Addressing Facts and Gaps in the Phenolics Chemistry of Winery By-Products
Abstract
:1. Introduction
2. Chemistry of Winery By-Products
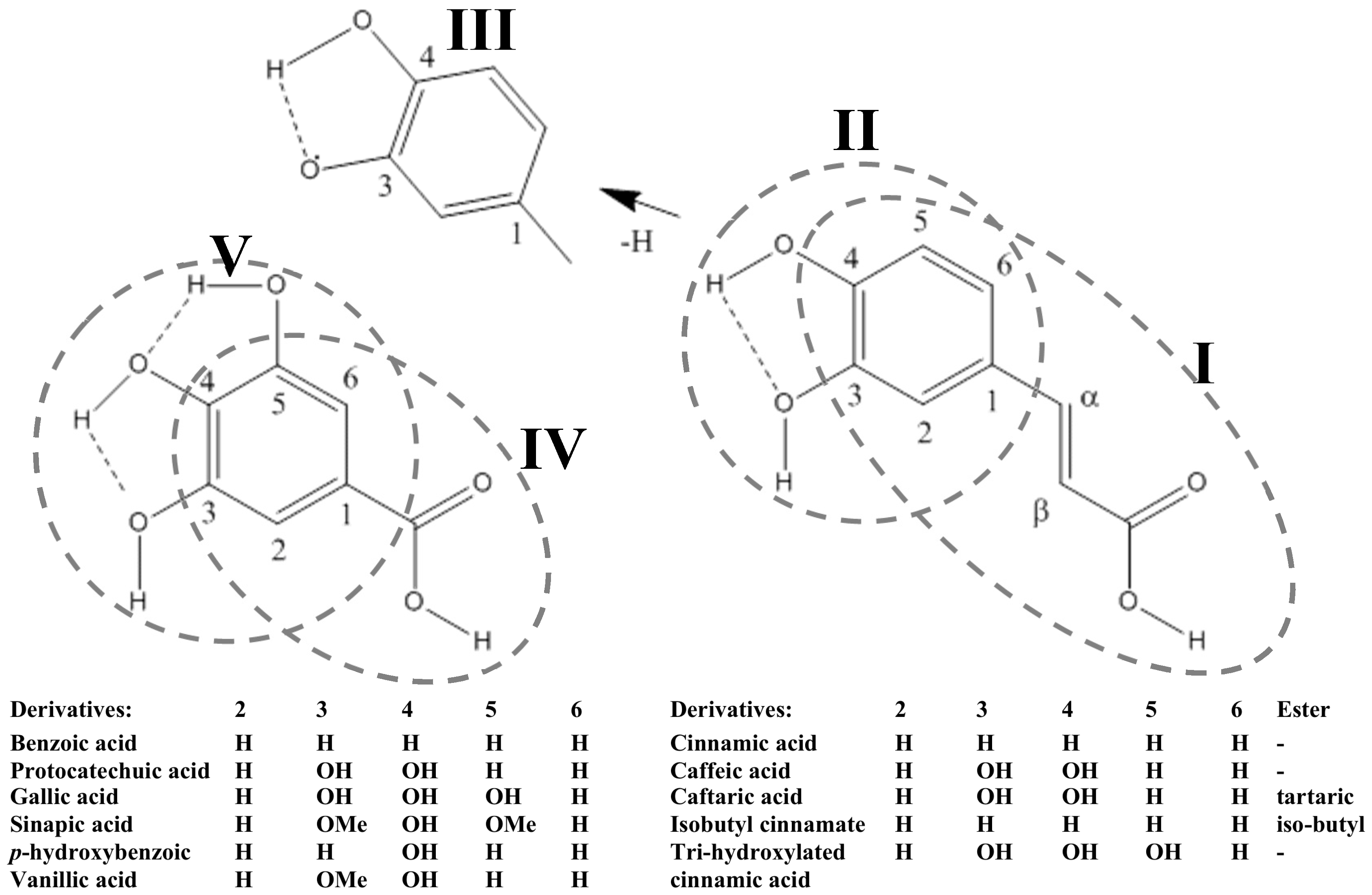
2.1. Phenolic Acids
2.2. Flavonoids
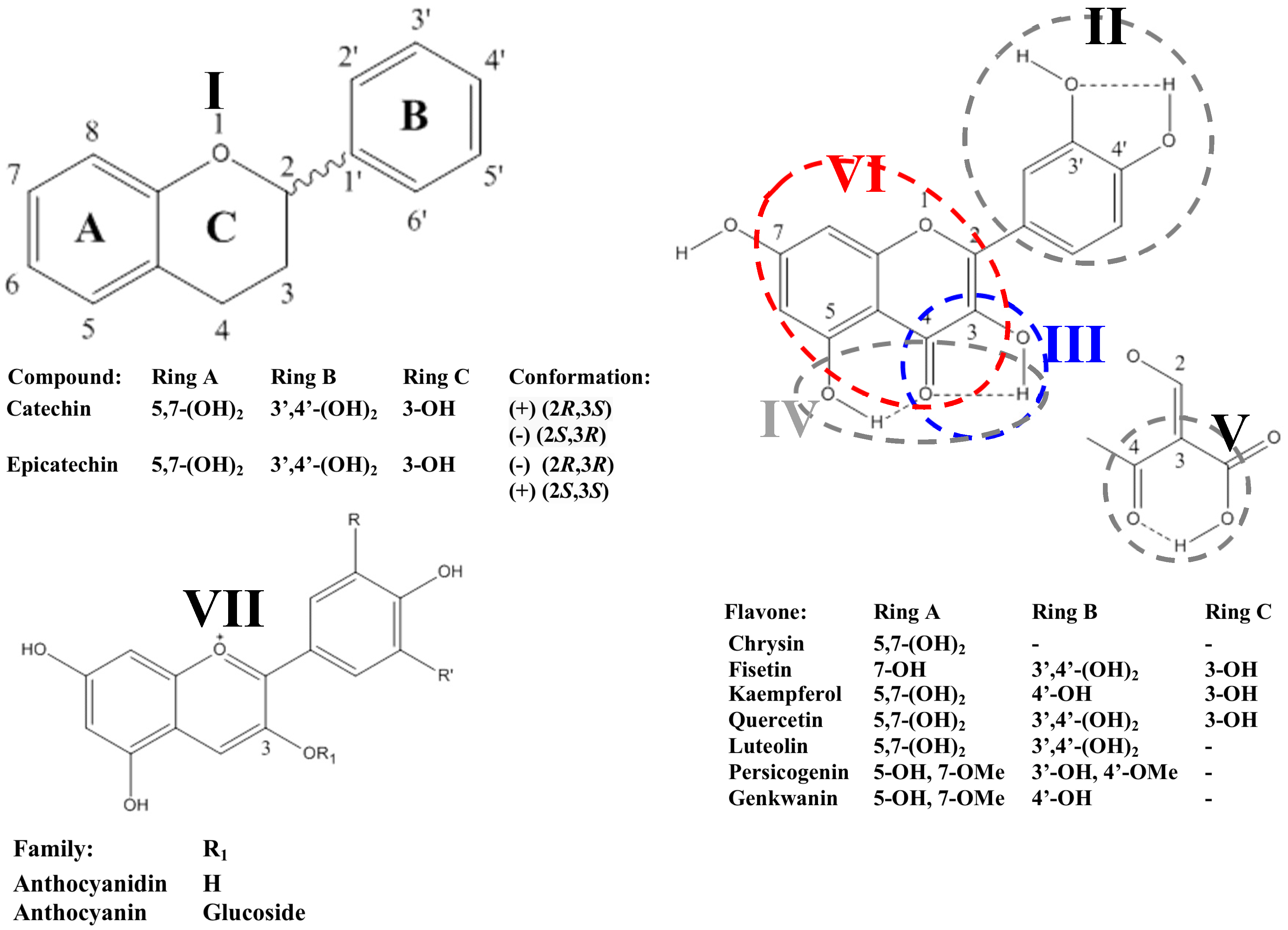
2.2.1. Flavanols
2.2.2. Flavonols
2.2.3. Anthocyanidins
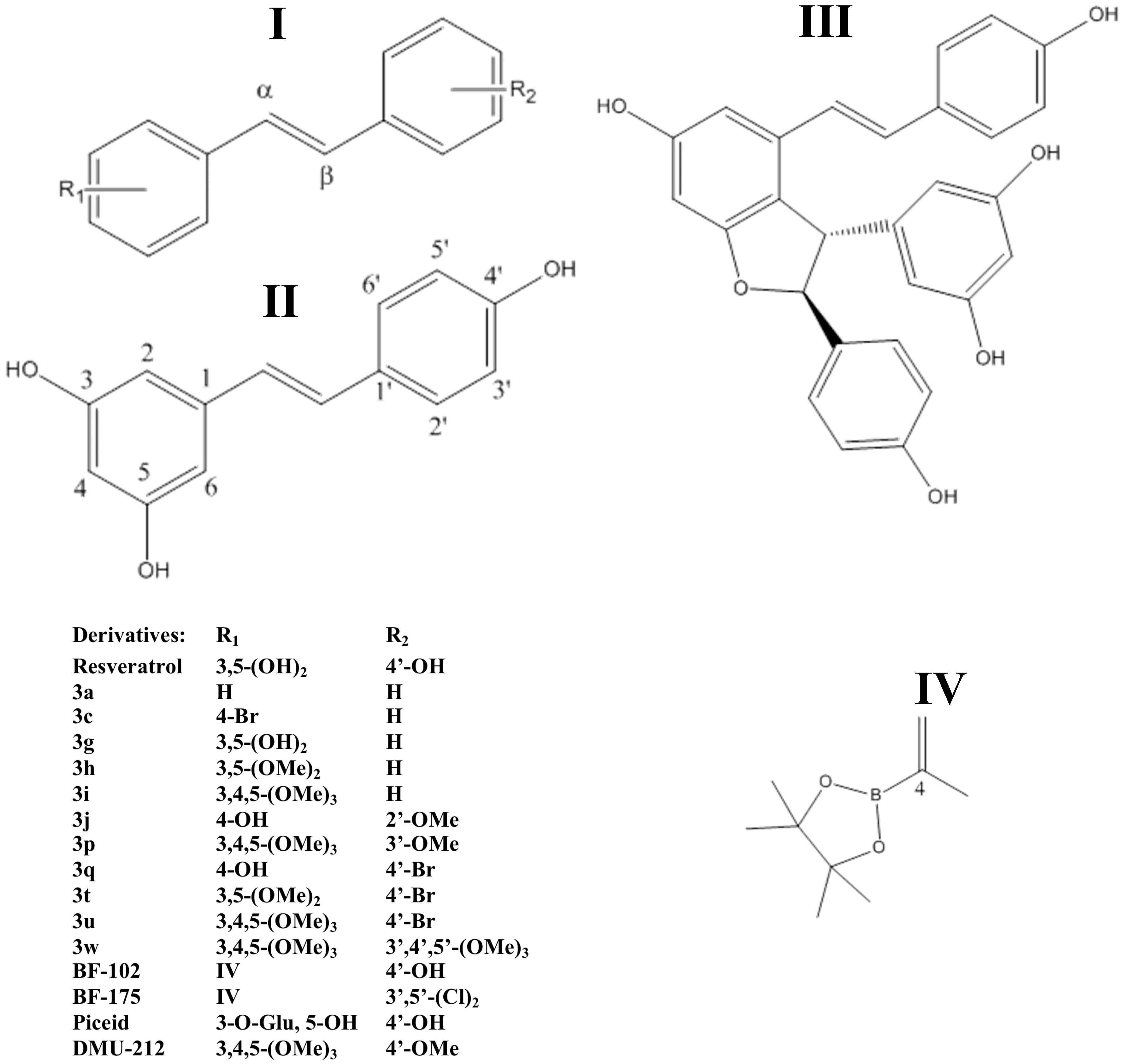
2.2.4. Stilbenoids
3. Use of Chemometrics for the Traceability of Winery By-Products
4. Processing Alternatives: Challenges for Improving Phytochemical Profile
4.1. Storage
4.2. Processing
4.2.1. Fermentation Processes
4.2.2. UV Radiation
4.2.3. Liberation of Compounds through Electricity Pulses, and Thermal and Enzymatic Treatments
5. Structure-Activity Relationship: What Do We Know?
5.1. Health Effects and Antioxidant Properties of Phenols
5.2. Importance of H-Bond Interactions for Conformation and Function
5.3. Main Computational Methods in Studying Phenolic Antioxidants
5.4. Presently Known SAR/QSAR for Compounds from Winery Industry
6. Conclusions and Future Prospects
Acknowledgments
Conflicts of Interest
References
- Barba, F.J.; Zhu, Z.; Koubaa, M.; de Souza Sant’Ana, A.; Orlien, V. Green alternative methods for the extraction of antioxidant 1 bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). Available online: http://www.oiv.int/ (accessed on 1 October 2016).
- Teixeira, A.; Baenas, N.; Domínguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; García-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed]
- El Achkar, J.H.; Lendormi, T.; Hobaika, Z.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.L. Anaerobic digestion of grape pomace: Biochemical characterization of the fractions and methane production in batch and continuous digesters. Waste Manag. 2016, 50, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Songa, J.; Smart, R.; Wang, H.; Dambergs, B.; Sparrow, A.; Qian, M.C. Effect of grape bunch sunlight exposure and UV radiation on phenolics and volatile composition of Vitis vinifera L. cv. Pinot noir wine. Food Chem. 2015, 173, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; da Ricardo Silva, J.M.; Spranger, M.I. Quantification of catechins and proanthocyanidins in several Portuguese grapevine varieties and red wine. Ciênc. Téc. Vitiviníc. 2001, 16, 23–34. [Google Scholar]
- Laufenberg, G.; Kuntz, B.; Nystroem, M. Transformation of vegetable waste into value added products: (A) the upgrading concept, (B) practical implementations. Bioresour. Technol. 2003, 87, 167–198. [Google Scholar] [CrossRef]
- Gil, M.; Bottini, R.; Berli, F.; Pontin, M.; Silva, M.F.; Piccoli, P. Volatile organic compounds characterized from grapevine (Vitis vinífera L. cv. Malbec) berries increase at pre-harvest and in response to UV-B radiation. Phytochemistry 2013, 96, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.J.; Maicas, S. Valorization of winery and oil mill wastes by microbial technologies. Food Res. Int. 2015, 73, 13–25. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Song, C.Z.; Yu, Y.; Hua, F.; Zhang, L.; Zhang, Z.W.; Xi, Z.M. Melatonin treatment of pre-veraison grape berries to increase size and synchronicity of berries and modify wine aroma components. Food Chem. 2015, 185, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ju, J.; Paul, S.; So, J.Y.; DeCastro, A.; Smolarek, A.; Lee, M.J.; Yang, C.S.; Newmark, H.L.; Suh, N. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-γ. Clin. Cancer Res. 2009, 15, 4242–4249. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A. Strategies for discovering drugs from previously unexplored natural products. Drug Discov. Today 2000, 5, 294–300. [Google Scholar] [CrossRef]
- Bindseil, K.U.; Jakupovic, J.; Wolf, D.; Lavayre, J.; Leboul, J.; van der Pyl, D. Pure compound libraries; a new perspective for natural product based drug discovery. Drug Discov. Today 2001, 6, 840–847. [Google Scholar] [CrossRef]
- Lam, K.L. New aspects of natural products in drug discovery. Trends Microbiol. 2007, 15, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Mutafova, B.; Christen, P. Molecular biodiversity and recent analytical developments: A marriage 3 of convenience. Biotechnol. Adv. 2014, 32, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Penner, M.H.; Zhao, W. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Paradelo, R.; Moldes, A.B.; Barral, M.T. Evolution of organic matter during the mesophilic composting of lignocellulosic winery wastes. J. Environ. Manag. 2013, 116, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Koubaa, M.; Mhemdi, H.; Vorobiev, E. Seed oil polyphenols: Rapid and sensitive extraction method and high resolution-mass spectrometry identification. Anal. Biochem. 2015, 476, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Ryean, D.; Robards, K.; Leave, S. Changes in phenolic contents in olives during maturation. Int. J. Food Sci. Technol. 1999, 34, 265–274. [Google Scholar] [CrossRef]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Garcia-Jares, C.; Vazquez, A.; Lamas, J.P.; Pajaro, M.; Alvarez-Casas, M.; Lores, M. Antioxidant White Grape Seed Phenolics: Pressurized Liquid Extracts from Different Varieties. Antioxidants 2015, 4, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- De la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, A.; Obreque-Sliera, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valero, J.R. Extraction and Analysis of Polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Casas, M.; García-Jares, C.; Llompart, M.; Lores, M. Effect of experimental parameters in the pressurized solvent extraction of polyphenolic compounds from white grape marc. Food Chem. 2014, 15, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Guendez, R.; Kallithraka, S.; Makris, D.P.; Kefalas, P. Determination of low molecular weight phenolic constituents in grape (Vitis vinifera sp.) seed extracts: Correlation with antiradical activity. Food Chem. 2005, 89, 1–9. [Google Scholar] [CrossRef]
