Bufalin Induces Apoptosis of Human Osteosarcoma U-2 OS Cells through Endoplasmic Reticulum Stress, Caspase- and Mitochondria-Dependent Signaling Pathways
Abstract
:1. Introduction
2. Results
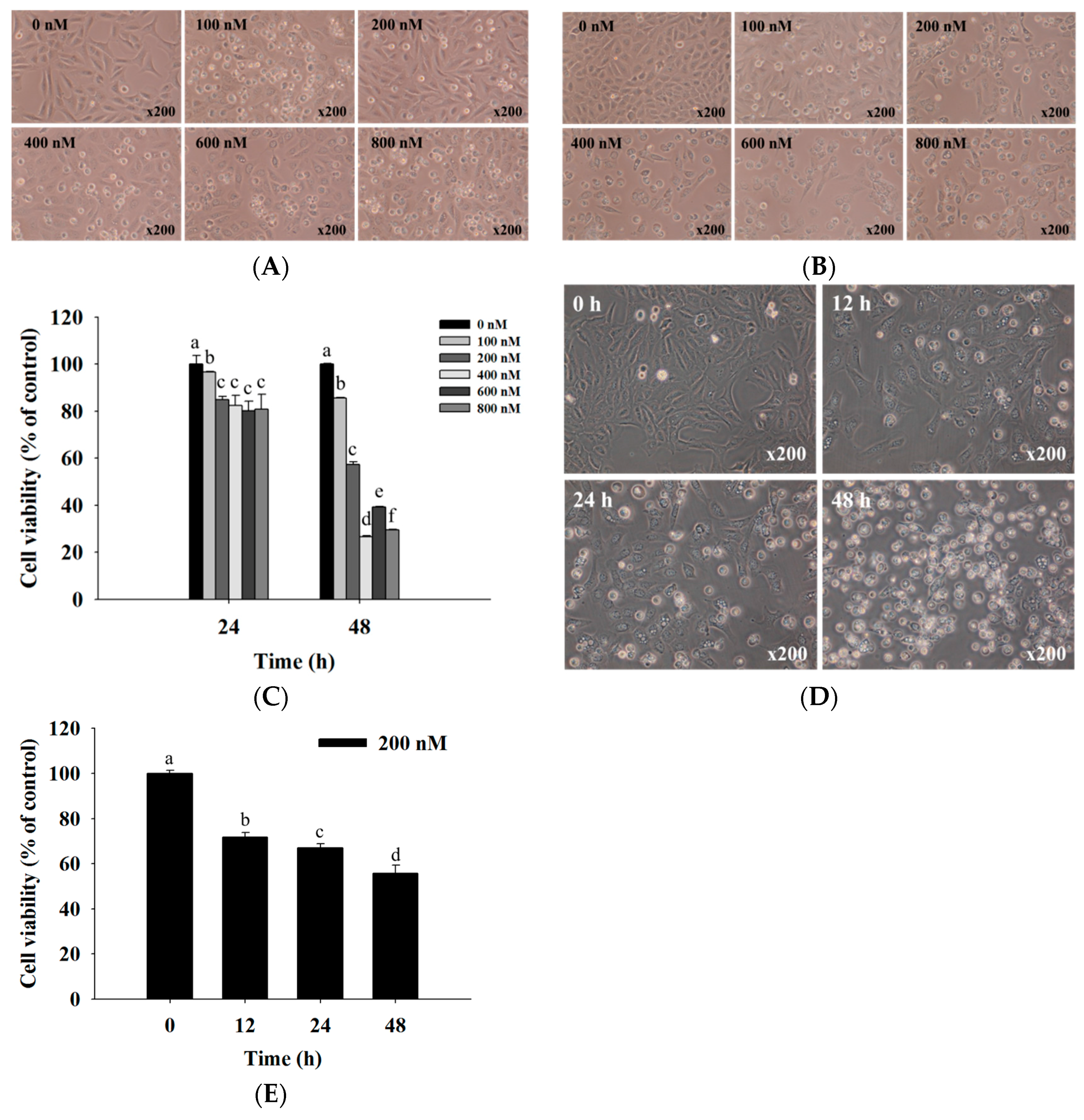
2.1. Bufalin Induced Cell Morphological