Zhu, H.; Zhou, Z.; Wang, Z.; Zhao, Y.; Xiao, W.; Lin, J.; Bi, K. Simultaneous determination of six bioactive constituents of Guizhi Fuling Capsule in rat plasma by UHPLC-MS/MS: Application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1001, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, Q.; Liang, K.; Zhao, L.; He, B.; Ji, W.; Chen, X.; Wang, Z.; Bi, K.; Jia, Y. Comparative pharmacokinetics of three triterpene acids in rat plasma after oral administration of Poria extract and its formulated herbal preparation: GuiZhi-FuLing capsule. Fitoterapia 2012, 83, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, I.; de Gregorio, V.; Baroni, A.; Tufano, M.A.; Donnarumma, G.; Perez, J.J. Amygdalin analogues inhibit IFN-gamma signalling and reduce the inflammatory response in human epidermal keratinocytes. Inflammation 2013, 36, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Fu, L.X.; Zhang, L.W.; Yin, B.; Zhou, P.M.; Cao, N.; Lu, Y.H. Paeoniflorin suppresses inflammatory response in imiquimod-induced psoriasis-like mice and peripheral blood mononuclear cells (PBMCs) from psoriasis patients. Can. J. Physiol. Pharmacol. 2016, 94, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Li, H.; Sun, J.; Wang, S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J. Ethnopharmacol. 2013, 150, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, M.J.; Giner, R.M.; Recio, M.C.; Just, M.J.; Manez, S.; Rios, J.L. Effect of the basidiomycete Poria cocos on experimental dermatitis and other inflammatory conditions. Chem. Pharm. Bull. 1997, 45, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Maurya, H.; Mangal, V.; Gandhi, S.; Prabhu, K.; Ponnudurai, K. Prophylactic antioxidant potential of gallic Acid in murine model of sepsis. Int. J. Inflamm. 2014, 2014, 580320. [Google Scholar] [CrossRef] [PubMed]
- Pal, C.; Bindu, S.; Dey, S.; Alam, A.; Goyal, M.; Iqbal, M.S.; Maity, P.; Adhikari, S.S.; Bandyopadhyay, U. Gallic acid prevents nonsteroidal anti-inflammatory drug-induced gastropathy in rat by blocking oxidative stress and apoptosis. Free Radic. Biol. Med. 2010, 49, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Rossato, M.F.; Tonello, R.; Hoffmeister, C.; Klafke, J.Z.; Rosa, F.; Pinheiro, K.V.; Pinheiro, F.V.; Boligon, A.A.; Athayde, M.L.; et al. Gallic acid functions as a TRPA1 antagonist with relevant antinociceptive and antiedematogenic effects in mice. Naunyn-Schmiedeberg's Arch. Pharmacol. 2014, 387, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.S.; Na, H.J.; Kim, Y.M.; Kwon, H.J. Antiangiogenic activity of 4-O-methylgallic acid from Canavalia gladiata, a dietary legume. Biochem. Biophys. Res. Commun. 2005, 330, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Na, H.J.; Lee, G.; Oh, H.Y.; Jeon, K.S.; Kwon, H.J.; Ha, K.S.; Lee, H.; Kwon, Y.G.; Kim, Y.M. 4-O-Methylgallic acid suppresses inflammation-associated gene expression by inhibition of redox-based NF-kappaB activation. Int. Immunopharmacol. 