Isolation and Structure Identification of Novel Brominated Diketopiperazines from Nocardia ignorata—A Lichen-Associated Actinobacterium
Abstract
:1. Introduction
2. Results and Discussion
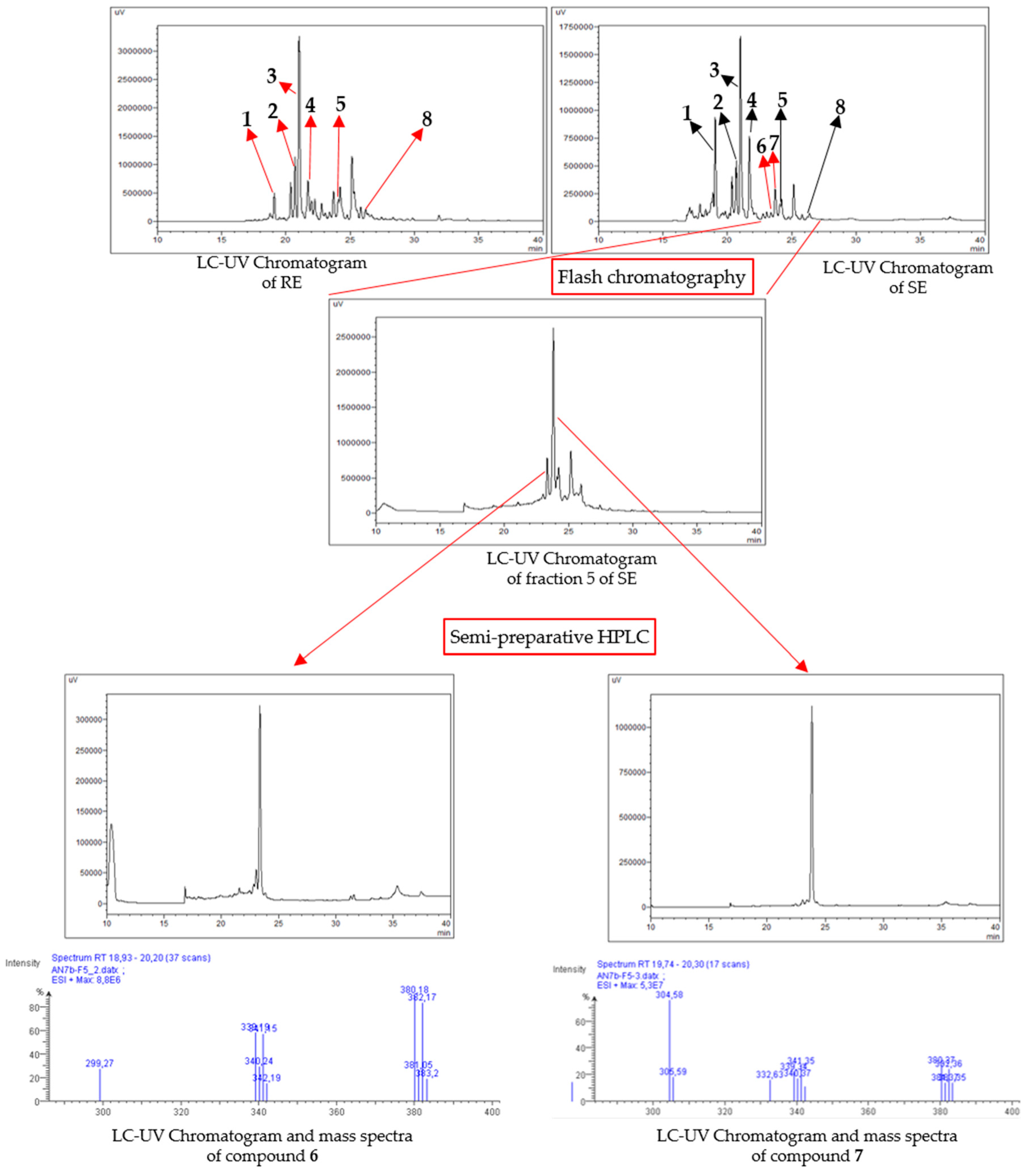
2.1. Chemical Profiling of Extracts and Purification
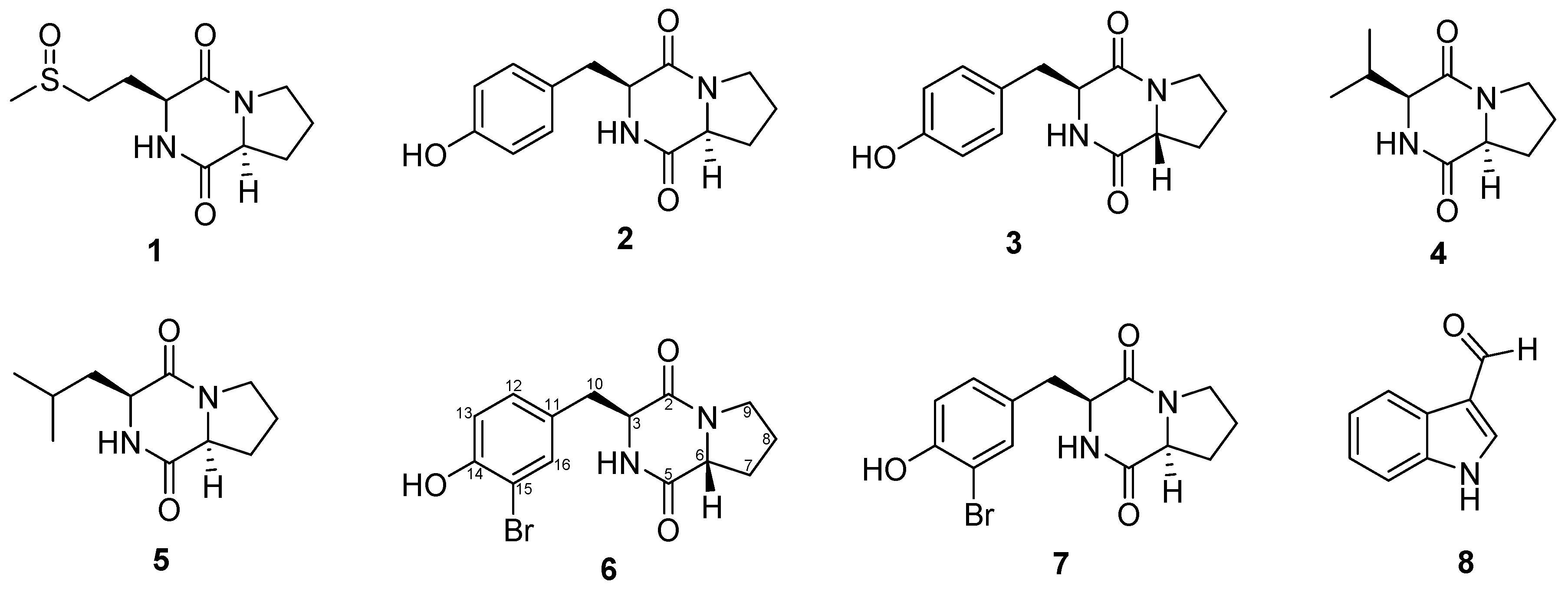
2.2. Structural Determination of Diketopiperazines 6 and 7
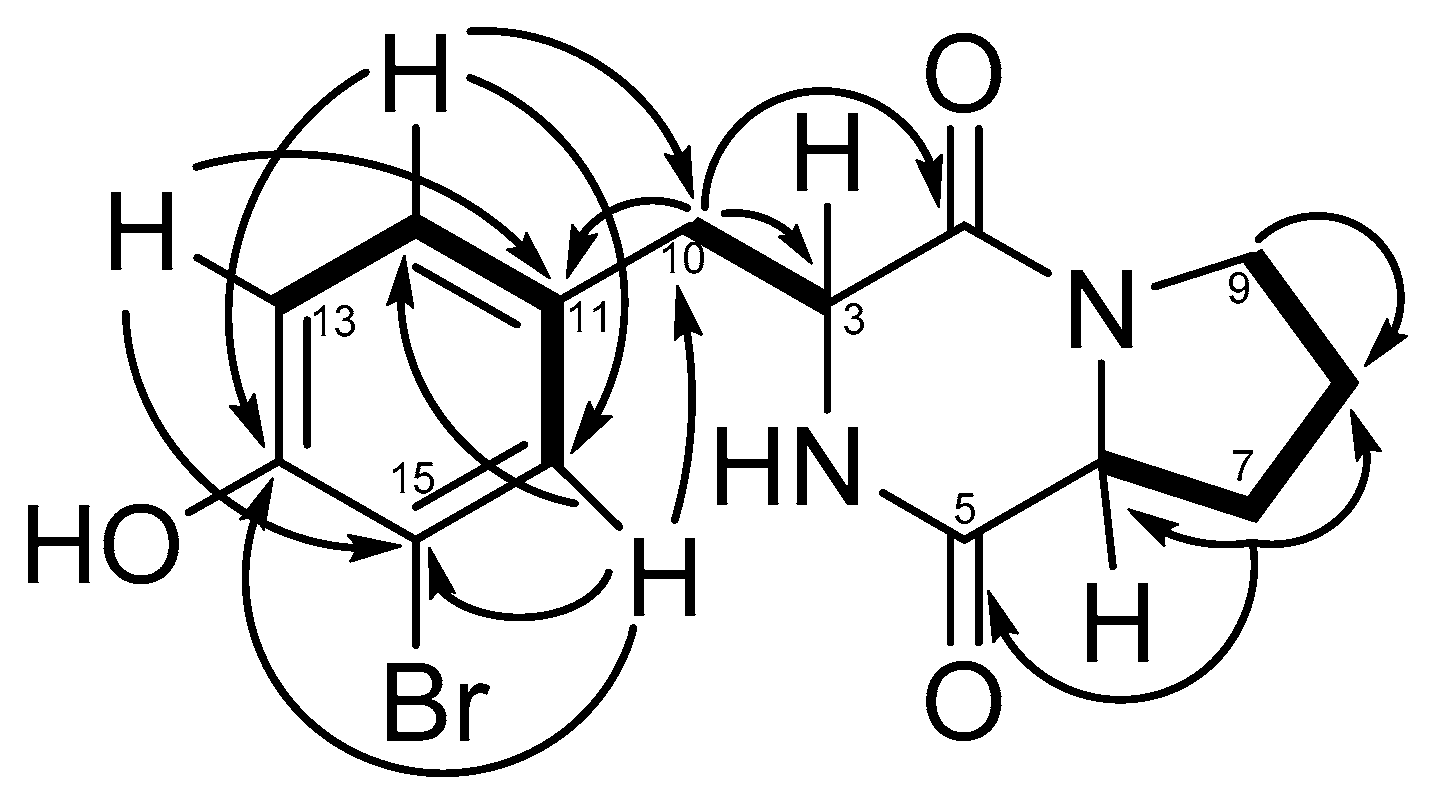
2.2.1. Structural Analysis
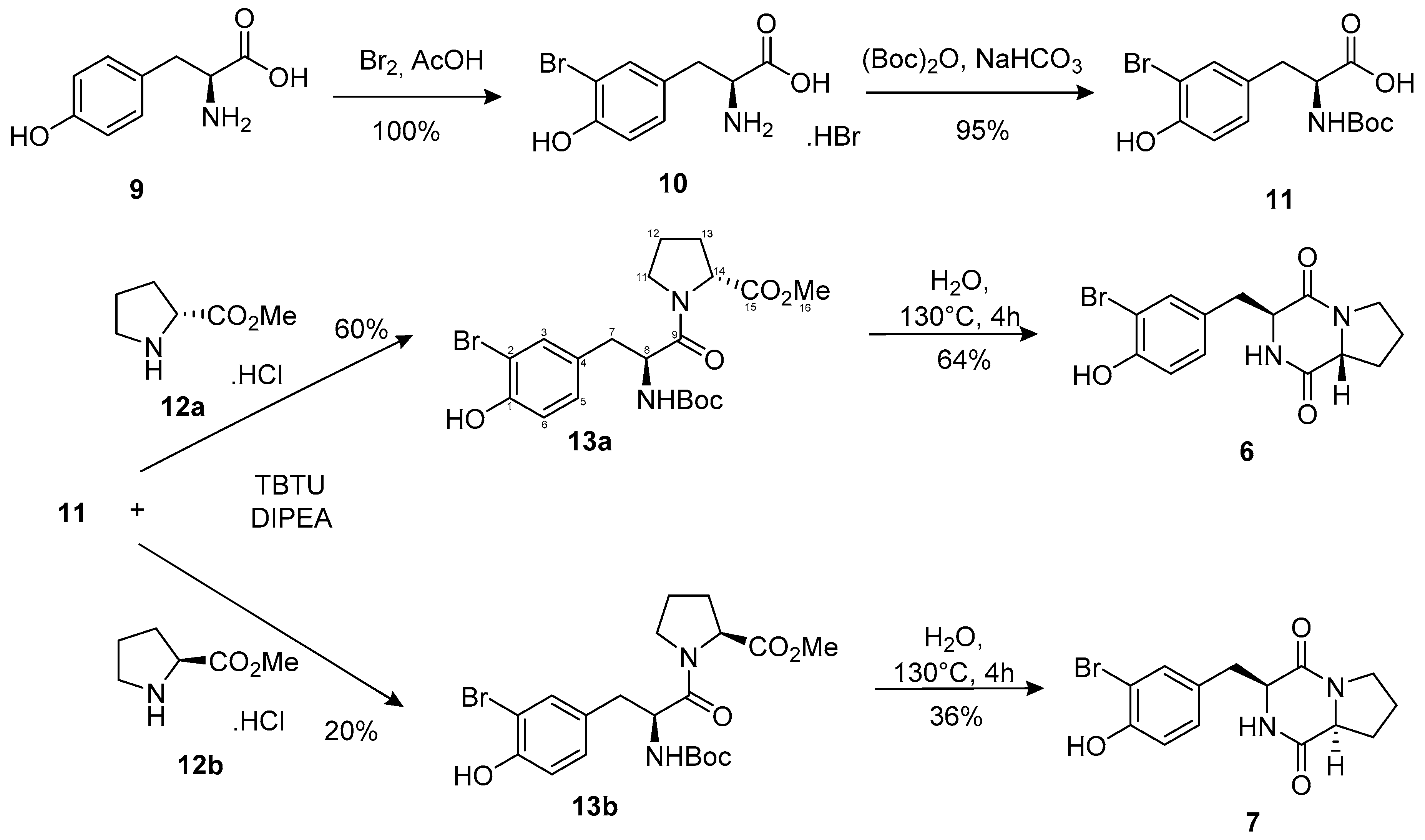
2.2.2. Synthesis
2.3. Cytotoxic Activity
3. Materials and Methods
3.1. General Experimental Procedures
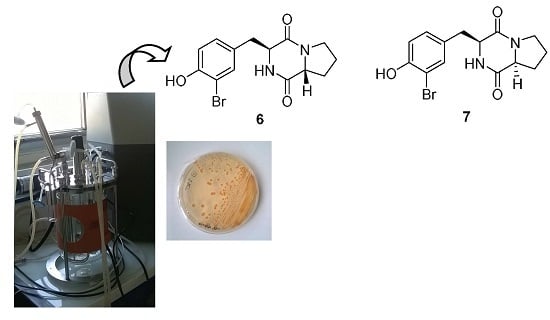
3.2. Microorganism
3.3. Fermentation of Nocardia ignorata DP94
3.4. Mass Spectrometry Analysis
3.5. Extraction and Isolation
3.6. Biological Assays: Cytotoxic Activities
3.7. Preparation of l-Bromo-Tyrosine (10)
3.8. Protection of the Amine Group (11)
3.9. Condensation of the Amino Acids
3.10. Deprotection and Cyclization
3.11. Analytical Data
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shukla, V.; Joshi, G.P.; Rawat, M.S.M. Lichens as a Potential Natural Source of Bioactive Compounds: A Review. Phytochem. Rev. 2010, 9, 303–314. [Google Scholar] [CrossRef]
- Parrot, D.; Antony-Babu, S.; Intertaglia, L.; Grube, M.; Tomasi, S.; Suzuki, M.T. Littoral Lichens as a Novel Source of Potentially Bioactive Actinobacteria. Sci. Rep. 2015, 5, 15839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinale, M.; Puglia, A.M.; Grube, M. Molecular