Changes and Decreased Total Viability of U-2 OS Cells
2.2. Bufalin Induced Cell Cycle Arrest in U-2 OS Cells
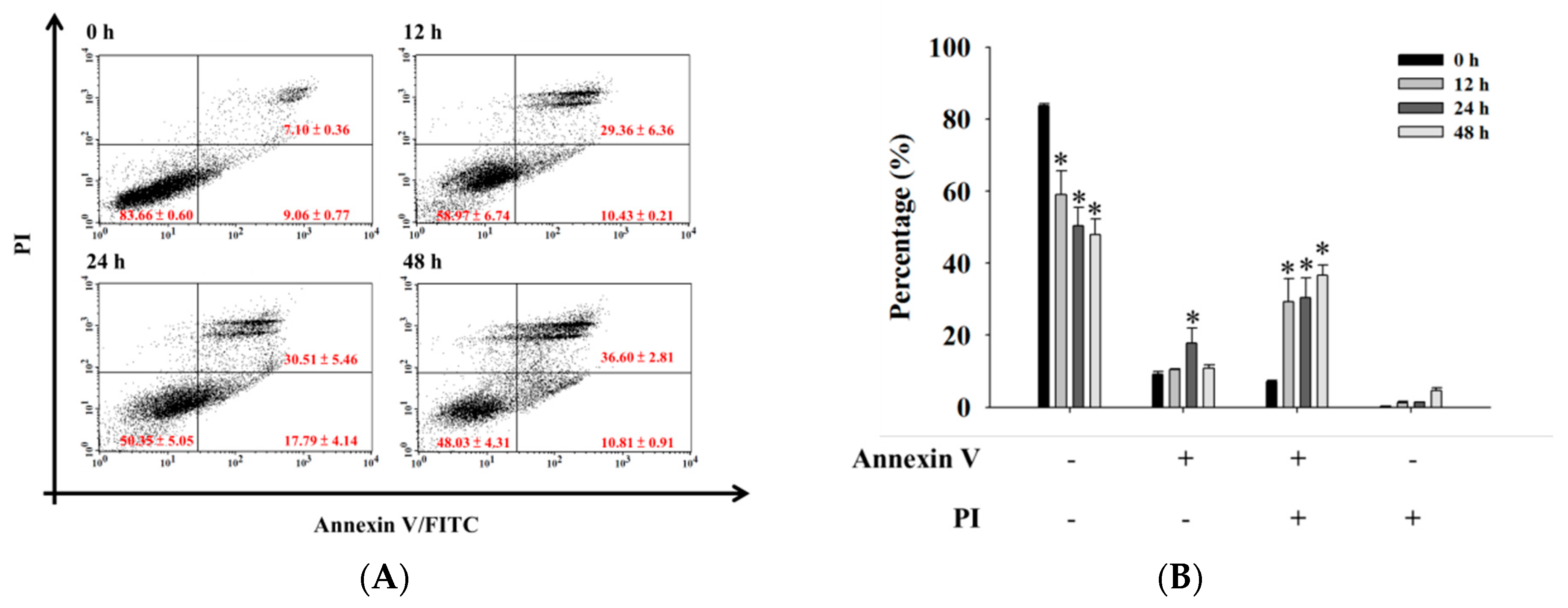