- Obreque-slier, E.; peñaneira, A.; López-Solís, R.; Zamora-Marín, Z.; Ricardo-da Silva, J.M.; Laureano, O. Comparative Study of the Phenolic Composition of Seeds and Skins from Carménère and Cabernet Sauvignon Grape Varieties (Vitis vinifera L.) during Ripening. J. Agric. Food Chem. 2010, 58, 3591–3599. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Chedea, V.S.; Echimi, C.; Braicu, C.; Andjelkovic, M.; Verhe, R.; Socaciu, C. Composition in polyphenols and stability of the aqueous grape seed extract from the romanian variety “Merlot Recas”. J. Food Biochem. 2011, 35, 92–108. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Peña-Neira, A.; López-Solís, R. Interactions of enological tannins with the protein fraction of saliva and astringency perception are affected by pH. LWT Food Sci. Technol. 2012, 45, 88–93. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Peña-Neira, A.; López-Solís, R.; Cáceres-Mella, A.; Toledo-Araya, H.; López-Rivera, A. Phenolic composition of skins from four Carmenet grape varieties (Vitis vinifera L.) during ripening. LWT Food Sci. Technol. 2013, 54, 404–413. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Antioxidant potential of white grape pomaces: Phenolic composition and antioxidant capacity measured by spectrophotometric and cyclic voltammetry methods. Food Res. Int. 2014, 66, 150–157. [Google Scholar] [CrossRef]
- Iora, S.R.F.; Maciel, G.M.; Zielinski, A.A.F.; da Silva, M.V.; Pontes, P.V.A.; Haminiuk, C.W.I.; Granato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Rodríguez-Pulido, F.J.; Hernán, D.; Escudero-Gilete, M.L.; Heredia, F.J. Determination of phenolic substances of seeds, skins and stems from white grape marc by near-infrared hyperspectral imaging. Aust. J. Grape Wine Res. 2016, 22, 11–15. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Dabić-Zagorac, D.Č.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Souquet, J.M.; Labarbe, B.; Guernevé, C.L.; Cheynier, V.; Moutounet, M. Phenolic composition of grape stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, A. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Barros, A.; Gironés-Vilaplana, A.; Teixeira, A.; Collado-González, J.; Moreno, D.; Gil-Izquierdo, A.; Rosa, E.; Domínguez-Perles, R. Evaluation of grape (Vitis vinifera L.) stems from Portuguese varieties as a resource of (poly)phenolic compounds: A comparative study. Food Res. Int. 2014, 65, 375–384. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinífera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. In vivo antioxidant activity of grape, pomace and wine from three red varieties grown in Argentina: Its relationship to phenolic profile. J. Funct. Foods 2016, 20, 332–345. [Google Scholar] [CrossRef]
- Kallithraka, S.; Salachaa, M.I.; Tzouroua, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agroindustrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Baderschneider, B.; Winterhalter, P. Isolation and characterization of novel benzoates, cinnamates, flavonoids, and lignans from Riesling wine and screening for antioxidant activity. J. Agric. Food Chem. 2001, 49, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T. Flavonoid function and activity to plants and other organisms. Biol. Sci. Space 2003, 17, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Montealegre, P.R.; Peces, R.R.; Vozmediano, J.L.C.; Gascueña, J.M.; Romero, G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Welch, C.R.; Wu, Q.; Simon, J.E. Recent Advances in Anthocyanin Analysis and Characterization. Curr. Anal. Chem. 2008, 4, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Lagana, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation. Foods Plants Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Evidence for enzyme complexes in the phenylpropanoid and flavonoid pathways. Physiol. Plant. 1999, 107, 142–149. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.L. Proanthocyanidin Composition and Antioxidant Potential of the Stem Winemaking Byproducts from 10 Different Grape Varieties (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 11850–11858. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.L. Characterization of Polyphenols and Antioxidant Potential of White Grape Pomace Byproducts (Vitis vinifera L.). J. Agric. Food Chem. 2013, 61, 11579–11587. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.; Justino, V.; Spranger, M.I.; Zhao, Y.Q.; Han, L.; Suna, B.S. Extraction yields and anti-oxidant activity of proanthocyanidins from different parts of grape pomace: Effect of mechanical treatments. Phytochem. Anal. 2014, 25, 134–140. [Google Scholar] [PubMed]
