Peripheral and Cerebral Resistance Arteries in the Spontaneously Hypertensive Heart Failure Rat: Effects of Stilbenoid Polyphenols
Abstract
:1. Introduction
2. Results
2.1. Body Weight and Blood Pressure
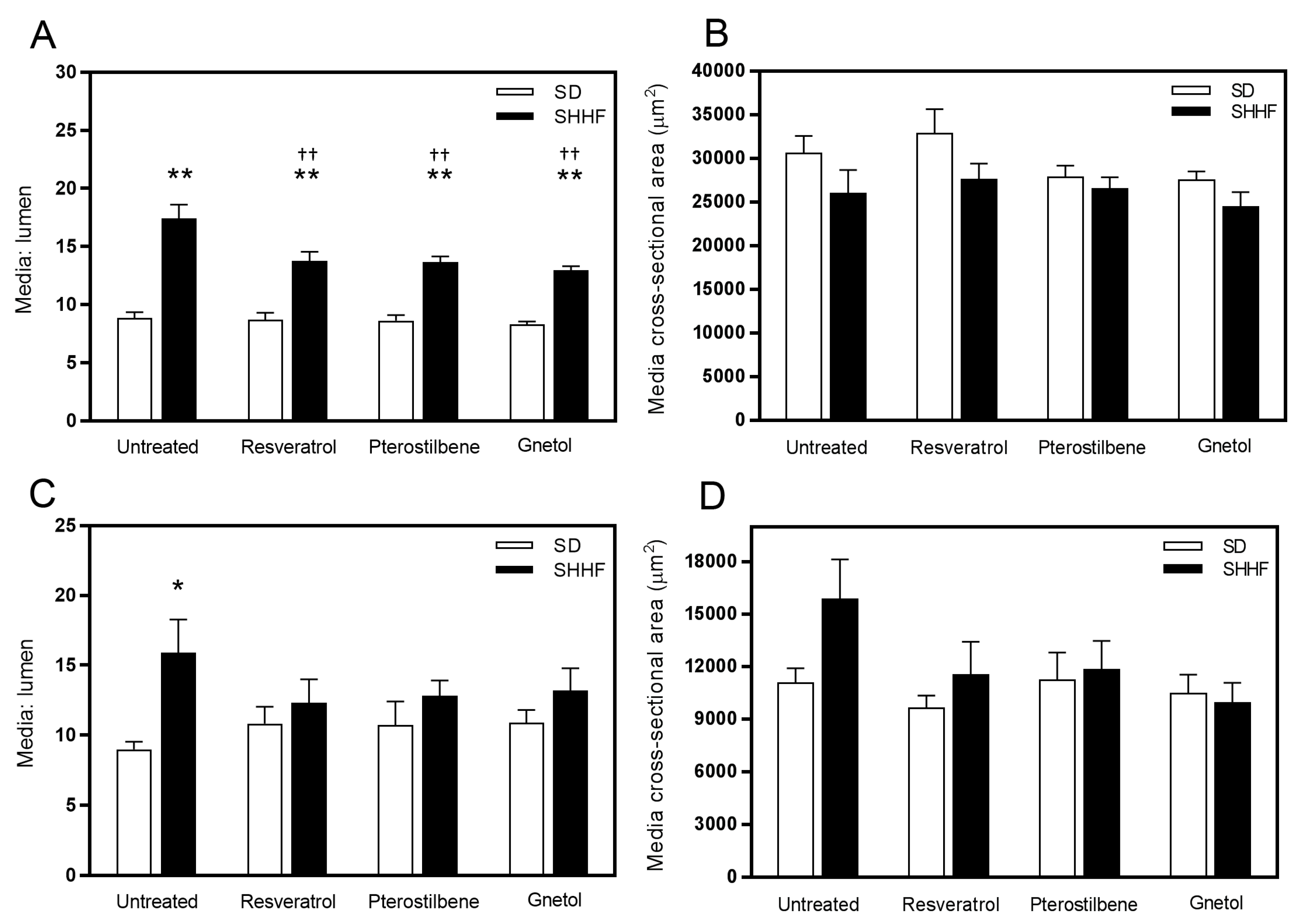
2.2. Vascular Geometry
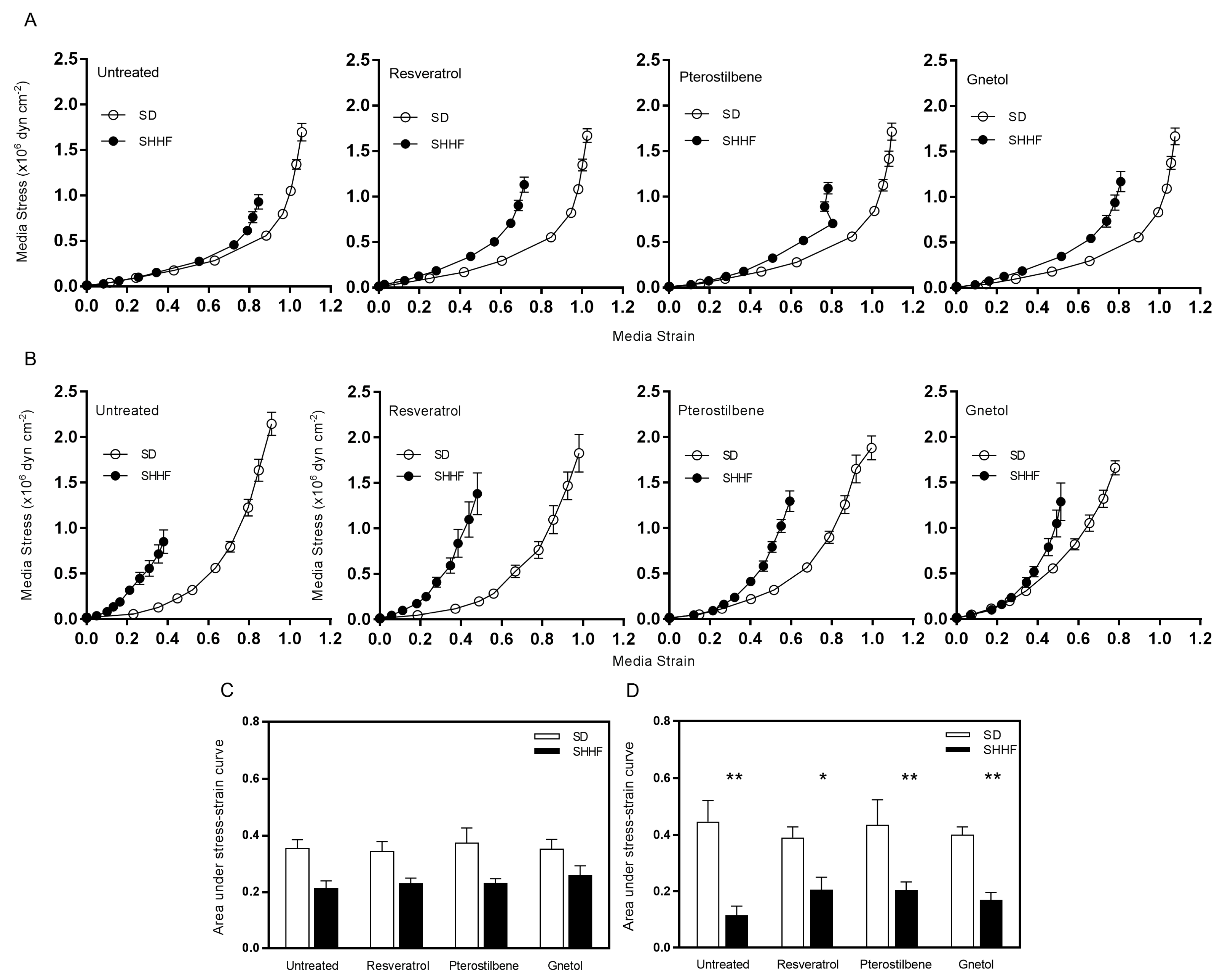
2.3. Vascular Compliance
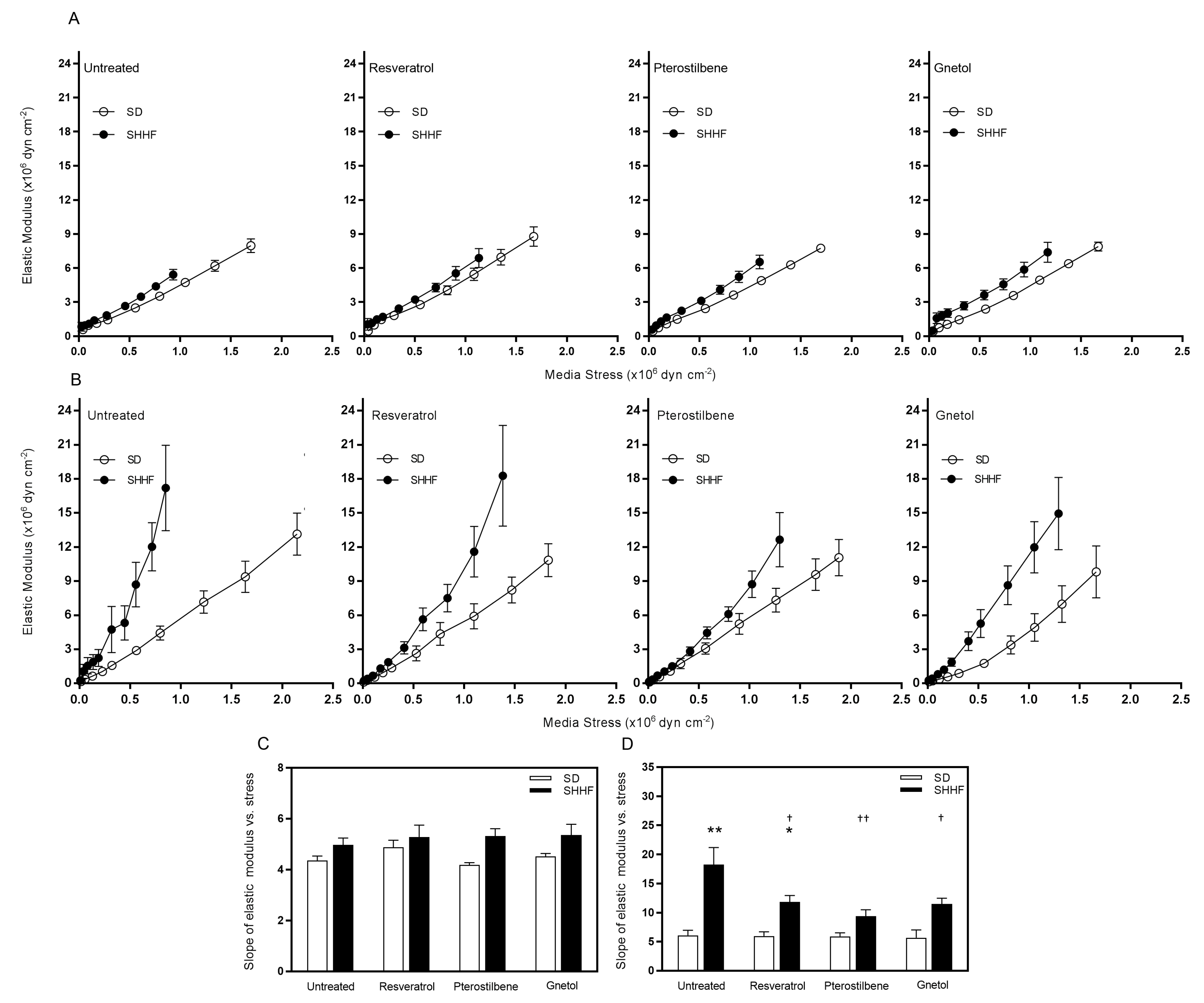
2.4. Arterial Wall Component Stiffness
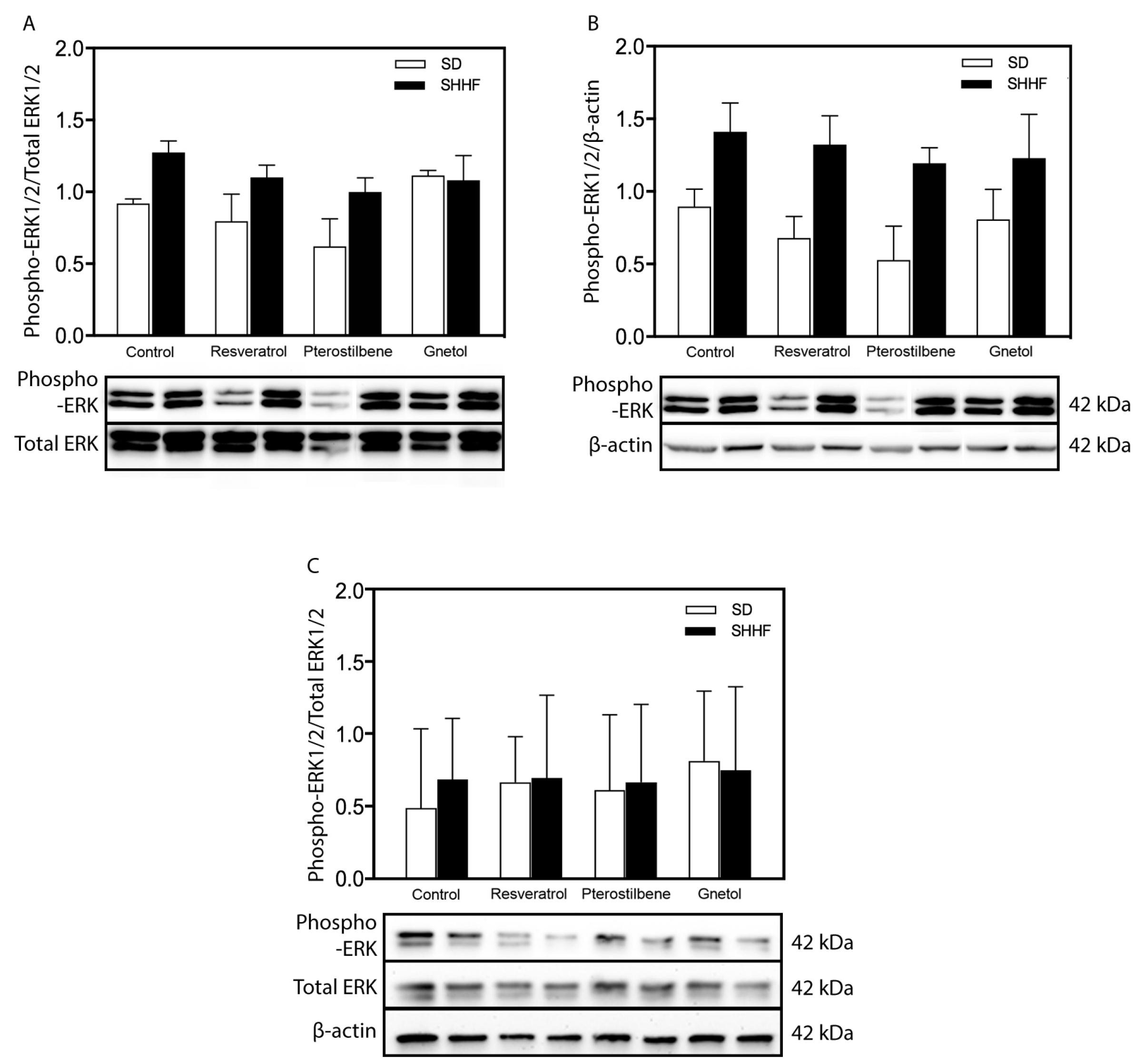
2.5. Signaling Effectors
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Pressure Myography
4.2.1. Arterial Segments
4.2.2. Vascular Geometry
4.2.3. Vascular Mechanics
4.2.4. Formulas
4.3. Western Blotting
4.4. Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intengan, H.D.; Schiffrin, E.L. Structure and mechanical properties of resistance arteries in hypertension: Role of adhesion molecules and extracellular matrix determinants. Hypertension 2000, 36, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L. Vascular changes in hypertension in response to drug treatment: Effects of angiotensin receptor blockers. Can. J. Cardiol. 2002, 18 (Suppl. A), 15A–18A. [Google Scholar] [PubMed]
- Mulvany, M.J.; Hansen, O.K.; Aalkjaer, C. Direct evidence that the greater contractility of resistance vessels in spontaneously hypertensive rats is associated with a narrowed lumen, a thickened media, and an increased number of smooth muscle cell layers. Circ. Res. 1978, 43, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J.; Korsgaard, N. Correlations and otherwise between blood pressure, cardiac mass and resistance vessel characteristics in hypertensive, normotensive and hypertensive/normotensive hybrid rats. J. Hypertens. 1983, 1, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J.; Baumbach, G.L.; Aalkjaer, C.; Heagerty, A.M.; Korsgaard, N.; Schiffrin, E.L.; Heistad, D.D. Vascular remodeling. Hypertension 1996, 28, 505–506. [Google Scholar] [PubMed]
- Hajdu, M.A.; Baumbach, G.L. Mechanics of large and small cerebral arteries in chronic hypertension. Am. J. Physiol. 1994, 266, H1027–H1033. [Google Scholar] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. Dash collaborative research group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- de Lorgeril, M.; Renaud, S.; Mamelle, N.; Salen, P.; Martin, J.L.; Monjaud, I.; Guidollet, J.; Touboul, P.; Delaye, J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994, 343, 1454–1459. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the lyon diet heart study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.M.; Vita, J.A. Grapes and cardiovascular disease. J. Nutr. 2009, 139, 1788S–1793S. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; Hollman, P.C.; Feskens, E.J.; Bueno de Mesquita, H.B.; Kromhout, D. Catechin intake and associated dietary and lifestyle factors in a representative sample of Dutch men and women. Eur. J. Clin. Nutr. 2001, 55, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M.; Launer, L.J.; Van der Kuip, D.A.; Hofman, A.; Witteman, J.C. Inverse association of tea and flavonoid intakes with incident myocardial infarction: The rotterdam study. Am. J. Clin. Nutr. 2002, 75, 880–886. [Google Scholar] [PubMed]
