Electrochemical Detecting Lung Cancer-Associated Antigen Based on Graphene-Gold Nanocomposite
Abstract
:1. Introduction
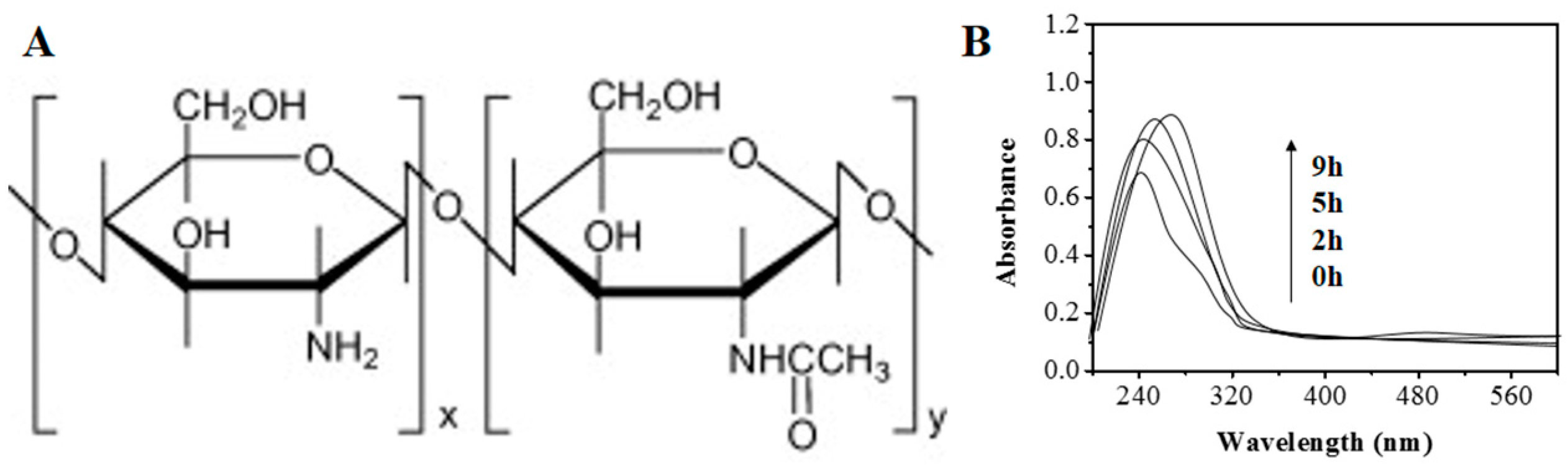
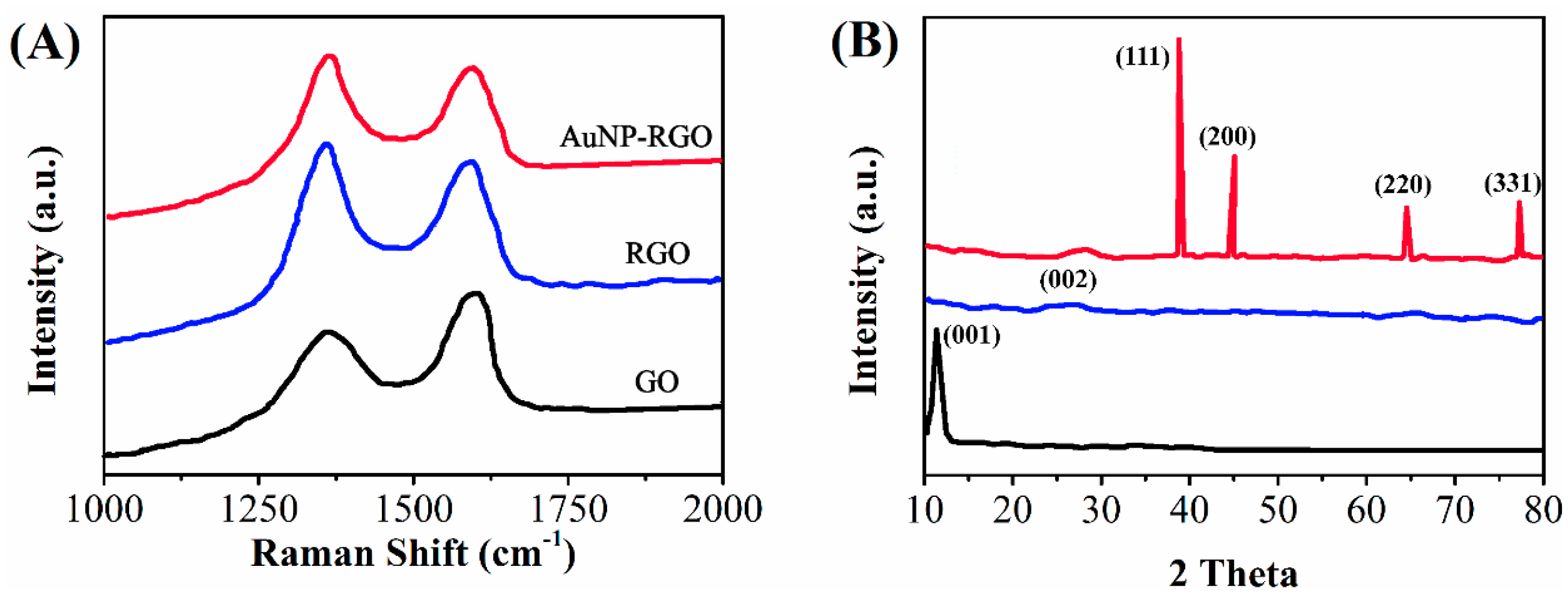
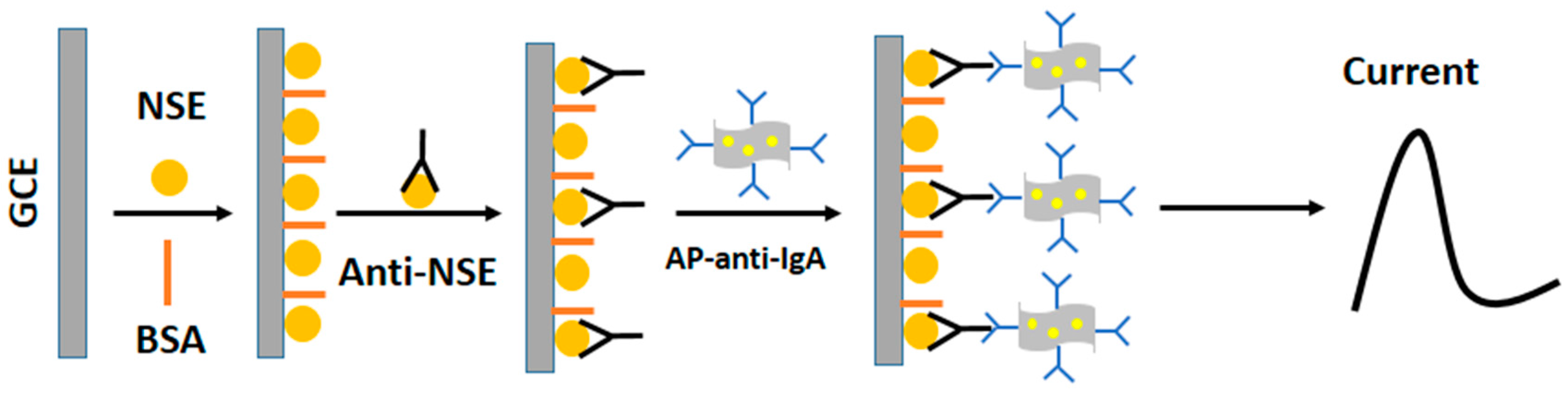
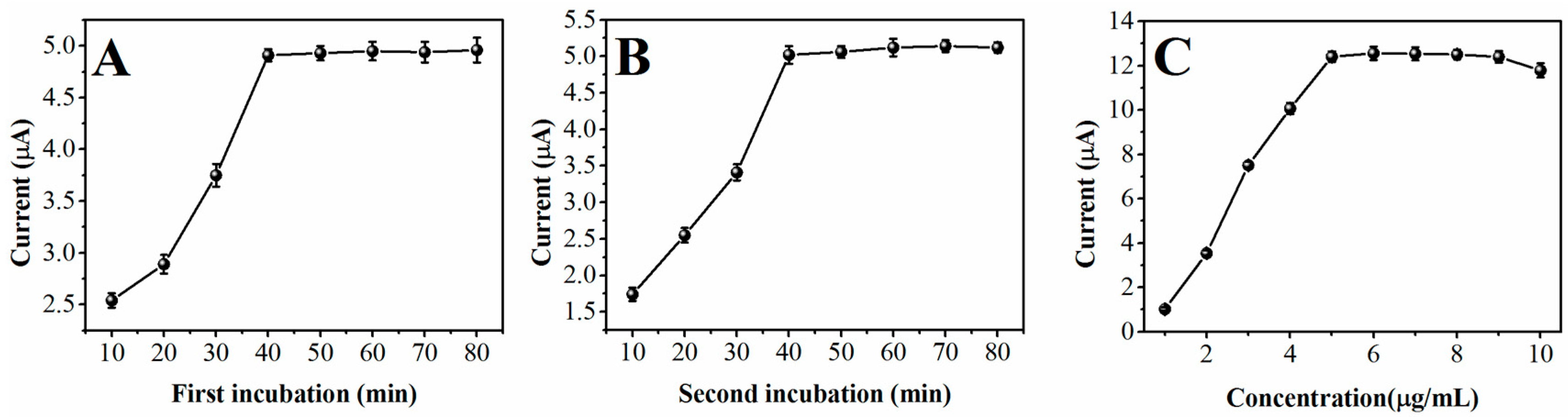
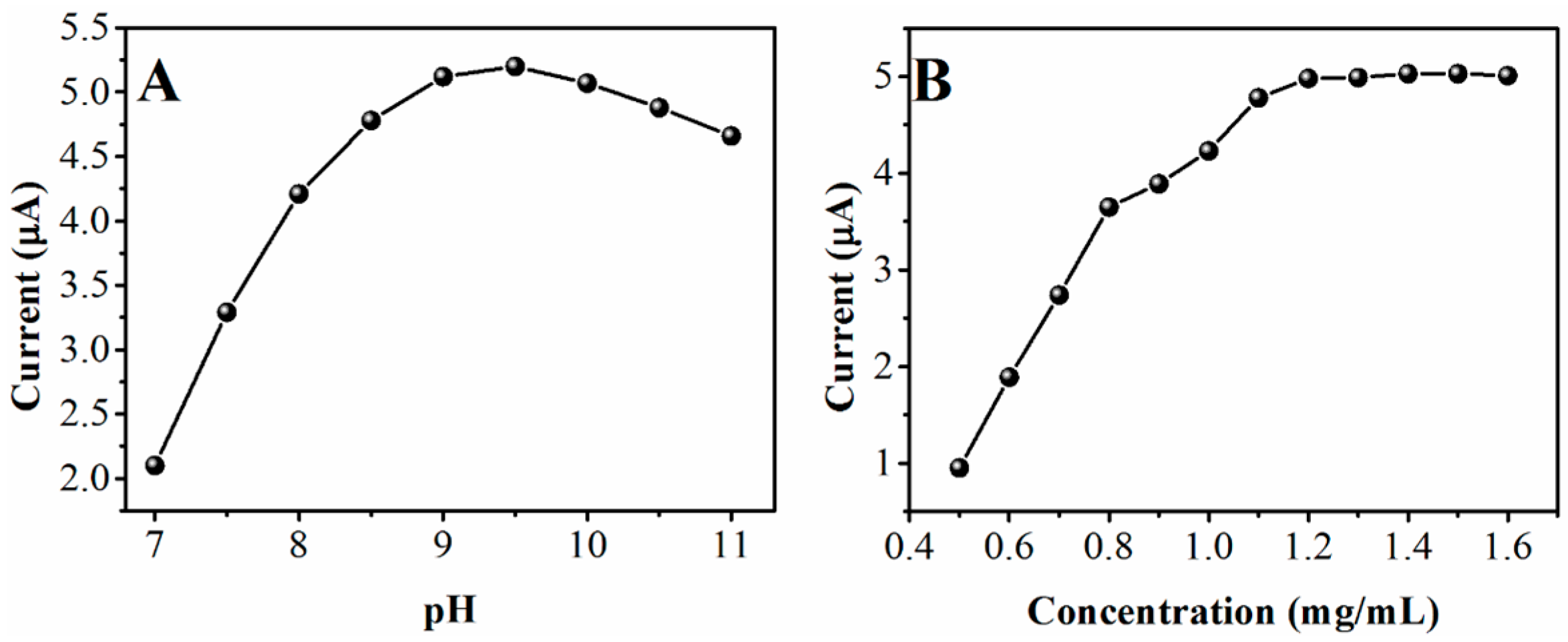
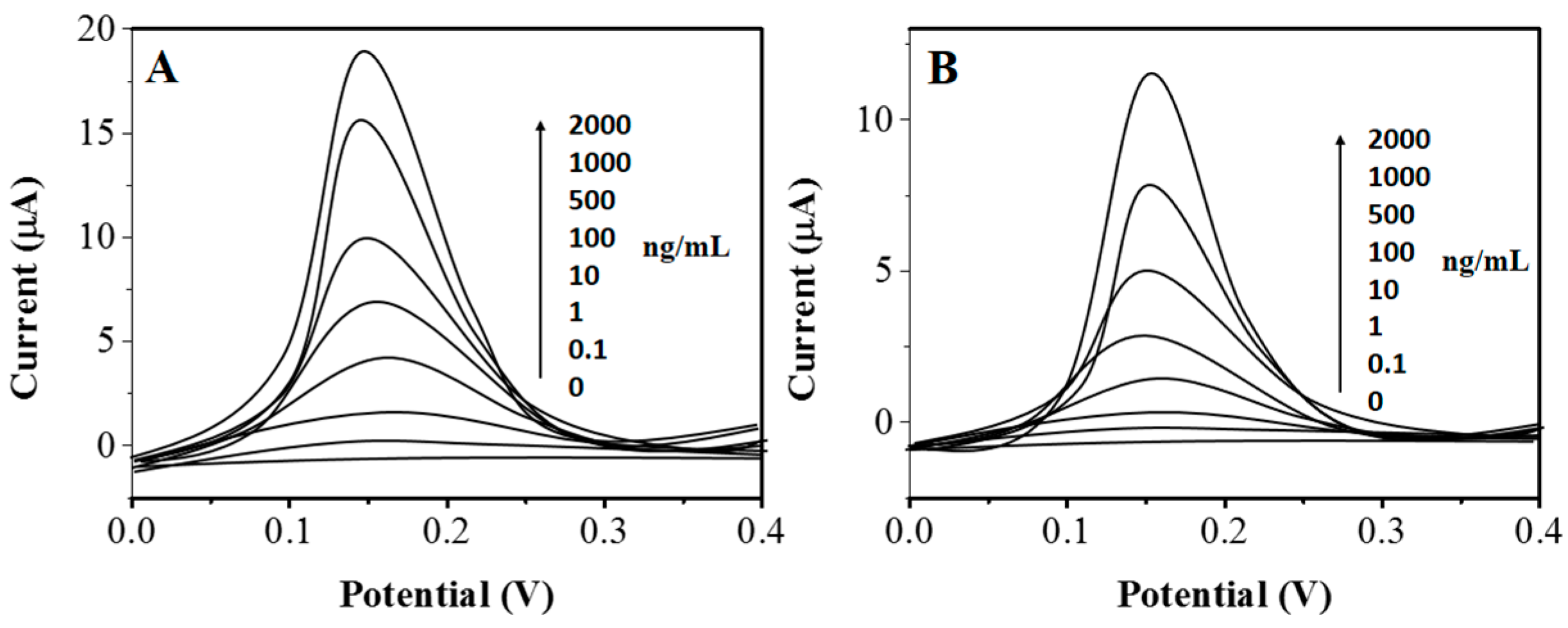
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Han, M.; Liu, Q.; Yu, J.; Zheng, S. Detection and significance of serum protein markers of small-cell lung cancer. J. Clin. Lab. Anal. 2008, 22, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Monnerat, C.; Turrisi, A.T.; Perry, M.C.; Leyvraz, S. Small cell lung cancer: State of the art and future perspectives. Lung Cancer 2004, 45, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Philipp, M.; Velcovsky, H.G.; Morr, H.; Katz, N. Pro-gastrin-releasing peptide (ProGRP), neuron specific enolase (NSE), carcinoembryonic antigen (CEA) and cytokeratin 19-fragments (CYFRA 21-1) in patients with lung cancer in comparison to other lung diseases. Anticancer Res. 2002, 23, 885–893. [Google Scholar]
- Stammet, P.; Collignon, O.; Hassager, C.; Wise, M.P.; Hovdenes, J.; Åneman, A.; Horn, J.; Devaux, Y.; Erlinge, D.; Kjaergaard, J. Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33 °C and 36 °C. J. Am. Coll. Cardiol. 2015, 65, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Quintard, H.; Heurteaux, C.; Ichai, C. Neuron specific enolase and Glasgow motor score remain useful tools for assessing neurological prognosis after out-of-hospital cardiac arrest treated with therapeutic hypothermia. Retour Au Numéro 2015, 34, 231–237. [Google Scholar]