Analysis of Lichen-Associated Bacterial Communities. FEMS Microbiol. Ecol. 2006, 57, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, T.; Grube, M.; Hoem, S.; Jorgensen, S.L.; Daae, F.L.; Thorseth, I.H.; Øvreås, L. Microbial Metacommunities in the Lichen-rock Habitat. Environ. Microbiol. Rep. 2011, 3, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.T.; Cropsey, G.W.G.; Caporaso, J.G.; Knight, R.; Fierer, N. Bacterial Communities Associated with the Lichen Symbiosis. Appl. Environ. Microbiol. 2011, 77, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.T.; Parrot, D.; Berg, G.; Grube, M.; Tomasi, S. Lichens as Natural Sources of Biotechnologically Relevant Bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 583–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selbmann, L.; Zucconi, L.; Ruisi, S.; Grube, M.; Cardinale, M.; Onofri, S. Culturable Bacteria Associated with Antarctic Lichens: Affiliation and Psychrotolerance. Polar Biol. 2010, 33, 71–83. [Google Scholar] [CrossRef]
- Grube, M.; Cardinale, M.; de Castro, J.V.; Müller, H.; Berg, G. Species-Specific Structural and Functional Diversity of Bacterial Communities in Lichen Symbioses. ISME J. 2009, 3, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Parrot, D.; Legrave, N.; Delmail, D.; Grube, M.; Suzuki, M.; Tomasi, S. Review—Lichen-Associated Bacteria as a Hot Spot of Chemodiversity: Focus on Uncialamycin, a Promising Compound