- Boussetta, N.; Vorobiev, E.; Deloison, V.; Pochez, F.; Falcimaigne-Cordin, E.; Lanoisellé, J.L. Valorisation of grape pomace by the extraction of phenolic antioxidants: Application of high voltage electrical discharges. Food Chem. 2011, 128, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Casas, M.; Pájaro, M.; Lores, M.; García-Jares, C. Characterization of grape marcs from native and foreign White varieties grown in northwestern Spain by their polyphenolic composition and antioxidant activity. Eur. Food Res. Technol. 2016, 242, 655–665. [Google Scholar] [CrossRef]
- Hellström, J.; Sinkkonen, J.; Karonen, M.; Mattila, P. Isolation and structure elucidation of procyanidin oligomers from saskatoon berries (Amelanchier alnifolia). J. Agric. Food Chem. 2007, 55, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.A.; Rodinõ-Janeiro, B.K.; Jerez, M.; Ucieda-Somoza, R.; Núñez, M.J.; González-Juanatey, J.R. Procyanidins From Grape Pomace Are Suitable Inhibitors of Human Endothelial NADPH Oxidase. J. Cell. Biochem. 2012, 113, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.W. Methods in Plant Biochemistry, I: Plant Phenolics; Harborne, J.B., Ed.; Academic Press: London, UK, 1989; p. 389. [Google Scholar]
- Okuda, T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry 2005, 66, 2012–2031. [Google Scholar] [CrossRef] [PubMed]
- Akaberi1, M.; Hosseinzadeh, H. Grapes (Vitis vinifera) as a Potential Candidate for the Therapy of the Metabolic Syndrome. Phytother. Res. 2016, 30, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. The Use of Grape Seed Byproducts Rich in Flavonoids to Improve the Antioxidant Potential of Red Wines. Molecules 2016, 21, 1526. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; González-Manzano, S.; Escudero-Gilete, M.L.; Hernanz, D.; Dueñas, M.; González-Paramás, A.M.; Heredia, F.J.; Santos-Buelga, C. Study of zalema grape pomace: Phenolic composition and biological effects in Caenorhabditis elegans. J. Agric. Food Chem. 2013, 61, 5114–5121. [Google Scholar] [CrossRef] [PubMed]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine by-products: Phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different french grape varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, D.W.; Parker, M.; Smith, P.A. Flavonol composition of Australian red and white wines determined by high-performance liquid chromatography. Aust. J. Grape Wine Res. 2008, 14, 153–161. [Google Scholar] [CrossRef]
- Di Lecce, G.; Arranz, S.; Jáuregui, O.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Lamuela-Raventós, R.M. Phenolic profiling of the skin, pulp and seeds of Albariño grapes using hybrid quadrupole time-of-flight and triple-quadrupole mass spectrometry. Food Chem. 2014, 145, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- De Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, C.; Domínguez-Perles, R.; Aires, A.; Teixeira, A.; Rosa, E.; Barros, A.; Saavedra, M.J. Phytochemistry and activity against digestive pathogens of grape (Vitis vinifera L.) stem’s (poly)phenolic extracts. LWT Food Sci. Technol. 2015, 61, 25–32. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Ramprasath, V.R.; Aluko, R.E.; Jones, P.J.H. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr. Rev. 2008, 66, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Mercolini, L.; Saracino, M.A.; Bugamelli, F.; Ferranti, A.; Malaguti, M.; Hrelia, S.; Raggi, M.A. HPLC-F analysis of melatonin and resveratrol isomers in wine using an SPE procedure. J. Sep. Sci. 2008, 31, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Kaga, S.; Zhan, L.; Bagchi, D.; Das, D.K.; Bertelli, A.; Maulik, N. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem. Biophys. 2006, 44, 43–49. [Google Scholar] [CrossRef]
- Bosso, A.; Guaita, M.; Petrozziello, M. Influence of solvents on the composition of condensed tannins in grape pomace seed extracts. Food Chem. 2016, 207, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Catana, F.; Yang, Y.; Roderick, R.; van Breemen, R.B. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 2002, 50, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjostrom, M.; Erikson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Dos Teixeira Santos, C.A.; Páscoa, R.N.M.J.; Lopes, J.A. A review on the application of vibrational spectroscopy in the wine industry: From soil to bottle. Trends Anal. Chem. 2016. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Paredes, C.; Morales, J.; Mayoral, A.M.; Moral, R. A Study of the composting process of winery and distillery wastes using multivariate techniques. Bioresour. Technol. 2009, 100, 4766–4772. [Google Scholar] [CrossRef] [PubMed]
- Moros, J.; Garrigues, S.; de la Guardia, M. Vibrational spectroscopy provides a green tool for multi-component analysis. Trends Anal. Chem. 2010, 29, 578–591. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Martínez-Carballo, E.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Pattern recognition of three Vitis vinifera L. red grapes varieties based on anthocyanin and flavonol profiles, with correlations between their biosynthesis pathways. Food Chem. 2012, 130, 9–19. [Google Scholar] [CrossRef]