- Knekt, P.; Jarvinen, R.; Reunanen, A.; Maatela, J. Flavonoid intake and coronary mortality in finland: A cohort study. BMJ 1996, 312, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Ness, A.R.; Powles, J.W. Fruit and vegetables, and cardiovascular disease: A review. Int. J. Epidemiol. 1997, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113 (Suppl. 9B), 71S–88S. [Google Scholar] [CrossRef]
- McCall, D.O.; McGartland, C.P.; McKinley, M.C.; Patterson, C.C.; Sharpe, P.; McCance, D.R.; Young, I.S.; Woodside, J.V. Dietary intake of fruits and vegetables improves microvascular function in hypertensive subjects in a dose-dependent manner. Circulation 2009, 119, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Diebolt, M.; Bucher, B.; Andriantsitohaina, R. Wine polyphenols decrease blood pressure, improve no vasodilatation, and induce gene expression. Hypertension 2001, 38, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Ikeda, K.; Kawai, Y.; Yamori, Y. Extract of wine phenolics improves aortic biomechanical properties in stroke-prone spontaneously hypertensive rats (shrsp). J. Nutr. Sci. Vitaminol. 1999, 45, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Bernatova, I.; Pechanova, O.; Babal, P.; Kysela, S.; Stvrtina, S.; Andriantsitohaina, R. Wine polyphenols improve cardiovascular remodeling and vascular function in no-deficient hypertension. Am. J. Physiol. 2002, 282, H942–H948. [Google Scholar] [CrossRef] [PubMed]
- Sarr, M.; Chataigneau, M.; Martins, S.; Schott, C.; El Bedoui, J.; Oak, M.H.; Muller, B.; Chataigneau, T.; Schini-Kerth, V.B. Red wine polyphenols prevent angiotensin II-induced hypertension and endothelial dysfunction in rats: Role of nadph oxidase. Cardiovasc. Res. 2006, 71, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Kim, J.S.; Kang, M.H. Concord grape juice supplementation reduces blood pressure in korean hypertensive men: Double-blind, placebo controlled intervention trial. Biofactors 2004, 22, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Clark, J.T.; Prasain, J.; Kim, H.; White, C.R.; Wyss, J.M. Antihypertensive and cognitive effects of grape polyphenols in estrogen-depleted, female, spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R771–R775. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [PubMed]
- Gocmez, S.S.; Scarpace, P.J.; Whidden, M.A.; Erdos, B.; Kirichenko, N.; Sakarya, Y.; Utkan, T.; Tumer, N. Age impaired endothelium-dependent vasodilation is improved by resveratrol in rat mesenteric arteries. J. Exerc. Nutr. Biochem. 2016, 20, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Jugder, B.E.; Poljak, A.; Jayasena, T.; Mansour, H.; Nabavi, S.M.; Sachdev, P.; Grant, R. Resveratrol as a potential therapeutic candidate for the treatment and management of alzheimer’s disease. Curr. Top. Med. Chem. 2016, 16, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.L.; Fernandez, M.L. Cardioprotective effects of dietary polyphenols. J. Nutr. 2005, 135, 2291–2294. [Google Scholar] [PubMed]