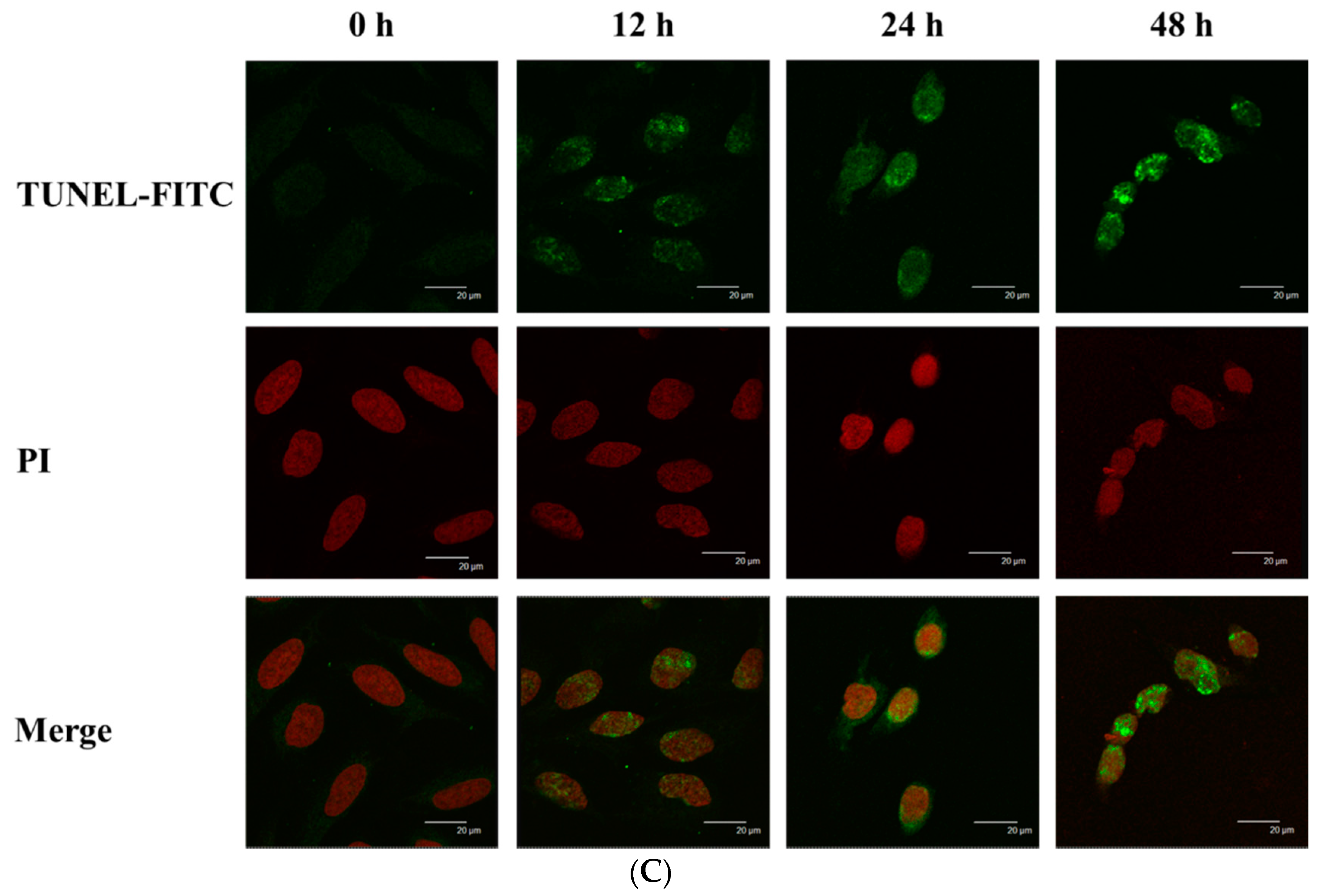
2.3. Bufalin Induced Apoptosis in U-2 OS Cells
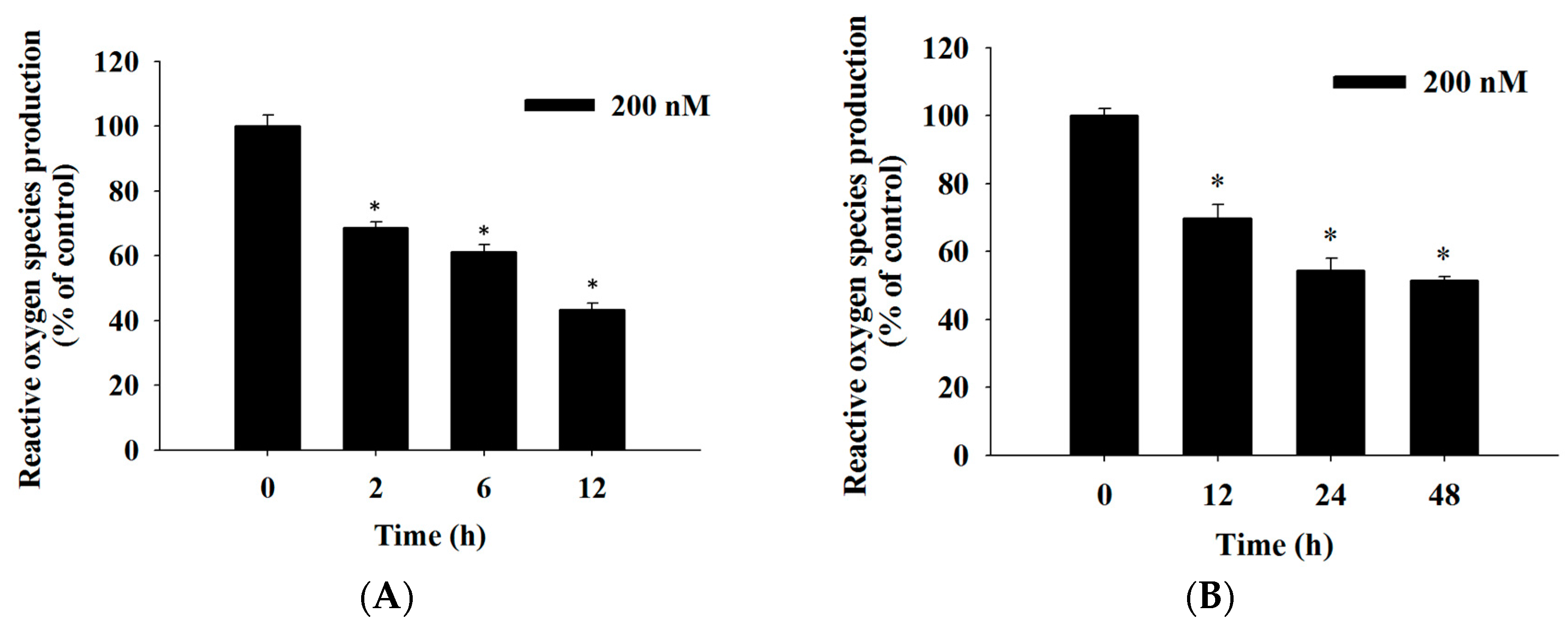