- Cozzolino, D. The Role of Visible and Infrared Spectroscopy Combined with Chemometrics to Measure Phenolic Compounds in Grape and Wine Samples. Molecules 2015, 20, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.A.; Suárez-Estrella, F.; Torrecillas, C.; Paredes, C.; Moral, R.; Moreno, J. Use of chemometrics in the chemical and microbiological characterization of composts from agroindustrial wastes. Bioresour. Technol. 2010, 101, 4068–4074. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, C.; Kokkotou, K.; Zoumpoulakis, P.; Zervou, M. NMR metabolite fingerprinting in grape derived products: An overview. Food Res. Int. 2013, 54, 1184–1194. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Tardaguila, J.; Fernández-Novales, J.; Diago, M.P. Support Vector Machine and Artificial Neural Network Models for the Classification of Grapevine Varieties Using a Portable NIR Spectrophotometer. PLoS ONE 2015, 10, e0143197. [Google Scholar] [CrossRef] [PubMed]
- Páscoa, R.N.; Machado, S.; Magalhães, L.M.; Lopes, J.A. Value adding to red grape pomace exploiting eco-friendly FT-NIR spectroscopy technique. Food Bioprocess Technol. 2015, 8, 865–874. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Determination of phenolic compounds of grape skins during ripening by NIR spectroscopy. LWT Food Sci. Technol. 2011, 44, 847–853. [Google Scholar] [CrossRef]
- Torchio, F.; Río-Segade, S.; Giacosa, S.; Gerbi, V.; Rolle, L. Effect of Growing Zone and Vintage on the Prediction of Extractable Flavanols in Winegrape Seeds by a FT-NIR Method. J. Agric. Food Chem. 2013, 61, 9076–9088. [Google Scholar] [CrossRef] [PubMed]
- Rolle, L.; Torchio, F.; Lorrain, B.; Giacosa, S.; Río Segade, S.; Cagnasso, F.; Gerbi, V.; Teissedre, P.L. Rapid methods for the evaluation of total phenol content and extractability in intact grape seeds of Cabernet-Sauvignon: Instrumental mechanical properties and FT-NIR spectrum. J. Int. Sci. Vigne Vin 2012, 46, 29–40. [Google Scholar] [CrossRef]
- Fotakis, C.; Christodouleas, D.; Kokkotou, K.; Zervou, M.; Zoumpoulakis, P.; Moulos, P.; Liouni, M.; Calokerinos, A. NMR metabolite profiling of Greek grape marc spirits. Food Chem. 2013, 138, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Serradilla, J.A.; de Luque Castro, M.D. Microwave-assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar] [CrossRef]
- Augustine, S.; Kudachikar, V.B.; Vanajakshi, V.; Ravi, R. Effect of combined preservation techniques on the stability and microbial quality and retention of anthocyanins in grape pomace stored at low temperatura. J. Food Sci. Technol. 2013, 50, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Litaor, M.I.; Meir-Dinar, N.; Castro, B.; Azaizeh, H.; Rytwo, G.; Levi, N.; Levi, M.; MarChaim, U. Treatment of winery wastewater with aerated cells mobile system Environmental. Nanotechnol. Monit. Manag. 2015, 4, 17–26. [Google Scholar]
- Conde, E.; Moure, A.; Domínguez, H.; Parajó, J.C. Production of antioxidants by non-isothermal autohydrolysis of lignocellulosic wastes. LWT Food Sci. Technol. 2011, 44, 436–442. [Google Scholar] [CrossRef]
- Huynh, N.T.; Van Camp, J.; Smagghe, G.; Raes, K. Improved Release and Metabolism of Flavonoids by Steered Fermentation Processes: A Review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, B.; Zepf, F.; Bai, Z.; Gao, B.; Zhu, N. A biotech-systematic approach to select fungi for bioconversion of winery biomass wastes to nutrient-rich feed. Process Saf. Environ. Prot. 2016, 103, 60–68. [Google Scholar] [CrossRef]
- Guzzo, F.; Cappello, M.S.; Azzolini, M.; Tosi, E.; Zapparoli, G. The inhibitory effects of wine phenolics on lysozyme activity against lactic acid bacteria. Int. J. Food Microbiol. 2011, 148, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Viveros, A.; Alvarez, I.; Vega, E.; Brenes, A. Changes in polyphenol and polysaccharide content of grape seed extract and grape pomace after enzymatic treatment. Food Chem. 2012, 133, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, J.; Martínez-Cordeiro, H.; Alvarez-Casas, M.; Lores, M. Vermicomposting grape marc yields high quality organic biofertiliser and bioactive polyphenols. Waste Manag. Res. 2014, 32, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.P.; Perin, E.H.; Bulsing Schott, I.; da Silva Rodrigues, R.; Lucchetta, L.; Manfroi, V.; Rombaldi, C.V. The effect of postharvest application of UV-C radiation on the phenolic compounds of conventional and organic grapes (Vitis labrusca cv. ‘Concord’). Postharvest Biol. Technol. 2016, 120, 84–91. [Google Scholar] [CrossRef]
- González-Barrio, R.; Beltrán, D.; Cantos, E.; Gil, M.I.; Espín, J.C.; Tomás-Barberán, F. Comparison of Ozone and UV-C Treatments on the Postharvest Stilbenoid Monomer, Dimer, and Trimer Induction in Var. ‘Superior’ White Table Grapes. J. Agric. Food Chem. 2006, 54, 4222–4228. [Google Scholar] [CrossRef] [PubMed]
- Ibarz, R.; Garvín, A.; Azuara, E.; Ibarz, A. Modelling of ochratoxin A photo-degradation by a UV multi-wavelength emitting lamp. LWT Food Sci. Technol. 2015, 61, 385–392. [Google Scholar] [CrossRef]
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Balasa, A.; Toepfl, S.; Knorr, D. Pulsed electric field treatment of grapes. In Proceedings of the Food Factory of the Future 3, Gothenburg, Sweden, 7–9 June 2006.