for Future Medicinal Applications. Planta Med. 2016, 82, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Bilyk, O.; Luzhetskyy, A. Metabolic Engineering of Natural Product Biosynthesis in Actinobacteria. Curr. Opin. Biotechnol. 2016, 42, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Sanchez, S. Microbial Drug Discovery: 80 Years of Progress. J. Antibiot. (Tokyo) 2009, 62, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Q.; Yang, Y.-B.; Zhou, H.; He, G.-W.; Zhao, L.-X.; Xu, L.-H.; Ding, Z.-T. New Megastigmane Glycoside and Alkaloids from Streptomyces Sp. YIM 63342. Nat. Prod. Res. 2013, 27, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Mohandas, C.; Nambisan, B.; Kumar, D.R.S.; Lankalapalli, R.S. Isolation of Proline-Based Cyclic Dipeptides from Bacillus Sp. N Strain Associated with Rhabitid Entomopathogenic Nematode and Its Antimicrobial Properties. World J. Microbiol. Biotechnol. 2012, 29, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.C.; Cardellina, J.H.; Strobel, G.A. Maculosin, a Host-Specific Phytotoxin for Spotted Knapweed from Alternaria Alternata. Proc. Natl. Acad. Sci. USA 1988, 85, 8008–8011. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Luis, S.; Gómez, J.F.; Spadafora, C.; Guzmán, H.M.; Gutiérrez, M. Antitrypanosomal Alkaloids from the Marine Bacterium Bacillus Pumilus. Molecules 2012, 17, 11146–11155. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Rivera, D.; González, O.; Guzmán-Rodríguez, J.; Díaz-Pérez, A.L.; Ochoa-Zarzosa, A.; López-Bucio, J.; Meza-Carmen, V.; Campos-García, J. Cytotoxicity of Cyclodipeptides from Pseudomonas Aeruginosa PAO1 Leads to Apoptosis in Human Cancer Cell Lines. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-S.; Cho, Y.-J.; Lee, K.; Yoon, S.-H.; Kim, M.; Na, H.; Park, S.-C.; Jeon, Y.S.; Lee, J.-H.; Yi, H.; et al. Introducing EzTaxon-E: A Prokaryotic 16S rRNA Gene Sequence Database with Phylotypes That Represent Uncultured Species. Int. J. Syst. Evol. Microbiol. 2012, 65, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Millot, M.; Tomasi, S.; Studzinska, E.; Rouaud, I.; Boustie, J. Cytotoxic Constituents of the Lichen Diploicia Canescens. J. Nat. Prod. 2009, 33, 2177–2180. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.; Mayor, S.; Rodríguez, K.; Lloyd-Williams, P.; Giralt, E. Racemization in Suzuki Couplings: A Quantitative Study Using 4-Hydroxyphenylglycine and Tyrosine Derivatives as Probe Molecules. J. Org. Chem. 2007, 72, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Thajudeen, H.; Park, K.; Moon, S.-S.; Hong, I.S. An Efficient Green Synthesis of Proline-Based Cyclic Dipeptides under Water-Mediated Catalyst-Free Conditions. Tetrahedron Lett. 2010, 51, 1303–1305. [Google Scholar] [CrossRef]