2006, 6, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, S.; Saravanan, R. Protective effects of azelaic acid against high-fat diet-induced oxidative stress in liver, kidney and heart of C57BL/6J mice. Mol. Cell. Biochem. 2013, 377, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Sieber, M.A.; Hegel, J.K. Azelaic acid: Properties and mode of action. Skin Pharmacol. Physiol. 2014, 27 (Suppl. 1), 9–17. [Google Scholar] [CrossRef] [PubMed]
- Schulte, B.C.; Wu, W.; Rosen, T. Azelaic acid: Evidence-based update on mechanism of action and clinical application. J. Drugs Dermatol. 2015, 14, 964–968. [Google Scholar]
- Akamatsu, H.; Komura, J.; Asada, Y.; Miyachi, Y.; Niwa, Y. Inhibitory effect of azelaic acid on neutrophil functions: A possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch. Dermatol. Res. 1991, 283, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Grange, P.A.; Chereau, C.; Raingeaud, J.; Nicco, C.; Weill, B.; Dupin, N.; Batteux, F. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathog. 2009, 5, e1000527. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Fukui, T.; Ouchi, M.; Watanabe, K.; Suzuki, T.; Yamamoto, S.; Yamamoto, T.; Hayashi, T.; Oba, K.; HIrano, T. Relationship between daily and day-to-day glycemic variability and increased oxidative stress in type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 122, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Cacciapaglia, F.; Anelli, M.G.; Rizzo, D.; Morelli, E.; Scioscia, C.; Mazzotta, D.; Iannone, F.; Lapadula, G. Influence of TNF-alpha inhibition on oxidative stress of rheumatoid arthritis patients. Reumatismo 2015, 67, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Montini, L.; DE Sole, P.; Pennisi, M.A.; Rossi, C.; Scatena, R.; DE Pascale, G.; Bello, G.; Cutuli, S.L.; Antonelli, M. Prognostic value of the reactive oxygen species in severe sepsis and septic shock patients: A pilot study. Min. Anestesiol. 2016, 82, 1306–1313. [Google Scholar]
- Fukuda, S.; Nojima, J.; Motoki, Y.; Yamaguti, K.; Nakatomi, Y.; Okawa, N.; Fujiwara, K.; Watanabe, Y.; Kuratsune, H. A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biol. Psychol. 2016, 118, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Koseki, J.; Matsumoto, T.; Matsubara, Y.; Tsuchiya, K.; Mizuhara, Y.; Sekiguchi, K.; Nishimura, H.; Watanabe, J.; Kaneko, A.; Hattori, T.; et al. Inhibition of rat 5alpha-reductase activity and testosterone-induced sebum synthesis in hamster sebocytes by an extract of Quercus acutissima cortex. Evid. Based Complement. Altern. Med. 2015, 2015, 853846. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, O.D.; Gargouri, B.; Trabelsi, S.K.; Bouaziz, M.; Abdelhedi, R. Synthesis of 3-O-methylgallic acid a powerful antioxidant by electrochemical conversion of syringic acid. Biochim. Biophys. Acta 2013, 1830, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Kim, S.H.; Hagerman, A.E.; Lu, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [PubMed]