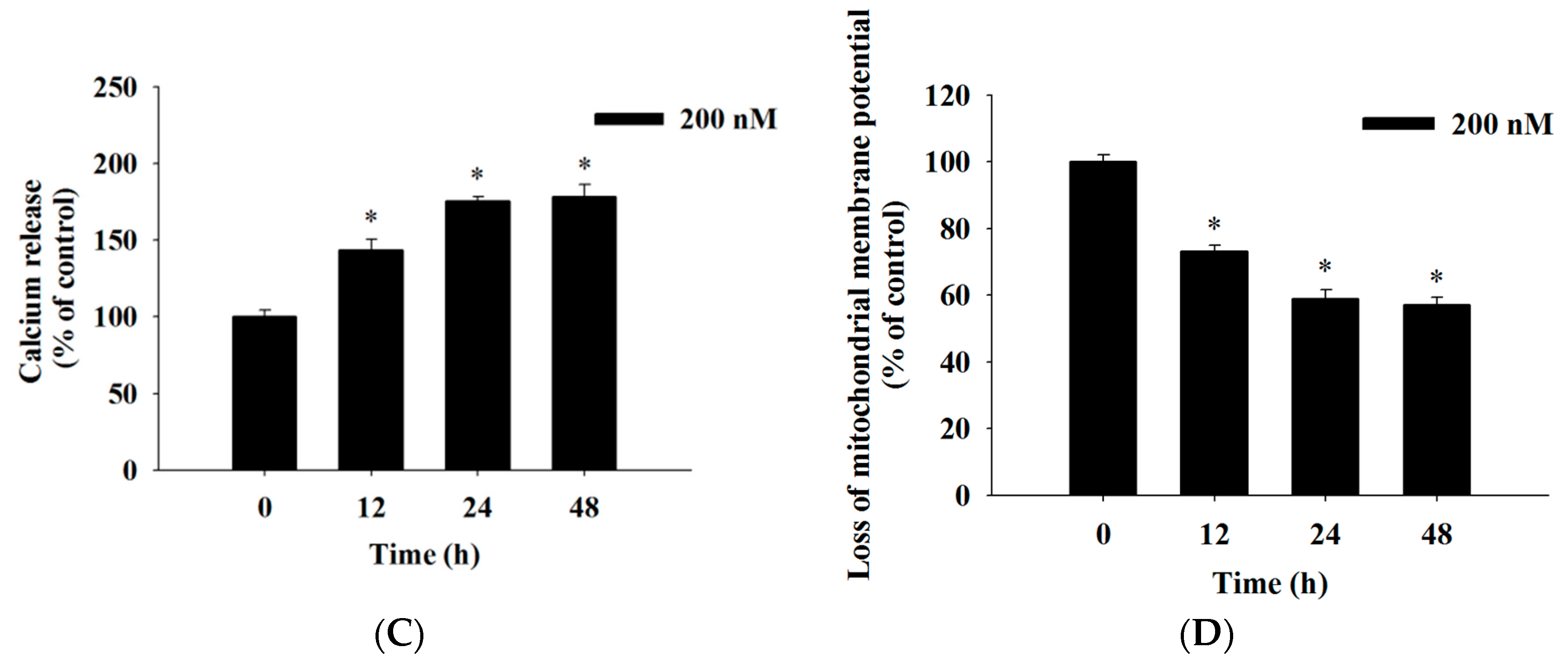
2.4. Bufalin Induced Reactive Oxygen Species and Ca2+ Productions and Decreased