- Boussetta, N.; Lebovka, N.; Vorobiev, E.; Adenier, H.; Bedel-Cloutour, C.; Lanoisellé, J.L. Electrically assistedextraction of soluble matter from chardonnay grape skins forpolyphenol recovery. J. Agric. Food Chem. 2009, 57, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Boussetta, N.; Vorobiev, E.; Le, L.H.; Cordin-Falcimaigne, A.; Lanoisellé, J.L. Application of electrical treatments inalcoholic solvent for polyphenols extraction from grape seeds. LWT Food Sci. Technol. 2012, 46, 127–134. [Google Scholar] [CrossRef]
- Alov, P.; Tsakovska, I.; Pajeva, I. Computational Studies of Free Radical-Scavenging Properties of Phenolic Compounds. Curr. Top. Med. Chem. 2015, 15, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In Vitro and in vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis vinifera) Pomace. Molecules 2014, 19, 21154–21167. [Google Scholar] [CrossRef] [PubMed]
- Dröse, S.; Brandt, U. Molecular Mechanisms of Superoxide Production by the Mitochondrial Respiratory Chain. In Mitochondrial Oxidative Phosphorylation; Kadenbach, B., Ed.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2012; pp. 145–169. [Google Scholar]
- Celik, H.; Arinç, E. Evaluation of the protective effects of quercetin, rutin, naringenin, resveratrol, and trolox against idarubicin-induced DNA damage. J. Pharm. Pharmacol. Sci. 2010, 13, 231–241. [Google Scholar] [CrossRef]
- Sies, H. Role of Metabolic H2O2 Generation: Redox Signaling and Oxidative Stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Antioxidant Properties of Phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. The Wanderings of a Free Radical. Free Radic. Biol. Med. 2009, 46, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Denisov, E.; Tumanov, E.V. Estimation of the bond dissociation energies from the kinetic characteristics of liquid-phase radical reactions. Russ. Chem. Rev. 2015, 74, 825–858. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Photoprotection by dietary carotenoids: Concept, mechanisms, evidence and future development. Mol. Nutr. Food Res. 2012, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Pick, A.; Muller, H.; Mayer, R.; Haenisch, B.; Pajeva, I.K.; Weigt, M.; Bönisch, H.; Müller, C.E.; Wiese, M. Structure-activity Relationships of Flavonoids as Inhibitors of Breast Cancer Resistance Protein (BCRP). Bioorg. Med. Chem. 2011, 19, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Mladěnka, P.; Zatloukalová, L.; Filipský, T.; Hrdina, R. Cardiovascular Effects of Flavonoids Are Not Caused Only by Direct Antioxidant Activity. Free Radic. Biol. Med. 2010, 49, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Haasio, K. Toxicology and Safety of COMT Inhibitors. In International Review of Neurobiology; Nissinen, E., Ed.; Basic Aspects of Catechol-O-Methyltransferase and the Clinical Applications of its Inhibitors; Academic Press: Cambridge, MA, USA, 2010; Volume 95, pp. 163–189. [Google Scholar]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Bair, L.; Dinkova-Kostova, A.T. The Cytoprotective Role of the Keap1-Nrf2 Pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P.E. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015, 183, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Gilli, G.; Gilli, P. The Nature of the hydrogen Bond, Outline of a Comrehensive Hydrogen Bond Theory; Clegg, W., Ed.; IUCr Monographs on Chritallography, 23; Oxford Science Publication: Oxford, UK, 2010. [Google Scholar]
- Dias, A.B.; Muller, C.M.O.; Larotonda, J.B. Mechanical and barrier properties of composite films ased on rice flour and cellulose fibers. Food Sci. Technol. 2011, 44, 535–542. [Google Scholar]
- Price, W.N.; Chen, Y.; Handelman, S.K.; Neely, H.; Manor, P.; Karlin, R.; Nair, R.; Liu, J.; Baran, M.; Everett, J.; et al. Understanding the physical properties that control protein crystallization by analysis of large-scale experimental data. Nat. Biotechnol. 2009, 27, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.F.V.; Melo, R.P.; Araujo, R.S.; Lunz, J.N.; Aguiar, V.O. Improvement of mechanical properties of natural fiber-polypropylene composites using successive alkaline treatments. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Rostkowska, H.; Nowak, M.J.; Lapinski, L.; Adamowicz, L. IR spectral and theoretical characterization of intramolecular hydrogen bonds closing five-membered rings. Phys. Chem. Chem. Phys. 2001, 3, 3012–3021. [Google Scholar] [CrossRef]
- Machado, N.F.L.; Batista de Carvalho, L.A.E.; Otero, J.C.; Marques, M.P.M. A conformational study of hydroxylated isoflavones by vibrational spectroscopy coupled with DFT calculations. Vib. Spectrosc. 2013, 68, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Machado, N.F.L.; Valero, R.; Domingos, H.S.; Tomkinson, J.; de Batista Carvalho, L.A.E.; Otero, J.C.; Marques, M.P.M. Conformational behavior of antioxidant chromones. A vibrational spectroscopy study. Vib. Spectrosc. 2012, 63, 325–337. [Google Scholar] [CrossRef]
- Pratt, D.A.; DiLabio, G.A.; Valgimigli, L.; Pedulli, G.F.; Ingold, K.U. Substituent Effects on the Bond Dissociation Enthalpies of Aromatic Amines. J. Am. Chem. Soc. 2002, 124, 11085–11092. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Modulation of the antioxidant activity of phenols by non-covalent interactions. Org. Biomol. Chem. 2012, 10, 4147–4158. [Google Scholar] [CrossRef] [PubMed]
- Musialik, M.; Kuzmicz, R.; Pawlowski, T.S.; Litwinienko, G. Acidity of Hydroxyl Groups: An Overlooked Influence on Antiradical properties of Flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability, and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Bugianesi, R.; Maiani, G.; Valtuena, S.; De Santis, S.; Crozier, A. Plasma antioxidants from chocolate. Nature 2003, 424, 1013. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Ingold, K.U.; Lusztyk, J. Antioxidant activities of vitamin E analogues in water and a Kalmet-T a Kamlet–Taft β-Value for Water. J. Am. Chem. Soc. 1996, 118, 3545–3549. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Mitroka, S.; Zimmeck, S.; Troya, D.; Tanko, J.M. How solvent modulates hydroxyl radical reactivity in hydrogen atom abstractions. J. Am. Chem. Soc. 2010, 132, 2907. [Google Scholar] [CrossRef] [PubMed]