- Cheng, F.; Yuan, Q.; Yang, J.; Wang, W.; Liu, H. The prognostic value of serum neuron-specific enolase in traumatic brain injury: Systematic review and meta-analysis. PLoS ONE 2014, 9, e106680. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lai, G.; Zhu, D.; Jia, B.; Malherbe, F.; Yu, A. Advanced catalytic and electrocatalytic performances of polydopamine functionalized reduced graphene oxide-palladium nanocomposites. ChemCatChem 2016, 8, 2975–2980. [Google Scholar] [CrossRef]
- Yildirim, A.O.; Eroglu, M.; Kaldirim, U.; Eyi, Y.E.; Simsek, K.; Durusu, M.; Yamanel, L.; Arziman, I.; Tuncer, S.K.; Toygar, M. Serum neuron-specific enolase and S-100β levels as prognostic follow-up markers for oxygen administered carbon monoxide intoxication cases. Indian J. Biochem. Biophys. 2015, 52, 29–33. [Google Scholar] [PubMed]
- Stefanvanstaden, R.I.; Comnea, I.; Vanstaden, J.; Stanciugavan, C. Stochastic microsensors as screening tools for neuron specific enolase. Rsc. Adv. 2014, 4, 26383–26388. [Google Scholar] [CrossRef]
- Ho, J.A.; Chang, H.-C.; Shih, N.-Y.; Wu, L.-C.; Chang, Y.-F.; Chen, C.-C.; Chou, C. Diagnostic detection of human lung cancer-associated antigen using a gold nanoparticle-based electrochemical immunosensor. Anal. Chem. 2010, 82, 5944–5950. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.A.; Hung, C.-H. Using liposomal fluorescent biolabels to develop an immunoaffinity chromatographic biosensing system for biotin. Anal. Chem. 2008, 80, 6405–6409. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Yu, S.; Thompson, L.; Yu, A. Development of a novel nitrite electrochemical sensor by stepwise in situ formation of palladium and reduced graphene oxide nanocomposites. Rsc. Adv. 2015, 5, 40111–40116. [Google Scholar] [CrossRef]