- Wu, J.M.; Wang, Z.R.; Hsieh, T.C.; Bruder, J.L.; Zou, J.G.; Huang, Y.Z. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review). Int. J. Mol. Med. 2001, 8, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, Y.; Zhang, X.; Liu, Z.; Zhang, W.; Mao, W.; Wang, W.; Cui, W.; Zhang, X.; Jia, X.; et al. Effects of trans-resveratrol on hypertension-induced cardiac hypertrophy using the partially nephrectomized rat model. Clin. Exp. Pharmacol. Physiol. 2005, 32, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Miatello, R.; Vazquez, M.; Renna, N.; Cruzado, M.; Zumino, A.P.; Risler, N. Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. Am. J. Hypertens. 2005, 18, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Aubin, M.C.; Lajoie, C.; Clement, R.; Gosselin, H.; Calderone, A.; Perrault, L.P. Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: Therapeutic potential of resveratrol. J. Pharmacol. Exp. Ther. 2008, 325, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, J.; Thandapilly, S.J.; Louis, X.L.; Huang, Y.; Shao, Z.; Kopilas, M.A.; Wojciechowski, P.; Netticadan, T.; Anderson, H.D. Resveratrol and small artery compliance and remodeling in the spontaneously hypertensive rat. Am. J. Hypertens. 2010, 23, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Gao, Y.; Zhao, J.; Zhang, J.; Li, Q.; Zhao, Z.; Liu, J. Preparation and optimization of resveratrol nanosuspensions by antisolvent precipitation using box-behnken design. AAPS PharmSciTech 2015, 16, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; McFadden, D. Pterostilbene and cancer: Current review. J. Surg. Res. 2012, 173, e53–e61. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Lim, Y.; Hong, J.T.; Yoo, H.S.; Lee, C.K.; Pyo, M.Y.; Yun, Y.P. Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking akt-dependent pathway. Vasc. Pharmacol. 2010, 53, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Riche, D.M.; Riche, K.D.; Blackshear, C.T.; McEwen, C.L.; Sherman, J.J.; Wofford, M.R.; Griswold, M.E. Pterostilbene on metabolic parameters: A randomized, double-blind, and placebo-controlled trial. Evid. Based Complement. Altern. Med. 2014, 2014, 459165. [Google Scholar] [CrossRef] [PubMed]
- Mačičková, T.; Pečivová, J.; Harmatha, J.; Sviteková, K.; Nosál, R. Effect of stilbene derivative on superoxide generation and enzyme release from human neutrophils in vitro. Interdiscip. Toxicol. 2012, 5, 71–75. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell. Longev. 2013, 2013, 575482. [Google Scholar] [CrossRef] [PubMed]
- Remsberg, C.M.; Martinez, S.E.; Akinwumi, B.C.; Anderson, H.D.; Takemoto, J.K.; Sayre, C.L.; Davies, N.M. Preclinical pharmacokinetics and pharmacodynamics and content analysis of gnetol in foodstuffs. Phytother. Res. 2015, 29, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Tokunaga, Y.; Sakan, F. Stilbenoids isolated from the seeds of melinjo (gnetum gnemon l.) and their biological activity. J. Agric. Food Chem. 2009, 57, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Jiang, B.; Li, X.M.; Zhang, H.J.; Zhao, Q.S.; Li, S.H.; Sun, H.D. Constituents of gnetum montanum. Fitoterapia 2002, 73, 40–42. [Google Scholar] [CrossRef]