the Levels of Mitochondrial Membrane Potential (ΔΨm) in U-2 OS Cells
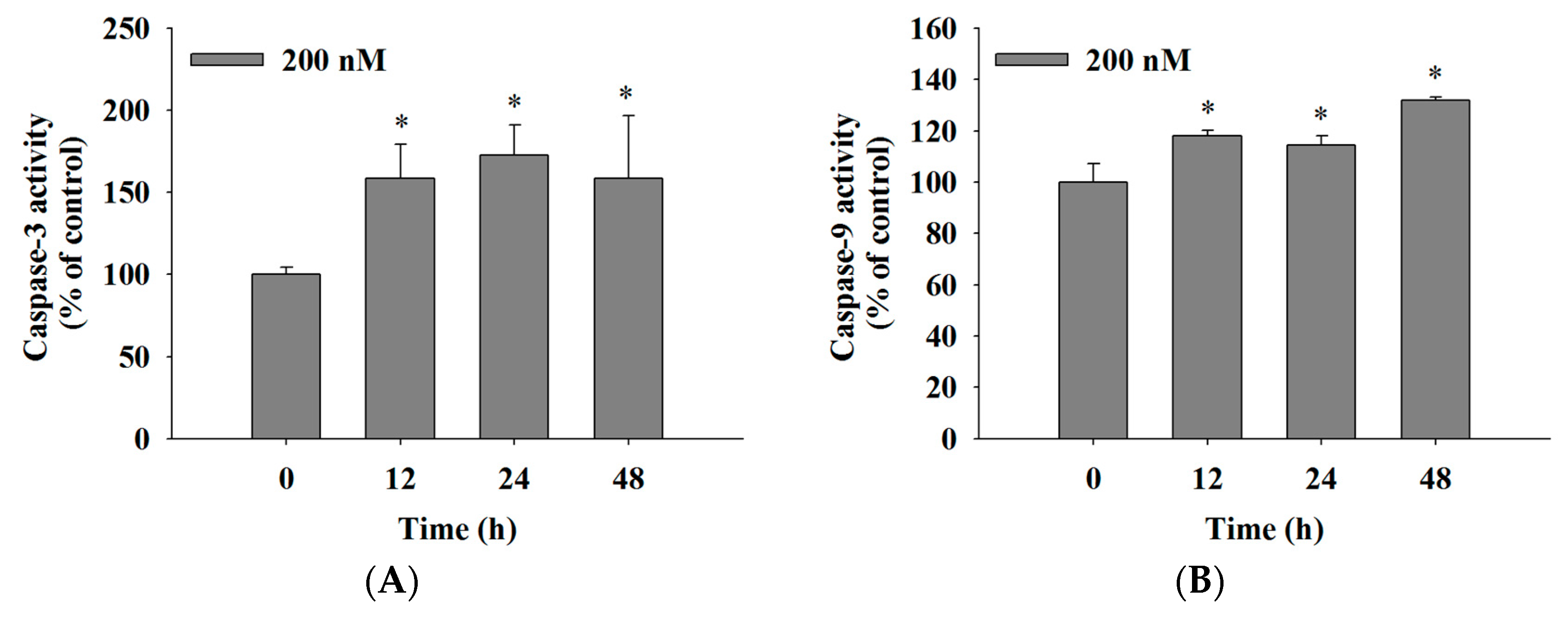
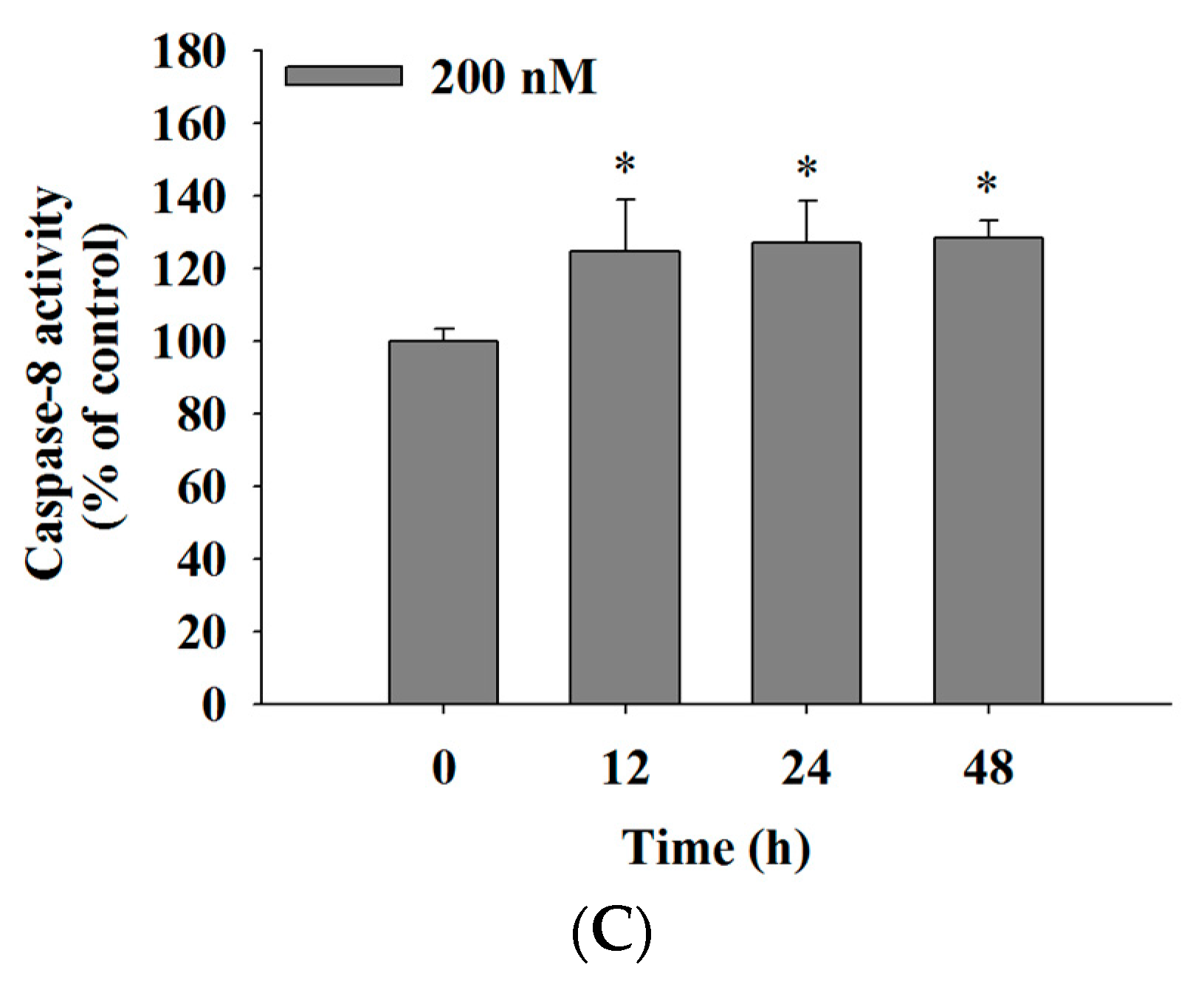