- Dequaire, M.; Degrand, C.; Limoges, B. An electrochemical metalloimmunoassay based on a colloidal gold label. Anal. Chem. 2000, 72, 5521–5528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Fu, L.; Wang, A. Poly (diallyldimethylammonium chloride) functionalized reduced graphene oxide based electrochemical sensing platform for luteolin determination. Int. J. Electrochem. Sci. 2015, 10, 3518–3529. [Google Scholar]
- Lyon, L.A.; Musick, M.D.; Natan, M.J. Colloidal Au-enhanced surface plasmon resonance immunosensing. Anal. Chem. 1998, 70, 5177–5183. [Google Scholar] [CrossRef] [PubMed]
- Muratsugu, M.; Ohta, F.; Miya, Y.; Hosokawa, T.; Kurosawa, S.; Kamo, N.; Ikeda, H. Quartz crystal microbalance for the detection of microgram quantities of human serum albumin: Relationship between the frequency change and the mass of protein adsorbed. Anal. Chem. 1993, 65, 2933–2937. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Z.; Peng, F.; Fu, L. One-pot synthesis of ZnO-Pd nanocomposite with high electrocatalytic activity towards quinoline yellow. Inorg. Nano-Met. Chem. 2007, 47, 934–937. [Google Scholar] [CrossRef]
- Viallard, J.; Murthy, M.V.; Dastugue, B. An ultramicro bioluminescence assay of enolase: Application to human cerebrospinal fluid. Neurochem. Res. 1985, 10, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.B.; Kachman, M.T.; Gong, S.; Hinderer, R.; Parus, S.; Misek, D.E.; Hanash, S.M.; Lubman, D.M. Isoelectric