- Narayanan, N.K.; Kunimasa, K.; Yamori, Y.; Mori, M.; Mori, H.; Nakamura, K.; Miller, G.; Manne, U.; Tiwari, A.K.; Narayanan, B. Antitumor activity of melinjo (gnetum gnemon l.) seed extract in human and murine tumor models in vitro and in a colon-26 tumor-bearing mouse model in vivo. Cancer Med. 2015, 4, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Fremont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Heyen, J.R.; Blasi, E.R.; Nikula, K.; Rocha, R.; Daust, H.A.; Frierdich, G.; Van Vleet, J.F.; De Ciechi, P.; McMahon, E.G.; Rudolph, A.E. Structural, functional, and molecular characterization of the shhf model of heart failure. Am. J. Physiol. 2002, 283, H1775–H1784. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, A.M.; Onodera, T.; Wang, X.; McCune, S.A. Myocyte remodeling during the progression to failure in rats with hypertension. Hypertension 1996, 28, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.J.; Teschke, S.R.; Reid, E.B.; Durham, K.K.; Kroetsch, J.T.; Rush, J.W. Amp-activated protein kinase activator aicar acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J. Hypertens. 2012, 30, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.; Dolinsky, V.W.; Soltys, C.L.; Viollet, B.; Baksh, S.; Light, P.E.; Dyck, J.R. Resveratrol inhibits cardiac hypertrophy via amp-activated protein kinase and akt. J. Biol. Chem. 2008, 283, 24194–24201. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, E.J.; Kim, D.I.; Park, S.K.; Kim, W.J.; Moon, S.K. Inhibition of proliferation and migration by piceatannol in vascular smooth muscle cells. Toxicol. In Vitro 2009, 23, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- El-Mowafy, A.M.; Alkhalaf, M.; Nassar, N.N. Resveratrol reverses et-1-evoked mitogenic effects in human coronary arterial cells by activating the kinase-g to inhibit erk-enzymes. Int. J. Cardiol. 2009, 136, 263–269. [Google Scholar] [CrossRef] [PubMed]
- El Mabrouk, M.; Touyz, R.M.; Schiffrin, E.L. Differential ang II-induced growth activation pathways in mesenteric artery smooth muscle cells from shr. Am. J. Physiol. 2001, 281, H30–H39. [Google Scholar]
- Intengan, H.D.; Thibault, G.; Li, J.S.; Schiffrin, E.L. Resistance artery mechanics, structure, and extracellular components in spontaneously hypertensive rats: Effects of angiotensin receptor antagonism and converting enzyme inhibition. Circulation 1999, 100, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Witters, L.A.; Kemp, B.E.; Means, A.R. Chutes and ladders: The search for protein kinases that act on ampk. Trends Biochem. Sci. 2006, 31, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Beauloye, C.; Bertrand, L.; Horman, S.; Hue, L. Ampk activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc. Res. 2011, 90, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Intengan, H.D.; Deng, L.Y.; Li, J.S.; Schiffrin, E.L. Mechanics and composition of human subcutaneous resistance arteries in essential hypertension. Hypertension 1999, 33, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Remsberg, C.M.; Yanez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. 2008, 22, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Chan, A.Y.; Robillard Frayne, I.; Light, P.E.; Des Rosiers, C.; Dyck, J.R. Resveratrol prevents the prohypertrophic effects of oxidative stress on lkb1. Circulation 2009, 119, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Mathiassen, O.N.; Buus, N.H.; Sihm, I.; Thybo, N.K.; Morn, B.; Schroeder, A.P.; Thygesen, K.; Aalkjaer, C.; Lederballe, O.; Mulvany, M.J.; et al. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J. Hypertens. 2007, 25, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Porteri, E.; Boari, G.E.; De Ciuceis, C.; Sleiman, I.; Muiesan, M.L.; Castellano, M.; Miclini, M.; Agabiti-Rosei, E. Prognostic significance of small-artery structure in hypertension. Circulation 2003, 108, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Chakrabarti, S.; Pereira, T.J.; Oka, T.; Levasseur, J.; Beker, D.; Zordoky, B.N.; Morton, J.S.; Nagendran, J.; Lopaschuk, G.D.; et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta 2013, 1832, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Thandapilly, S.J.; Louis, X.L.; Behbahani, J.; Movahed, A.; Yu, L.; Fandrich, R.; Zhang, S.; Kardami, E.; Anderson, H.D.; Netticadan, T. Reduced hemodynamic load aids low-dose resveratrol in reversing cardiovascular defects in hypertensive rats. Hypertens. Res. 2013, 36, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.N.; Groma, G.; Spijkers, L.J.; de Vos, J.; van Weert, A.; van Veen, H.; Everts, V.; Arribas, S.M.; VanBavel, E. Heterogeneity in arterial remodeling among sublines of spontaneously hypertensive rats. PLoS ONE 2014, 9, e107998. [Google Scholar] [CrossRef] [PubMed]
- Laurant, P.; Touyz, R.M.; Schiffrin, E.L. Effect of pressurization on mechanical properties of mesenteric small arteries from spontaneously hypertensive rats. J. Vasc. Res. 1997, 34, 117–125. [Google Scholar] [PubMed]
- Izzard, A.S.; Horton, S.; Heerkens, E.H.; Shaw, L.; Heagerty, A.M. Middle cerebral artery structure and distensibility during developing and established phases of hypertension in the spontaneously hypertensive rat. J. Hypertens. 2006, 24, 875–880. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Vingrys, A.J.; Armitage, J.A.; Bui, B.V. The role of blood pressure in glaucoma. Clin. Exp. Optom. 2011, 94, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.T., Jr.; Bakris, G.; Greene, T.; Agodoa, L.Y.; Appel, L.J.; Charleston, J.; Cheek, D.; Douglas-Baltimore, J.G.; Gassman, J.; Glassock, R.; et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the aask trial. JAMA 2002, 288, 2421–2431. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Williams, B.; Ritz, E. Essential hypertension. Lancet 2007, 370, 591–603. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Dahlof, B. Prevention of stroke in patients with hypertension. Am. J. Cardiol. 2007, 100, 17J–24J. [Google Scholar] [CrossRef] [PubMed]
- Tzourio, C. Hypertension, cognitive decline, and dementia: An epidemiological perspective. Dialogues Clin. Neurosci. 2007, 9, 61–70. [Google Scholar] [PubMed]