- Tropsha, A. Best Practices for QSAR Model Development, Validation, and Exploitation. Mol. Inform. 2010, 29, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, S. 3D-QSAR—Applications, Recent Advances, and Limitations: An interdisciplinary overview on recent advances in Quantitative Structure-Activity Relationships (QSAR) studies. In Recent Advances in QSAR; Puzyn, T., Leszczynsky, J., Cronin, M., Eds.; IUCr Monographs on Chritallography, 23; Springer: Jackson, USA, 2010; pp. 103–125. [Google Scholar]
- Todeschini, R.; Consonni, V. Handbook of Molecular Descriptors; Methods and Principles in Medicinal Chemistry; Wiley-VCH Verlag GmbH: Dresden, Germany, 2000; Volume 11. [Google Scholar]
- Lapointe, S.M.; Weaver, D.F. A Review of Density Functional Theory Quantum Mechanics as Applied to Pharmaceutically Relevant Systems. Curr. Comput. Aided Drug Des. 2007, 3, 290–296. [Google Scholar] [CrossRef]
- Voorhis, T.; Scuseria, G.E. A novel form for the exchange-correlation energy functional. J. Chem. Phys. 1998, 109, 400–410. [Google Scholar] [CrossRef]
- Merrick, J.P.; Moran, D.; Radom, L. An evaluation of harmonic vibrational frequency scale factors. J. Phys. Chem. 2007, 111, 11683–11700. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, L.; Pons, Z.; Margalef, M.; Arola-Arnal, A.; Muguerza, B. Regulation of vascular endothelial genes by dietary flavonoids: Structure-expression relationship studies and the role of the transcription factor KLF-2. J. Nutr. Biochem. 2015, 26, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Amić, D.; Davidović-Amić, D.; Beslo, D.; Rastija, V.; Lucić, B.; Trinajstić, N. SAR and QSAR of the Antioxidant Activity of Flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Mitra, I. Advances in Quantitative Structure-activity Relationship Models of Antioxidants. Expert Opin. Drug Discov. 2009, 4, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Abdullah, A.H.; Mohamad, M.M.; Qureshi, K.N.; Hussain, K. Adaptive Interface Reconfiguration in Low-Rate Mesh WPANs. J. Comput. Theor. Nanosci. 2016, 13, 4703–4710. [Google Scholar] [CrossRef]
- Guzmán, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2015, 19, 19292–19349. [Google Scholar] [CrossRef] [PubMed]
- Marques, V.; Farah, A. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chem. 2012, 113, 1370–1376. [Google Scholar] [CrossRef]
- Taofiq, O.; calhelha, R.C.; Heleno, S.; Barros, L.; Martins, A.; Santos-Buelga, C.; Qeiroz, M.J.; Ferreira, I.C.F.R. The contribution of phenolic acids to the anti-inflammatory activity of mushrooms: Screening in phenolic extracts, individual parent molecules and synthesized glucuronated and methylated derivatives. Food Res. Int. 2015, 76, 821–827. [Google Scholar] [CrossRef] [Green Version]
- Mudnic, I.; Modun, D.; Rastija, V.; BVrizic, I.; Katalinic, V.; Kozina, B.; Medic-Saric, M.; Boban, M. Antioxidative and vasodilatory effects of phenolic acids in wine. Food Chem. 2010, 119, 1205–1210. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A Review. Food Chem. 2012, 130, 797–830. [Google Scholar]
- Kasiotis, K.M.; Pratsinis, H.; Kletsas, D.; Haroutounian, S.A. Resveratrol and related stilbenes: Their antiaging and atiangiogenic properties. Food Chem. Toxicol. 2013, 61, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Etienne-Selloum, N.; Brasse, D.; Khallouf, H.; Bronner, C.; Rio, M.C.; Beretz, A.; Schini-Kerth, V.B. Intake of grape-derived polyphenols reduces C26 tumor growth by inhibiting angiogenesis and inducing apoptosis. FASEB J. 2010, 24, 3360–3369. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.C.; Zhao, X.; Tang, X.Y.; Yang, F. Design, Synthesis and Biological Study of Pinacolylboronate-substituted Stilbenes as Novel Lipogenic Inhibitors. Biorg. Med. Chem. Lett. 2011, 21, 5638–5641. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Chen, S.A.; Wu, S.N. Evidence for the stimulatory effect of resveratrol on Ca(2+)-activated K+ current in vascular endothelial cells. Cardiovasc. Res. 2000, 45, 1035–1045. [Google Scholar] [CrossRef]
- Orallo, F.; Álvarez, E.; Camiña, M.; Leiro, J.M.; Gómez, E.; Fernández, P. The possible implication of trans-resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol. Pharmacol. 2002, 61, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.A.; Arango, M.; Abderrahmane, S.; Lambert, E.; Tourette, C.; Catoire, H.; Néri, C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat. Genet. 2005, 37, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G. A review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: Part 1. Altern. Med.Rev. 2010, 15, 245–263. [Google Scholar] [PubMed]
- Chung, J.H.; Manganiello, V.; Dyck, J.R. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends Cell Biol. 2012, 22, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Frojdo, S.; Durand, C.; Molin, L.; Carey, A.L.; El-Osta, A.; Kingwell, B.A.; Febbraio, M.A.; Solari, F.; Vidal, H.; Pirola, L. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol. Cell. Endocrinol. 2011, 335, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.K.; Tucker, G.A.; Brameld, J.M. Physiological concentrations of dietary polyphenols regulate vascular endothelial cell expression of genes important in cardiovascular health. Br. J. Nutr. 2010, 103, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Kaga, S.; Zhan, L.; Matsumoto, M.; Maulik, N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J. Mol. Cell. Cardiol. 2005, 39, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Klingue, C.M.; Blankenship, K.A.; Risinger, K.E.; Bhatnagar, S.; Noisin, E.L.; sumanasekera, W.K.; Zhao, L.; Brey, D.M.; Keyton, R.S. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receeptors alpha and beta in endothelial cells. J. Biol. Chem. 2005, 580, 7460–7468. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Jannin, B.; Latruffe, N. Resveratrol: Preventing properties against vascular alterations and ageing. Mol. Nutr. Food Res. 2005, 49, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liang, J.L.; Kang, L.Z.; Huang, X.Y.; Huang, J.J.; Ye, Z.W.; Guo, L.Q.; Lin, J.F. Increased resveratrol production in wines using engineered wine strains Saccharomyces cerevisiae EC1118 and relaxed antibiotic or auxotrophic selection. Biotechnol. Prog. 2015, 31, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, M.; Kumar, R.; Ahmad, N. Resveratrol in cancer management: Where are we and where we go from here? Ann. N. Y. Acad. Sci. 2011, 1215, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, L.; Cheng, Y.; Jiang, J.; Chen, Y.; Jiang, H.; Yu, H.; Shan, A.; Cheng, B. Resveratrol, a Natural Antioxidant, Has a Protective Effect on Liver Injury Induced by Inorganic Arsenic Exposure. Biomed. Res. Int. 2014, 2014, 617202. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Justo, M.; Claro, C.; Vila, E.; Parrado, J.; Herrera, M.; Alvarez de Sotomayor, M. Endothelium-dependent vasodilator and antioxidant properties of a novel enzymatic extract of grape pomace from wine industrial waste. Food Chem. 2012, 135, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Oblak, M.; Randic, M.; Solmajer, T. Quantitative structure-activity relationship of flavonoids analogues. 3. Inhibition of p56lck protein tyrosine kinase. J. Chem. Inf. Model. 2000, 40, 994–1001. [Google Scholar]
- Banner, D.W.; D’Arcy, A.; Chene, C.; Winkler, F.K.; Guha, A.; Konigsberg, W.H. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature 1996, 380, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.W.; Kou, J.P.; Zhang, Z.; Yu, B.Y. The effects of twelve representative flavonoids on tissue factor expression in human monocytes: Structure-activity relationships. Thromb. Res. 2009, 124, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure-activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Zabotin, A.I.; Barisheva, T.S.; Larskaya, I.A.; Toroshina, T.E.; Trofimova, O.V.; Hahn, M.G.; Zabotina, O.A. Oligosaccharin: A new systemic factor in the acquisition of freeze tolerance in winter plants. Plant Biosyst. 2005, 139, 36–41. [Google Scholar] [CrossRef]
- Křen, V. Glycoside vs. aglycon: The role of glycosidic residue in biological activity. In Glycoscience: Chemistry and Chemical Biology, 2nd ed.; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2008; pp. 2589–2644. [Google Scholar]
- Amico, V.; Napoli, E.M.; Renda, A.; Ruberto, G.; Spatafora, C.; Tringali, C. Constituents of grape pomace from the Sicilian cultivar “Nerello Mascalese”. Food Chem. 2004, 88, 599–607. [Google Scholar] [CrossRef]
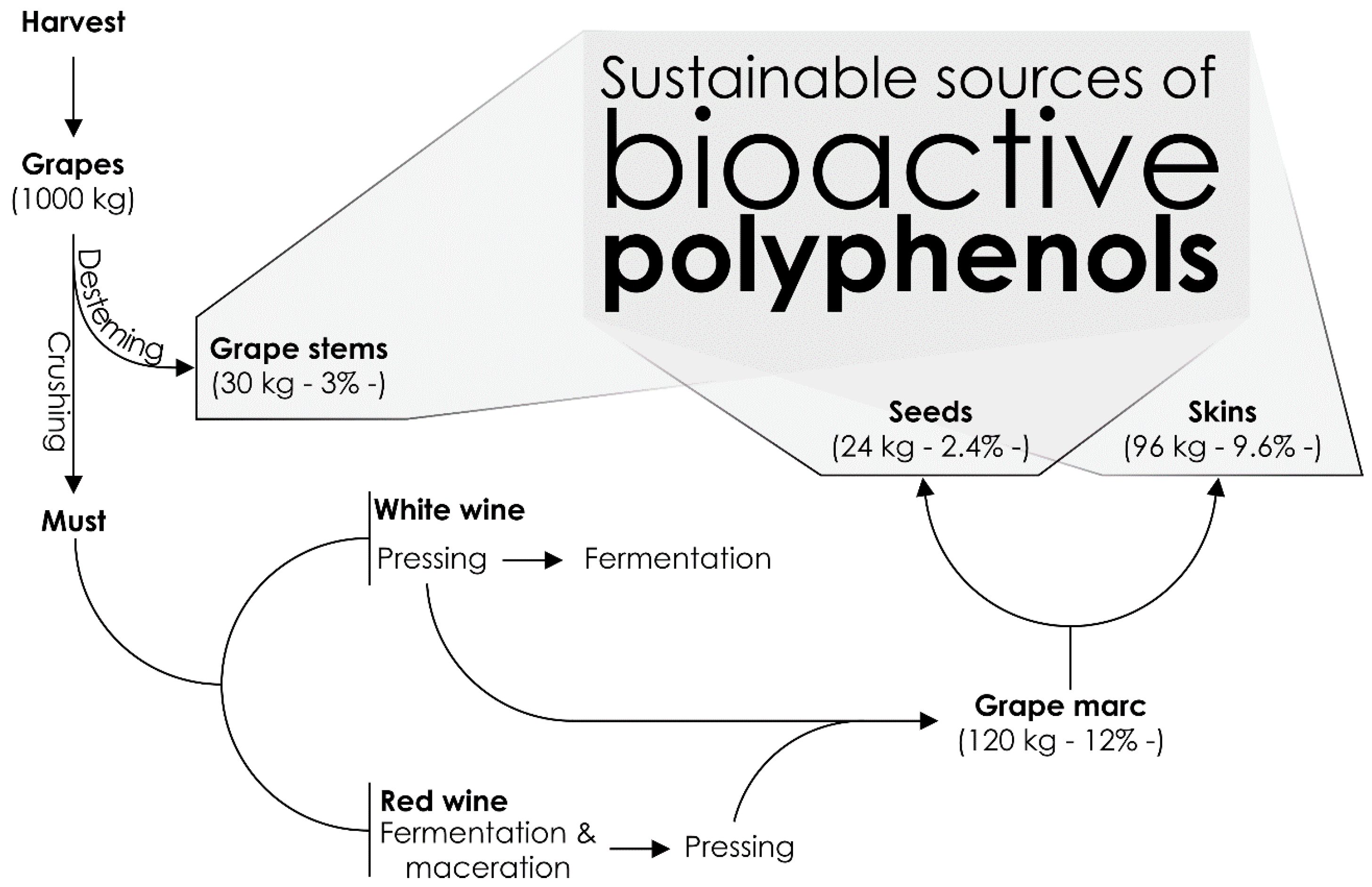
| Compound | Concentration (microg·g−1 dw) | Analytical Approach Z | Reference | |||
|---|---|---|---|---|---|---|
| Skins | Seeds | Stems | Marc | |||
| Hydroxybenzoic acids | ||||||
| Gallic acid | - | - | - | 82.00–99.00 | LC-MS/MS | [29] |
| 2.20–82.00 | 6.50–224.00 | 70.40–469.00 | 10.50–459.00 | HPLC-DAD | [2,27,30,31,32,33,34,35,36,37,38] | |
| 0.38–0.74 | - | - | - | HPLC-DAD-ESi-MSn | [24,39] | |
| Gentisic acid | 1.10–1.61 | - | - | - | HPLC-DAD-ESi-MSn | [39] |
| Hydroxybenzoic acid | 0.02–0.43 | - | - | HPLC-DAD-ESi-MSn | [39] | |
| Protocatechuic acid | - | - | - | 4.10–5.00 | LC-MS/MS | [29] |
| 6.00–13.00 | 2.00–10.00 | 66.00–115.00 | 0.50–4.20 | HPLC-DAD | [27,38] | |
| 0.08–0.13 | - | - | - | HPLC-DAD-ESi-MSn | [24,39] | |
| Syringic acid | 1.60–3.30 | - | - | - | HPLC-DAD | [31,35] |
| Vanillic acid | 3.00–7.10 | - | - | 8.40–154.20 | HPLC-DAD | [27,31,35,37] |
| Hydroxycinnamic acids | ||||||
| Caffeic acid | 1.00–21.00 | 2.00–9.00 | 5.00–40.00 | - | HPLC-DAD | [38] |
| 0.15–2.72 | - | - | - | HPLC-DAD-ESi-MSn | [39,40] | |
| Caftaric acid | - | - | - | 21.00–33.00 | LC-MS/MS | [29] |
| 0.60–24.00 | 2.00–11.00 | 15.10–274.00 | 2.60–277.20 | HPLC-DAD | [27,31,36,38,41] | |
| - | 11.80–40.40 | 8.20–70.60 | 0.20–1.60 | HPLC-DAD-ESi-MSn | [24,42] | |
| Chlorogenic acid | 0.29–0.32 | - | - | - | HPLC-DAD-ESi-MSn | [40] |
| 40.50–231.10 | 28.70–68.00 | - | - | HPLC-DAD | [43] | |
| trans-Coutaric acid | 0.10–5.50 | 0.40–7.50 | 6.90–20.70 | 6.10–63.40 | HPLC-DAD | [36,38,44] |
| Fertaric acid | - | - | - | 4.80–5.90 | HPLC-DAD | [36] |
| Ferulic acid | - | - | - | 6.70–27.80 | HPLC-DAD | [37] |
| 0.23–3.65 | - | - | - | HPLC-DAD-ESi-MSn | [39] | |
| Compound | Concentration (microg·g−1 dw) | Analytical Approach Z | Ref. | |||
|---|---|---|---|---|---|---|
| Skins | Seeds | Stems | Marc | |||
| Flavanols | ||||||
| Catechin | - | - | - | 1986.00–2124.00 | LC-MS/MS | [29] |
| 0.50–1300.00 | 158.00–4540.00 | 60.00–1858.00 | 87.70–2635.40 | HPLC-DAD | [27,30,31,32,34,35,36,38,40,41,43,55,56,57,65,66] | |
| - | - | - | 19.60–89.70 | HPLC-DAD-ESi-MSn | [24,33,44,57] | |
| Epicatechin | - | - | - | 849.00–1030.00 | LC-MS/MS | [27,29,35] |
| 2.00–850.00 | 100.00–2700.00 | 5.00–189.00 | 57.10–864.70 | HPLC-DAD | [30,31,32,34,36,38,41,55,56,57,58,65,66,67] | |
| - | - | 2200.90–3181.50 | 17.30–112.80 | HPLC-DAD-ESi-MSn | [24,33,42,44] | |
| Hydrolyzable tannins | ||||||
| Epicatechin-gallate | - | - | - | 91.00–119.00 | LC-MS/MS | [29] |
| - | 1.40–489.00 | 34.20–130.00 | 24.40–59.20 | HPLC-DAD | [27,30,34,41,65,66] | |
| - | - | - | 10.50–45.60 | HPLC-DAD-ESi-MSn | [44] | |
| Epigallocatechin | 0.37–0.42 | - | - | - | HPLC-DAD-ESi-MSn | [39] |
| - | ≤129.00 | - | - | [30] | ||
| Epigallocatechin gallate | 0.39–0.49 | - | - | - | HPLC-DAD-ESi-MSn | [39] |
| - | 0.50–156.00 | - | - | HPLC-DAD | [30] | |
| Gallocatechin gallate | 1.69–2.04 | - | - | - | HPLC-DAD-ESi-MSn | [39] |
| Condensed tannins | ||||||
| Procyanidin B1 | - | - | - | 467.00–556.00 | LC-MS/MS | [29] |
| 27.00–480.00 | 29.20–1020.00 | 133.00–1958.00 | 10.60–1346.1 | HPLC-DAD | [27,30,31,34,36,38,55,56,65,66] | |
| - | - | 228.60–761.20 | - | HPLC-DAD-ESi-MSn | [42] | |
| Procyanidin B2 | - | - | - | 384.00–444.00 | LC-MS/MS | [29] |
| 3.00–650.00 | 0.80–910.70 | 11.00–103.00 | 47.00–244.10 | HPLC-DAD | [27,30,31,34,38,41,55,56,57,65,68] | |
| - | 322.00–667.00 | - | - | HPLC-DAD-ESi-MSn | [24] | |
| Procyanidin B2-3-O-gallate | - | ≤738.50 | - | - | HPLC-DAD | [65] |
| Procyanidin B3 | 0.60–350.00 | 26.00–315.00 | 20.00–993.00 | 9.20–342.00 | HPLC-DAD | [27,31,34,36,38,41,55,56,57,65,66] |
| Procyanidin B4 | 2.00–300.00 | 34.00–310.00 | 30.00–139.00 | 17.00–515.20 | HPLC-DAD | [31,34,37,38,55,65,66] |
| Procyanidin B5 | - | 70.00–356.70 | - | - | HPLC-DAD | [34] |
| Procyanidin B7 | - | - | - | ≤90.00 | HPLC-DAD | [36] |