focusing nonporous RP HPLC: A two-dimensional liquid-phase separation method for mapping of cellular proteins with identification using MALDI-TOF mass spectrometry. Anal. Chem. 2000, 72, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lai, G.; Mahon, P.; Wang, J.; Zhu, D.; Malherbe, F.; Yu, A. Carbon nanotube and graphene oxide directed electrochemical synthesis of silver dendrites. Rsc. Adv. 2014, 4, 39645–39650. [Google Scholar] [CrossRef]
- Das, J.; Aziz, M. A.; Yang, H. A nanocatalyst-based assay for proteins: DNA-free ultrasensitive electrochemical detection using catalytic reduction of p-nitrophenol by gold-nanoparticle labels. J. Am. Chem. Soc. 2006, 128, 16022–16023. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, H.; Zheng, Y.; Wu, Z.; Jiang, J.; Shen, G.; Yu, R. A sensitive electrochemical immunosensor for α-Fetoprotein detection with colloidal gold-based dentritical enzyme complex amplification. Electroanalysis 2010, 22, 244–250. [Google Scholar] [CrossRef]
- Lin, J.; Ju, H. Electrochemical and chemiluminescent immunosensors for tumor markers. Biosens. Bioelectron. 2005, 20, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Yuan, P.-X.; Yuan, R.; Chai, Y.-Q.; Hong, C.-L. Nanostructured conductive material containing ferrocenyl for reagentless amperometric immunosensors. Biomaterials 2008, 29, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Jv, Y.; Li, B.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef] [PubMed]