- Beason-Held, L.L.; Moghekar, A.; Zonderman, A.B.; Kraut, M.A.; Resnick, S.M. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke 2007, 38, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Birns, J.; Kalra, L. Cognitive function and hypertension. J. Hum. Hypertens. 2009, 23, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Waldstein, S.R.; Rice, S.C.; Thayer, J.F.; Najjar, S.S.; Scuteri, A.; Zonderman, A.B. Pulse pressure and pulse wave velocity are related to cognitive decline in the baltimore longitudinal study of aging. Hypertension 2008, 51, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, I.; Goldstein, F.C.; Martin, G.S.; Quyyumi, A.A. Roles of arterial stiffness and blood pressure in hypertension-associated cognitive decline in healthy adults. Hypertension 2016, 67, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Trojano, L.; Antonelli Incalzi, R.; Acanfora, D.; Picone, C.; Mecocci, P.; Rengo, F. Cognitive impairment: A key feature of congestive heart failure in the elderly. J. Neurol. 2003, 250, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Winblad, B.; Marengoni, A.; Klarin, I.; Fastbom, J.; Fratiglioni, L. Heart failure and risk of dementia and alzheimer disease: A population-based cohort study. Arch. Intern. Med. 2006, 166, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simone, G.; Ford, E.S.; et al. Heart disease and stroke statistics—2011 update: A report from the american heart association. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Nedergaard, M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007, 10, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Baumbach, G.L.; Walmsley, J.G.; Hart, M.N. Composition and mechanics of cerebral arterioles in hypertensive rats. Am. J. Pathol. 1988, 133, 464–471. [Google Scholar] [PubMed]
- Hart, M.N.; Heistad, D.D.; Brody, M.J. Effect of chronic hypertension and sympathetic denervation on wall/lumen ratio of cerebral vessels. Hypertension 1980, 2, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.L.; Bohlen, H.G. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension 1984, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Baumbach, G.L.; Dobrin, P.B.; Hart, M.N.; Heistad, D.D. Mechanics of cerebral arterioles in hypertensive rats. Circ. Res. 1988, 62, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, A.; Wiesel, P.; Aubert, J.F.; Brunner, H.R.; Hayoz, D. Time course changes of the mechanical properties of the carotid artery in renal hypertensive rats. Hypertension 1997, 29, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Kontos, H.A.; Wei, E.P.; Navari, R.M.; Levasseur, J.E.; Rosenblum, W.I.; Patterson, J.L., Jr. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am. J. Physiol. 1978, 234, H371–H383. [Google Scholar] [PubMed]
- Rush, J.W.; Quadrilatero, J.; Levy, A.S.; Ford, R.J. Chronic resveratrol enhances endothelium-dependent relaxation but does not alter enos levels in aorta of spontaneously hypertensive rats. Exp. Biol. Med. 2007, 232, 814–822. [Google Scholar]
- Rodriguez-Vita, J.; Sanchez-Lopez, E.; Esteban, V.; Ruperez, M.; Egido, J.; Ruiz-Ortega, M. Angiotensin ii activates the smad pathway in vascular smooth muscle cells by a transforming growth factor-β-independent mechanism. Circulation 2005, 111, 2509–2517. [Google Scholar] [CrossRef] [PubMed]
- Carver, K.A.; Smith, T.L.; Gallagher, P.E.; Tallant, E.A. Angiotensin-(1-7) prevents angiotensin ii-induced fibrosis in cremaster microvessels. Microcirculation 2015, 22, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Lee Kraus, W. Nuclear receptors, coactivators and chromatin: New approaches, new insights. Trends Endocrinol. Metab. 2001, 12, 191–197. [Google Scholar] [CrossRef]