- Lopez de Felipe, F.; de Las Rivas, B.; Munoz, R. Bioactive compounds produced by gut microbial tannase: Implications for colorectal cancer development. Front. Microbiol. 2014, 5, 684. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wahala, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In vitro metabolism of plant lignans: New precursors of mamMalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q. MamMalian phytoestrogens: Enterodiol and enterolactone. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 777, 289–309. [Google Scholar] [CrossRef]
- Liu, Z.; Saarinen, N.M.; Thompson, L.U. Sesamin is one of the major precursors of mamMalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J. Nutr. 2006, 136, 906–912. [Google Scholar] [PubMed]
- Thornton, M.J. Estrogens and aging skin. Derm. Endocrinol. 2013, 5, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.Y.; Maibach, H.I. Estrogen and skin: Therapeutic options. Am. J. Clin. Dermatol. 2011, 12, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Puzzolo, D.; Rinaldi, M.; Irrera, N.; Marini, H.; Arcoraci, V.; Bitto, A.; Crea, G.; Pisani, A.; Squadrito, F.; et al. ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxid. Med. Cell. Longev. 2016, 2016, 2183026. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, K.; Ohashi, M.; Yamashita, S.; Kojima, M.; Sato, K.; Ueda, R.; Dohi, Y. Increased oxidative stress impairs endothelial modulation of contractions in arteries from spontaneously hypertensive rats. J. Hypertens. 2007, 25, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Sandu, O.; Peppa, M.; Goldberg, T.; Vlassara, H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann. N. Y. Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Mizawa, M.; Makino, T.; Hikiami, H.; Shimada, Y.; Shimizu, T. Effectiveness of keishibukuryogan on chronic-stage lichenification associated with atopic dermatitis. ISRN Dermatol. 2012, 2012, 158598. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
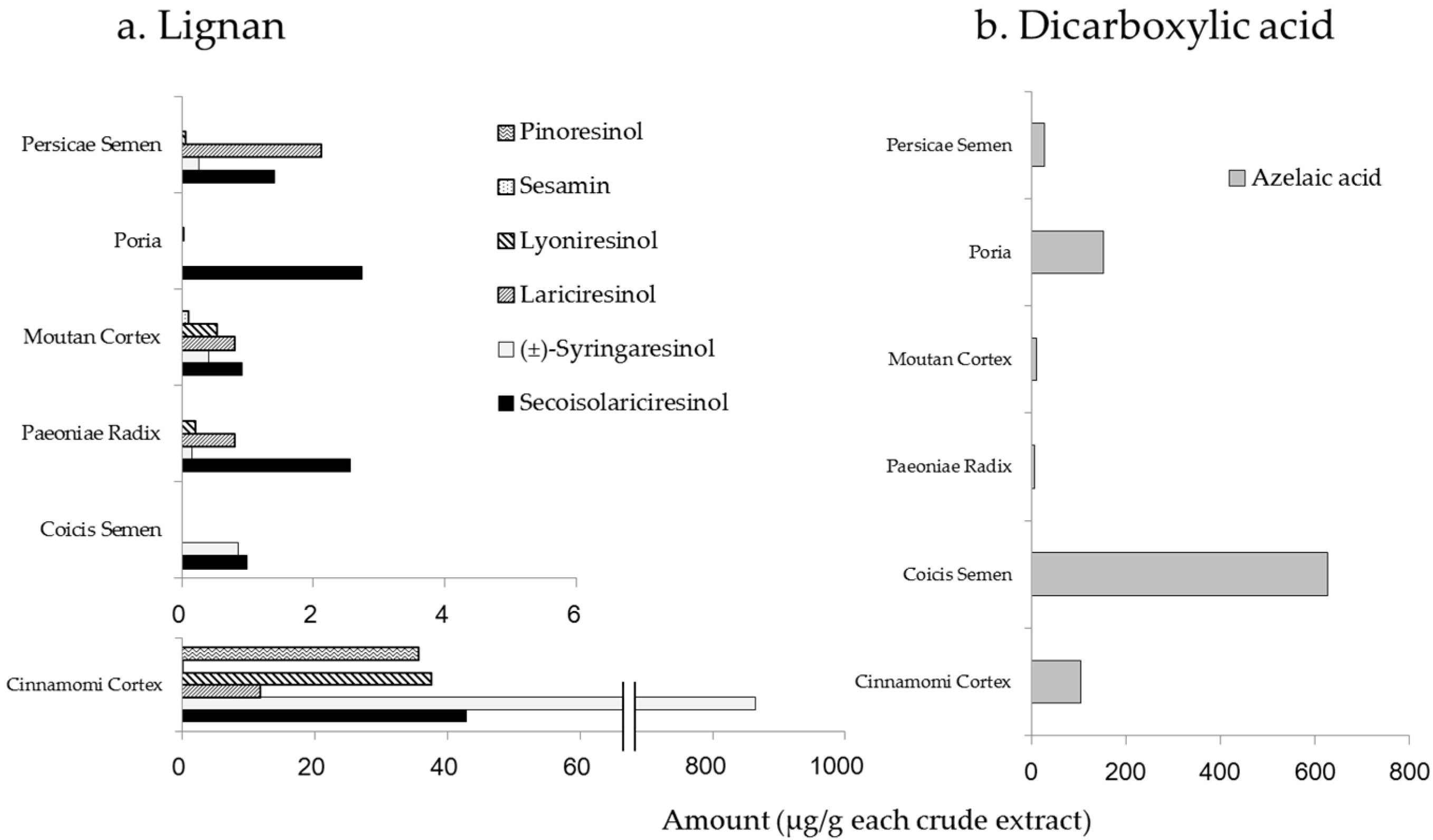

| Compound Name | Amount (µg/g KBGY) | Compound Name | Amount (µg/g KBGY) |
|---|---|---|---|
| Monoterpenoid | Flavonoid | ||
| Paeoniflorin a | 17,000 | (+)-Catechin c | 388 |
| Albiflorin a | 5340 | Phenylpropanoid | |
| Paeonimetabolin I | 275 | Cinnamaldehyde d | 1260 |
| Triterpenoid | (E)-Cinnamic acid f | 358 | |
| Tumulosic acid b | 302 | Cinnamylacetate e | 287 |
| Pachymic acid b | 289 | Cinnamyl alcohol d | 192 |
| Dehydropachymic acid b | 91.2 | 2-Methoxycinnamaldehyde e | 70.6 |