- Riskin, M.; Tel-Vered, R.; Bourenko, T.; Granot, E.; Willner, I. Imprinting of molecular recognition sites through electropolymerization of functionalized Au nanoparticles: Development of an electrochemical TNT sensor based on π-donor-acceptor interactions. J. Am. Chem. Soc. 2008, 130, 9726–9733. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, E. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Kajiya, K.; Nukaga, M.; Suga, Y.; Watanabe, T.; Nakamura, N.; Ohno, H. A simple fabrication method for three-dimensional gold nanoparticle electrodes and their application to the study of the direct electrochemistry of cytochrome c. Electroanalysis 2010, 22, 185–190. [Google Scholar] [CrossRef]
- Scodeller, P.; Flexer, V.; Szamocki, R.; Calvo, E.; Tognalli, N.; Troiani, H.; Fainstein, A. Wired-enzyme core-shell Au nanoparticle biosensor. J. Am. Chem. Soc. 2008, 130, 12690–12697. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Long, J.; Zhao, W.; Wang, L.; Chen, G. pH-responsive chitosan-mediated graphene dispersions. Langmuir 2010, 26, 16771–16774. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tian, J.; Wang, L.; Sun, X. A method for the production of reduced graphene oxide using benzylamine as a reducing and stabilizing agent and its subsequent decoration with Ag nanoparticles for enzymeless hydrogen peroxide detection. Carbon 2011, 49, 3158–3164. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Yin, J. Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites. ACS Appl. Mater. Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Paredes, J.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascon, J. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Merino, M.; Guardia, L.; Paredes, J.; Villar-Rodil, S.; Solis-Fernandez, P.; Martinez-Alonso, A.; Tascon, J. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved Raman spectroscopy of single- and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Zhao, Y.; Wu, W.; Chen, J.; Meng, H. Green synthesis and photo-catalytic performances for ZnO-reduced graphene oxide nanocomposites. J. Colloid Interface Sci. 2013, 411, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Tien, H.N.; Luan, V.H.; Hoa, L.T.; Khoa, N.T.; Hahn, S.H.; Chung, J.S.; Shin, E.W.; Hur, S.H. One-pot synthesis of a reduced graphene oxide-zinc oxide sphere composite and its use as a visible light photocatalyst. Chem. Eng. J. 2013, 229, 126–133. [Google Scholar] [CrossRef]
- Shervedani, R.K.; Amini, A. Novel graphene-gold hybrid nanostructures constructed via sulfur modified graphene: Preparation and characterization by surface and electrochemical techniques. Electrochim. Acta 2014, 121, 376–385. [Google Scholar] [CrossRef]
- Lee, J.; Novoselov, K.S.; Shin, H.S. Interaction between metal and graphene: Dependence on the layer number of graphene. ACS Nano 2010, 5, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Mabuchi, A.; Hagiwara, R. A new structure model of graphite oxide. Carbon 1988, 26, 357–361. [Google Scholar] [CrossRef]
- Chen, D.; Li, L.; Guo, L. An environment-friendly preparation of reduced graphene oxide nanosheets via amino acid. Nanotechnology 2011, 22, 325601. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhou, Z.; Cao, X.; Liu, S. Electrochemiluminescence immunosensor for ultrasensitive detection of biomarker using Ru(bpy)32+-encapsulated silica nanosphere labels. Anal. Chim. Acta 2010, 665, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, F.J.; Messina, G.A.; Molina, P.G.; Zón, M.A.; Raba, J.; Fernández, H. Determination of progesterone (P4) from bovine serum samples using a microfluidic immunosensor system. Talanta 2010, 80, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Shan, J.; Zhang, Z.; Qing, Y.; Wang, D. The signal-enhanced label-free immunosensor based on assembly of Prussian Blue-SiO2 nanocomposite for amperometric measurement of neuron-specific enolase. Electroanalytical 2010, 22, 2569–2575. [Google Scholar] [CrossRef]
- Han, J.; Zhuo, Y.; Chai, Y.Q.; Yuan, Y.L.; Yuan, R. Novel electrochemical catalysis as signal amplified strategy for label-free detection of neuron-specific enolase. Biosens. Bioelectron. 2012, 31, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Zhang, J.; Zhang, A.; Wang, Y.; Cai, X. Electrochemical Detecting Lung Cancer-Associated Antigen Based on Graphene-Gold Nanocomposite. Molecules 2017, 22, 392. https://doi.org/10.3390/molecules22030392
Wei Z, Zhang J, Zhang A, Wang Y, Cai X. Electrochemical Detecting Lung Cancer-Associated Antigen Based on Graphene-Gold Nanocomposite. Molecules. 2017; 22(3):392. https://doi.org/10.3390/molecules22030392
Chicago/Turabian StyleWei, Zheng, Junping Zhang, Aihua Zhang, Yanchun Wang, and Xiaoping Cai. 2017. "Electrochemical Detecting Lung Cancer-Associated Antigen Based on Graphene-Gold Nanocomposite" Molecules 22, no. 3: 392. https://doi.org/10.3390/molecules22030392