| Procyanidin B1-gallate | 310.00–350.00 | 660.00–820.00 | ≤40.00 | - | HPLC-DAD | [69] |
| Procyanidin B2-gallate | 23.00–230.00 | 34.50–505.00 | 20.00–336.00 | 135.20–1372.90 | HPLC-DAD | [31,36,38,57,66] |
| Procyanidin B4-gallate | - | 9.20–20.80 | - | - | HPLC-DAD | [31,36] |
| Procyanidin C1 | 10.00–360.00 | 16.10–600.00 | 46.00–190.00 | 17.90–201.10 | HPLC-DAD | [27,31,34,36,38,57,66] |
| Procyanidin C1-gallate | - | 15.10–31.90 | - | - | HPLC-DAD | [31] |
| Procyanidin C2 | 11.00–81.00 | 152.00–476.00 | 20.00–115.00 | 36.50–449.70 | HPLC-DAD | [36,38,66] |
| Procyanidin D1 | 41.00–320.00 | 45.00–370.00 | 10.00–547.00 | 16.40–852.50 | HPLC-DAD | [36,38,66,69] |
| Procyanidin D2 | 35.00–79.00 | 14.00–137.00 | 37.00–101.00 | 43.60–266.70 | HPLC-DAD | [36,38,66] |
| Compound Z | Concentration (microg·g−1 dw) | Analytical Approach Z | Reference | |||
|---|---|---|---|---|---|---|
| Skins | Seeds | Stems | Marc | |||
| Astilbin | - | - | - | 2.50–7.60 | HPLC-DAD-ESi-MSn | [44] |
| - | - | ≤35.00 | - | HPLC-DAD | [40] | |
| Isorhamnetin | - | - | - | 12.50–20.50 | HPLC-DAD-ESi-MSn | [44] |
| Isoquercetin | - | - | - | 16.00–26.50 | HPLC-DAD-ESi-MSn | [44] |
| I-3-Glc | 1.00–23.10 | - | - | 3.20–63.80 | HPLC-DAD | [31,35,36,66,69] |
| I-3-Fer-Glc | - | - | 6.90–9.10 | HPLC-DAD-ESi-MSn | [42] | |
| I-3-Gluc | 3.50–9.40 | - | - | 1.90–10.60 | HPLC-DAD | [35,36] |
| Kaempferol | 0.20–13.60 | - | 0.60–15.50 | ≤2.37 | HPLC-DAD | [38,69] |
| - | - | 9.80–34.20 | HPLC-DAD-ESi-MSn | [44] | ||
| K-3-Gal | 0.10–28.00 | - | 2.00–15.00 | 4.00–47.40 | HPLC-DAD | [31,35,36] |
| K-3-Glc | 1.90–79.00 | - | 7.00–26.00 | 6.00–15.80 | HPLC-DAD | [38,58,66] |
| - | 1.50–8.00 | 18.40–62.40 | HPLC-DAD-ESi-MSn | [36,42] | ||
| K-3-Gluc | 1.00–19.00 | - | 1.00–14.00 | 2.60–13.10 | HPLC-DAD | [36,38,66] |
| K-3-Rut | - | - | 1.80–12.10 | HPLC-DAD-ESi-MSn | [42] | |
| Laricitrin | - | - | - | 0.10–0.30 | HPLC-DAD-ESi-MSn | [44] |
| L-3-Glc | - | - | - | 2.90–6.40 | HPLC-DAD-ESi-MSn | [44] |
| - | - | - | ≤3.84 | HPLC-DAD | [69] | |
| Myrcetin | - | - | - | 2.20–7.20 | HPLC-DAD-ESi-MSn | [44] |
| M-3-Gal | 1.90–3.90 | - | - | - | HPLC-DAD | [35] |
| M-3-Glc | 2.40–13.80 | - | - | ≤21.30 | HPLC-DAD | [31,35,69] |
| - | - | - | 3.60–11.40 | HPLC-DAD-ESi-MSn | [44] | |
| M-3-Gluc | - | - | - | 0.50–1.80 | HPLC-DAD-ESi-MSn | [44] |
| Quercetin | 4.00–21.00 | 1.00–16.00 | - | 495.00–634.00 | LC-MS/MS | [29,38] |
| - | - | - | ≤15.30 | HPLC-DAD | [69] | |
| - | - | - | 93.00–163.60 | HPLC-DAD-ESi-MSn | [44] | |
| Q-3-Gal | 2.20–22.10 | - | 3.00–20.00 | 12.20–63.80 | HPLC-DAD | [31,35,36,66] |
| Q-3-Glc | - | - | - | 389.00–704.00 | LC-MS/MS | [29] |
| 0.90–200.00 | ≤10.00 | 18.00–170.00 | 26.00–549.70 | HPLC-DAD | [31,35,36,38,40,58,66,69] | |
| - | 958.00–12,744.00 | - | - | HPLC-DAD-ESi-MSn | [24] | |
| Q-3-Gluc | - | - | - | 243.00–424.00 | LC-MS/MS | [1] |
| 66.80–184.20 | 0.20–9.00 | ≤200.00 | 93.50–522.50 | HPLC-DAD | [31,36,38,68,69] | |
| - | 2.60–33.30 | 42.60–141.90 | 31.90–81.40 | HPLC-DAD-ESi-MSn | [24,42,44] | |
| Q-3-Pen | 0.40–12.80 | - | - | 1.80–4.00 | HPLC-DAD | [36,38,66] |
| Q-3-Rut | - | - | - | 27.00–70.00 | LC-MS/MS | [29] |
| 18.00–570.40 | 25.70–90.50 | 14.00–21.00 | 13.00–414.30 | HPLC-DAD | [32,36,38,43,66] | |
| - | 0.40–3.30 | 2.00–9.70 | - | HPLC-DAD-ESi-MSn | [24,42] | |
| Syringetin | - | - | - | 0.40–0.50 | HPLC-DAD-ESi-MSn | [44] |
| S-3-Glc | - | - | - | 4.20–12.00 | HPLC-DAD-ESi-MSn | [44] |
| Compound Z | Concentration (microg·g−1 dw) | Analytical Approach Y | Reference | ||
|---|---|---|---|---|---|
| Skins | Stems | Marc | |||
| D-3-Glc | 39.30–142.10 | - | - | HPLC-DAD | [35] |
| C-3-Glc | 3.40–83.90 | - | - | HPLC-DAD | [35] |
| Mv-3-Glc | 51.70–124.80 | - | - | HPLC-DAD | [35] |
| - | 22.90–80.20 | 55.80–142.20 | HPLC-DAD-ESi-MSn | [43,45] | |
| P-3-Glc | 45.80–220.40 | - | - | HPLC-DAD | [35] |
| - | - | 0.80–1.70 | HPLC-DAD-ESi-MSn | [45] | |
| Pt-3-Glc | 507.60–684.80 | - | - | HPLC-DAD | [35] |
| - | - | 0.10–0.90 | HPLC-DAD-ESi-MSn | [45] | |
| D-3-Ac-Glc | 2.20–15.50 | - | - | HPLC-DAD | [35] |
| C-3-Ac-Glc | 2.20–12.40 | - | - | HPLC-DAD | [35] |
| Mv-3-Ac-Glc | 12.20–29.80 | - | - | HPLC-DAD | [35] |
| - | - | 28.40–195.00 | HPLC-DAD-ESi-MSn | [45] | |
| P-3-Ac-Glc | 9.70–36.20 | - | - | HPLC-DAD | [35] |
| - | - | 0.20–3.30 | HPLC-DAD-ESi-MSn | [45] | |
| Pt-3-Ac-Glc | 156.10–300.70 | - | - | HPLC-DAD | [35] |
| - | - | 0.03–0.90 | HPLC-DAD-ESi-MSn | [45] | |
| C-3-Cou-Glc | 2.10–8.90 | - | - | HPLC-DAD | [35] |
| D-3-Cou-Glc | - | - | 0.30–43.90 | HPLC-DAD-ESi-MSn | [45] |
| Mv-3-Caff-Glc | - | - | 2.60–23.80 | HPLC-DAD-ESi-MSn | [45] |
| Mv-3-Cou-Glc | 0.60–3.30 | - | - | HPLC-DAD | [35] |
| - | - | 67.50–238.90 | HPLC-DAD-ESi-MSn | [45] | |
| P-3-Cou-Glc | 0.70–7.50 | - | - | HPLC-DAD | [35] |
| - | - | 1.60–42.70 | HPLC-DAD-ESi-MSn | [45] | |
| Pt-3-Cou-Glc | 21.90–33.90 | - | - | HPLC-DAD | [35] |
| - | - | 1.40–72.90 | HPLC-DAD-ESi-MSn | [45] | |
| Compound | Concentration (microg·g−1 dw) | Analytical Approach Z | Reference | ||
|---|---|---|---|---|---|
| Skins | Stems | Marc | |||
| trans-Piceid | ≤6400.00 | 74.00–266.00 | - | HPLC-DAD | [40,75] |
| trans-Resveratrol | 90.00–124,100.00 | - | 5.80–64.00 | HPLC-DAD | [32,37,55] |
| - | - | 29.00–53.00 | HPLC-DAD-ESi-MSn | [43] | |
| Σ-Viniferin | - | 167.00–499.00 | - | HPLC-DAD | [40] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, N.F.L.; Domínguez-Perles, R. Addressing Facts and Gaps in the Phenolics Chemistry of Winery By-Products. Molecules 2017, 22, 286. https://doi.org/10.3390/molecules22020286
Machado NFL, Domínguez-Perles R. Addressing Facts and Gaps in the Phenolics Chemistry of Winery By-Products. Molecules. 2017; 22(2):286. https://doi.org/10.3390/molecules22020286
Chicago/Turabian StyleMachado, Nelson F. L., and Raúl Domínguez-Perles. 2017. "Addressing Facts and Gaps in the Phenolics Chemistry of Winery By-Products" Molecules 22, no. 2: 286. https://doi.org/10.3390/molecules22020286
APA StyleMachado, N. F. L., & Domínguez-Perles, R. (2017). Addressing Facts and Gaps in the Phenolics Chemistry of Winery By-Products. Molecules, 22(2), 286. https://doi.org/10.3390/molecules22020286