- Diep, Q.N.; Schiffrin, E.L. Increased expression of peroxisome proliferator-activated receptor-alpha and -gamma in blood vessels of spontaneously hypertensive rats. Hypertension 2001, 38, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Diep, Q.N.; El Mabrouk, M.; Cohn, J.S.; Endemann, D.; Amiri, F.; Virdis, A.; Neves, M.F.; Schiffrin, E.L. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: Role of peroxisome proliferator-activated receptor-gamma. Circulation 2002, 105, 2296–2302. [Google Scholar] [CrossRef] [PubMed]
- Iglarz, M.; Touyz, R.M.; Amiri, F.; Lavoie, M.F.; Diep, Q.N.; Schiffrin, E.L. Effect of peroxisome proliferator-activated receptor-alpha and -gamma activators on vascular remodeling in endothelin-dependent hypertension. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Benkirane, K.; Viel, E.C.; Amiri, F.; Schiffrin, E.L. Peroxisome proliferator-activated receptor gamma regulates angiotensin II-stimulated phosphatidylinositol 3-kinase and mitogen-activated protein kinase in blood vessels in vivo. Hypertension 2006, 47, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Calleri, E.; Pochetti, G.; Dossou, K.S.; Laghezza, A.; Montanari, R.; Capelli, D.; Prada, E.; Loiodice, F.; Massolini, G.; Bernier, M.; et al. Resveratrol and its metabolites bind to ppars. Chembiochem 2014, 15, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (ppargamma): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Jiang, X.F.; Katayama, T.; Osada, S.; Umesono, K.; Namura, S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci. Lett. 2003, 352, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Lu, Y.; Rodrigues, G.A. Resveratrol protects rpe cells from sodium iodate by modulating pparalpha and ppardelta. Exp. Eye Res. 2014, 118, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Bhavsar, V.; Jaykaran; Kantharia, N.D. Effect of antihypertensive therapy on cognitive functions of patients with hypertension. Ann. Indian Acad. Neurol. 2010, 13, 180–183. [Google Scholar] [PubMed]
- Murray, M.D.; Lane, K.A.; Gao, S.; Evans, R.M.; Unverzagt, F.W.; Hall, K.S.; Hendrie, H. Preservation of cognitive function with antihypertensive medications: A longitudinal analysis of a community-based sample of african americans. Arch. Intern. Med. 2002, 162, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Ruben, Z.; Miller, J.E.; Rohrbacher, E.; Walsh, G.M. A potential model for a human disease: Spontaneous cardiomyopathy-congestive heart failure in SHR/N-CP rats. Hum. Pathol. 1984, 15, 902–903. [Google Scholar] [CrossRef]
- Louis, W.J.; Howes, L.G. Genealogy of the spontaneously hypertensive rat and wistar-kyoto rat strains: Implications for studies of inherited hypertension. J. Cardiovasc. Pharmacol. 1990, 16 (Suppl. 7), S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Alibin, C.P.; Kopilas, M.A.; Anderson, H.D. Suppression of cardiac myocyte hypertrophy by conjugated linoleic acid: Role of peroxisome proliferator-activated receptors alpha and gamma. J. Biol. Chem. 2008, 283, 10707–10715. [Google Scholar] [CrossRef] [PubMed]