- Campbell, J.; Lin, Q.; Geske, G.D.; Blackwell, H.E. New and Unexpected Insights into the Modulation of LuxR-Type Quorum Sensing by Cyclic Dipeptides. ACS Chem. Biol. 2009, 4, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.G.; Ram Chhabra, S.; De Nys, R.; Stead, P.; Bainton, N.J.; Hill, P.J.; Manefield, M.; Kumar, N.; Labatte, M.; England, D.; et al. Quorum-Sensing Cross Talk: Isolation and Chemical Characterization of Cyclic Dipeptides from Pseudomonas Aeruginosa and Other Gram-Negative Bacteria. Mol. Microbiol. 1999, 33, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, S.; Sivaranjani, M.; Kamaladevi, A.; Ravi, A.V.; Balamurugan, K.; Karutha Pandian, S.; Coenye, T. Cyclic Dipeptide Cyclo(l-Leucyl-l-Prolyl) from Marine Bacillus Amyloliquefaciens Mitigates Biofilm Formation and Virulence in Listeria Monocytogenes. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.-H. Cyclic Dipeptides Exhibit Synergistic, Broad Spectrum Antimicrobial Effects and Have Anti-Mutagenic Properties. Int. J. Antimicrob. Agents 2004, 24, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Yang, C.-Y.; Fang, S.-T.; Lu, J.; Quan, C.-S. Inhibition of Biofilm in Bacillus Amyloliquefaciens Q-426 by Diketopiperazines. World J. Microbiol. Biotechnol. 2016, 32. [Google Scholar] [CrossRef] [PubMed]
- Fdhila, F.; Vázquez, V.; Sánchez, J.L.; Riguera, R. DD-Diketopiperazines: Antibiotics Active against Vibrio Anguillarum Isolated from Marine Bacteria Associated with Cultures of Pecten Maximus. J. Nat. Prod. 2003, 66, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 6, 7, 13a and 13b are available from the authors.