| Oleanolic acid and/or Ursolic acid a | 31.3 | 3-Phenylpropyl acetate f | 20.5 |
| Dehydrotumulosic acid b | 9.76 | Methylcinnamate f | 5.59 |
| Eburicoic acid b | BQL | Eugenol e | 2.18 |
| Gallotannin | Lignan | ||
| Pentagalloyl glucose a | 3190 | (±)-Syringaresinol | 199 |
| Tetragalloyl glucose a | 1260 | Pinoresinol | 15.0 |
| Phenol | Lyoniresinol | 7.60 | |
| Gallic acid a | 2460 | Secoisolariciresinol | 7.09 |
| Paeonol a | 578 | Matairesinol | 0.217 |
| Salicylaldehyde d | 557 | Lariciresinol | BQL |
| 3-O-Methylgallic acid | 110 | Enterolactone | BQL |
| 4-O-Methylgallic acid | 27.4 | Enterodiol | BQL |
| Pyrogallol | 9.44 | Dicarboxylic acid | |
| Resorcinol | BQL | Azelaic acid | 75.2 |
| 5-Pentadecylresorcinol | BQL | Glutaric acid | 50.8 |
| 5-Heneicosylresorcinol | BQL | Suberic acid | 47.6 |
| 5-Tricosylresorcinol | BQL | Pimelic acid | 13.3 |
| Cyanogenic glycoside | Adipic acid | 12.7 | |
| Amygdalin g | 9760 | Sebacic acid | BQL |
| Prunasin g | 1260 | ||
| Mandelonitrile | 189 |
| Compound Name | Cmax (ng/mL) (μmol/L) | AUC0–last (ng·h/mL) | tmax (h) | t1/2 (h) |
|---|---|---|---|---|
| Poria | ||||
| Dehydrotumulosic acid | 15.5 (0.032) | 39.5 | 0.5 | 3.81 |
| Pachymic acid | 1.21 (0.002) | 5.29 | 4 | - |
| Tumulosic acid | 200 (0.411) | 700 | 0.5 | 4.66 |
| Paeoniae Radix & Moutan Cortex | ||||
| (+)-Catechin | 22.6(0.078) | 65.1 | 2 | - |
| 3-O-Methylgallic acid | 12.7 (0.069) | 58.6 | 1 | 6.59 |
| 4-O-Methylgallic acid | 159 (0.863) | 479 | 1 | 0.906 |
| Albiflorin | 16.1 (0.034) | 64.9 | 0.5 | 1.6 |
| Gallic acid | 84.9 (0.499) | 172 | 0.5 | 0.772 |
| Paeoniflorin | 80.2 (0.167) | 299 | 0.5 | 2.72 |
| Oleanolic acid and/or ursolic acid | 0.927 (0.002) | 0.116 | 0.5 | - |
| Persicae Semen | ||||
| Amygdalin | 37.7 (0.082) | 79.9 | 0.5 | 0.657 |
| Manderonitrile | 165 (1.239) | 251 | 1 | - |
| Prunasin | 1450 (4.910) | 3700 | 1 | 1.16 |
| Cinnamomi Cortex | ||||
| (±)-Syringaresinol | 0.213 (0.001) | 0.0771 | 0.25 | - |
| (E)-Cinnamic acid | 579 (3.908) | 916 | 0.25 | 23 |
| Cinnamaldehyde | 11.5 (0.087) | 51.6 | 1 | 5.21 |
| Lyoniresinol | 0.0408 (0.0001) | 0.3 | 1 | 11.4 |
| Lariciresinol | 1.68 (0.005) | 9.84 | 4 | - |
| Enterodiol | 1.03 (0.003) | 2.91 | 0.5 | 27.3 |
| Enterolactone | 1.07 (0.004) | 1.33 | 4 | - |
| Coicis Semen | ||||
| Adipic acid | 14.4 (0.099) | 17.1 | 6 | - |
| Azelaic acid | 54.7 (0.291) | 39.9 | 0.25 | - |
| Test Compound | Concentration (μmol/L) | Antioxidant Activity (% of Control) | |
|---|---|---|---|
| ROS Scavenge | LPO Generation | ||
| Gallic acid | 10 | 14.5 ± 0.7 | 74.0 ± 2.5 |
| 3-O-methylgallic acid | 10 | C.I. | 77.8 ± 4.3 |
| 4-O-methylgallic acid | 10 | 86.8 ± 0.2 | 105.4 ± 2.5 |
| Paeoniflorin | 10 | 100.2 ± 1.4 | 92.7 ± 0.9 |
| Alibiflorin | 10 | 104.2 ± 1.1 | 101.6 ± 2.2 |