- Akinwumi, B.C.; Raj, P.; Lee, D.I.; Acosta, C.; Yu, L.; Thomas, S.M.; Nagabhushanam, K.; Majeed, M.; Davies, N.M.; Netticadan, T.; et al. Disparate effects of stilbenoid polyphenols on hypertrophic cardiomyocytes in vitro vs. In the spontaneously hypertensive heart failure rat. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- H’Doubler, P.B., Jr.; Peterson, M.; Shek, W.; Auchincloss, H.; Abbott, W.M.; Orkin, R.W. Spontaneously hypertensive and wistar kyoto rats are genetically disparate. Lab. Anim. Sci. 1991, 41, 471–473. [Google Scholar] [PubMed]
- Kurtz, T.W.; Morris, R.C., Jr. Biological variability in wistar-kyoto rats. Implications for research with the spontaneously hypertensive rat. Hypertension 1987, 10, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Thandapilly, S.J.; Wojciechowski, P.; Behbahani, J.; Louis, X.L.; Yu, L.; Juric, D.; Kopilas, M.A.; Anderson, H.D.; Netticadan, T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the shr without lowering blood pressure. Am. J. Hypertens. 2010, 23, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compound gnetol is available from the authors.

| Parameter | SD | SHHF | ||||||
|---|---|---|---|---|---|---|---|---|
| C | R | P | G | C | R | P | G | |
| body weight, g | 564 ± 15 | 574 ± 19 | 552 ± 24 | 600 ± 38 | 375 ± 10 ** | 363 ± 14 ** | 351 ± 12 ** | 364 ± 9 ** |
| systolic BP | 142 ± 6 | 132 ± 7 | 136 ± 3 | 142 ± 5 | 194 ± 3 ** | 187 ± 5 ** | 190 ± 3 ** | 192 ± 4 ** |
| mesenteric arteries—slope of EM vs. stress | 4.4 ± 0.2 | 4.9 ± 0.3 | 4.2 ± 0.1 | 4.5 ± 0.1 | 5.0 ± 0.3 | 5.3 ± 0.4 | 5.3 ± 0.3 | 5.4 ± 0.4 |
| cerebral arteries—slope of EM vs. stress | 6.1 ± 0.9 | 6.0 ± 0.7 | 5.9 ± 0.6 | 5.7 ± 1.3 | 18.2 ± 2.9 ** | 11.8 ± 1.1 *,† | 9.4 ± 1.1 †† | 11.5 ± 1.0 † |
| Arteries | Growth Index | Remodeling Index | ||||||
|---|---|---|---|---|---|---|---|---|
| C | R | P | G | C | R | P | G | |
| Mesenteric arteries | 3.9% | 5.6% | 19.4% | 14.0% | 97.4% | 96.2% | 79.9% | 90.2% |
| Middle cerebral arteries | 43.6% | 4.3% | 7.0% | -10.3% | 58.0% | 54.5% | 48.6% | 39.0% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.I.; Acosta, C.; Anderson, C.M.; Anderson, H.D. Peripheral and Cerebral Resistance Arteries in the Spontaneously Hypertensive Heart Failure Rat: Effects of Stilbenoid Polyphenols. Molecules 2017, 22, 380. https://doi.org/10.3390/molecules22030380
Lee DI, Acosta C, Anderson CM, Anderson HD. Peripheral and Cerebral Resistance Arteries in the Spontaneously Hypertensive Heart Failure Rat: Effects of Stilbenoid Polyphenols. Molecules. 2017; 22(3):380. https://doi.org/10.3390/molecules22030380
Chicago/Turabian StyleLee, Danielle I., Crystal Acosta, Christopher M. Anderson, and Hope D. Anderson. 2017. "Peripheral and Cerebral Resistance Arteries in the Spontaneously Hypertensive Heart Failure Rat: Effects of Stilbenoid Polyphenols" Molecules 22, no. 3: 380. https://doi.org/10.3390/molecules22030380
APA StyleLee, D. I., Acosta, C., Anderson, C. M., & Anderson, H. D. (2017). Peripheral and Cerebral Resistance Arteries in the Spontaneously Hypertensive Heart Failure Rat: Effects of Stilbenoid Polyphenols. Molecules, 22(3), 380. https://doi.org/10.3390/molecules22030380