| No. | 6 | 7 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | - | - | - | - |
| 2 | - | 167.3 | - | 166.7 |
| 3 | 4.14, t (4.7) | 59.8 | 4.38, td (4.7, 2.0) | 57.7 |
| 4 | - | - | - | - |
| 5 | - | 171.1 | - | 170.7 |
| 6 | 2.82, m | 59.3 | 4.07, ddd (10.9, 6.3, 2.0) | 60.0 |
| 7 | 1.70, m | 29.9 | 1.25, m | 29.5 |
| 2.12, m | 2.13, m | |||
| 8 | 1.95, m 1.70, m | 22.5 | 1.84, m | 22.6 |
| 9 | 3.36, m | 46.2 | 3.38, m | 45.9 |
| 3.56, m | 3.57, m | |||
| 10 | 2.88, dd (13.9, 4.9) | 39.7 | 2.99, dd (14.2, 4.7) | 37.1 |
| 3.09, dd (13.9, 4.9) | 3.13, dd (14.2, 4.7) | |||
| 11 | - | 128.9 | - | 129.6 |
| 12 | 6.98, dd (8.2, 2.1) | 131.3 | 7.03, dd (8.3, 2.2) | 131.4 |
| 13 | 6.83, d (8.2) | 117.2 | 6.81, d (8.3) | 116.9 |
| 14 | - | 110.8 | - | 110.6 |
| 15 | - | 155.0 | - | 154.5 |
| 16 | 7.29, d (2.1) | 135.5 | 7.35, d (2.2) | 135.4 |
| Compounds | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 13a | 13b | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) | HaCaT | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 79 ± 6 | >200 | 7 ± 2.5 |
| B16 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 72 ± 6 | >200 | 18 ± 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noël, A.; Ferron, S.; Rouaud, I.; Gouault, N.; Hurvois, J.-P.; Tomasi, S. Isolation and Structure Identification of Novel Brominated Diketopiperazines from Nocardia ignorata—A Lichen-Associated Actinobacterium. Molecules 2017, 22, 371. https://doi.org/10.3390/molecules22030371
Noël A, Ferron S, Rouaud I, Gouault N, Hurvois J-P, Tomasi S. Isolation and Structure Identification of Novel Brominated Diketopiperazines from Nocardia ignorata—A Lichen-Associated Actinobacterium. Molecules. 2017; 22(3):371. https://doi.org/10.3390/molecules22030371
Chicago/Turabian StyleNoël, Alba, Solenn Ferron, Isabelle Rouaud, Nicolas Gouault, Jean-Pierre Hurvois, and Sophie Tomasi. 2017. "Isolation and Structure Identification of Novel Brominated Diketopiperazines from Nocardia ignorata—A Lichen-Associated Actinobacterium" Molecules 22, no. 3: 371. https://doi.org/10.3390/molecules22030371
APA StyleNoël, A., Ferron, S., Rouaud, I., Gouault, N., Hurvois, J.-P., & Tomasi, S. (2017). Isolation and Structure Identification of Novel Brominated Diketopiperazines from Nocardia ignorata—A Lichen-Associated Actinobacterium. Molecules, 22(3), 371. https://doi.org/10.3390/molecules22030371