| (+)-Catechin | 10 | 7.3 ± 1.1 | 95.4 ± 3.9 |
| Prunasin | 10 | 102.2 ± 1.1 | 97.9 ± 3.3 |
| Amygdalin | 10 | 106.4 ± 0.5 | 102.0 ± 2.1 |
| Mandelonitrile | 10 | 99.7 ± 0.6 | 104.6 ± 1.2 |
| Cinnamaldehyde | 10 | 103.8 ± 1.1 | 109.8 ± 3.9 |
| (E)-Cinnamic acid | 10 | 108.7 ± 2.2 | 104.3 ± 2.3 |
| Tumulosic acid | 10 | 105.9 ± 2.1 | 99.4 ± 2.3 |
| Dehydrotumulosic acid | 10 | 106.0 ± 0.4 | 97.4 ± 3.1 |
| Azelaic acid | 10 | 100.4 ± 0.7 | 97.9 ± 4.4 |
| Adipic acid | 10 | 97.0 ± 0.9 | 103.6 ± 2.9 |
| (+)-Ascorbic acid | 114 | 14.5 ± 0.6 | 27.6 ± 1.7 |
| Test Compound | Concentration (μmol/L) | Antioxidant Activity (% of Control) | |
|---|---|---|---|
| ROS Scavenge | LPO Generation | ||
| (±)-Syringaresinol | 1 | 39.4 ± 2.4 | 107.4 ± 4.2 |
| 10 | C.I. | 68.4 ± 3.3 | |
| Lariciresinol | 1 | C.I. | 98.3 ± 1.4 |
| 10 | C.I. | 79.5 ± 1.9 | |
| Lyoniresinol | 1 | 89.3 ± 2.0 | 96.8 ± 2.9 |
| 10 | C.I. | 57.4 ± 1.6 | |
| Enterodiol | 1 | 67.6 ± 2.4 | 95.2 ± 4.4 |
| 10 | C.I. | 84.9 ± 3.1 | |
| Enterolactone | 1 | 81.6 ± 2.9 | 102.1± 2.7 |
| 10 | C.I. | 86.1 ± 0.9 | |
| (+)-Ascorbic acid | 114 | 20.2 ± 0.6 | 9.9 ± 1.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsubara, Y.; Matsumoto, T.; Sekiguchi, K.; Koseki, J.; Kaneko, A.; Yamaguchi, T.; Kurihara, Y.; Kobayashi, H. Oral Administration of the Japanese Traditional Medicine Keishibukuryogan-ka-yokuinin Decreases Reactive Oxygen Metabolites in Rat Plasma: Identification of Chemical Constituents Contributing to Antioxidant Activity. Molecules 2017, 22, 256. https://doi.org/10.3390/molecules22020256
Matsubara Y, Matsumoto T, Sekiguchi K, Koseki J, Kaneko A, Yamaguchi T, Kurihara Y, Kobayashi H. Oral Administration of the Japanese Traditional Medicine Keishibukuryogan-ka-yokuinin Decreases Reactive Oxygen Metabolites in Rat Plasma: Identification of Chemical Constituents Contributing to Antioxidant Activity. Molecules. 2017; 22(2):256. https://doi.org/10.3390/molecules22020256
Chicago/Turabian StyleMatsubara, Yosuke, Takashi Matsumoto, Kyoji Sekiguchi, Junichi Koseki, Atsushi Kaneko, Takuji Yamaguchi, Yumiko Kurihara, and Hiroyuki Kobayashi. 2017. "Oral Administration of the Japanese Traditional Medicine Keishibukuryogan-ka-yokuinin Decreases Reactive Oxygen Metabolites in Rat Plasma: Identification of Chemical Constituents Contributing to Antioxidant Activity" Molecules 22, no. 2: 256. https://doi.org/10.3390/molecules22020256
APA StyleMatsubara, Y., Matsumoto, T., Sekiguchi, K., Koseki, J., Kaneko, A., Yamaguchi, T., Kurihara, Y., & Kobayashi, H. (2017). Oral Administration of the Japanese Traditional Medicine Keishibukuryogan-ka-yokuinin Decreases Reactive Oxygen Metabolites in Rat Plasma: Identification of Chemical Constituents Contributing to Antioxidant Activity. Molecules, 22(2), 256. https://doi.org/10.3390/molecules22020256