2.5. Bufalin Increased the Activities of Caspase-3, -9 and -8 in U-2 OS Cells
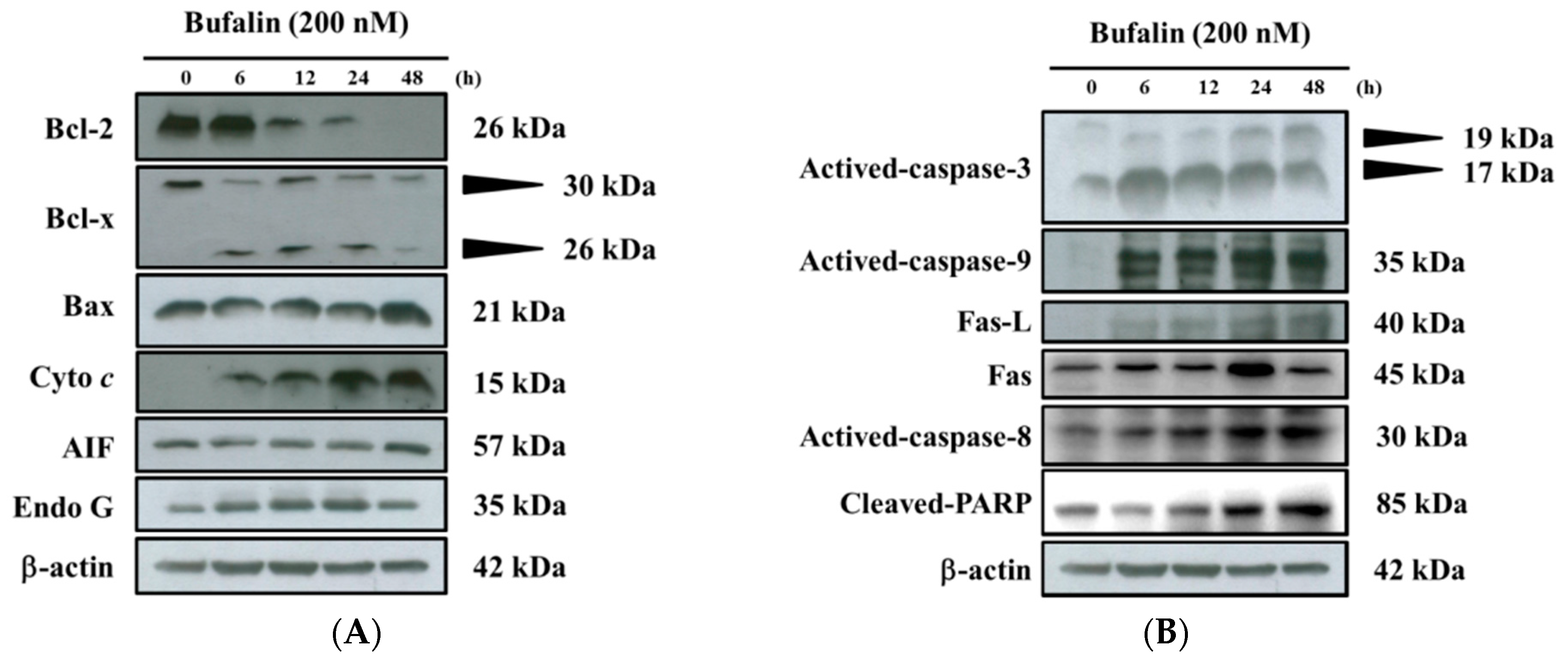
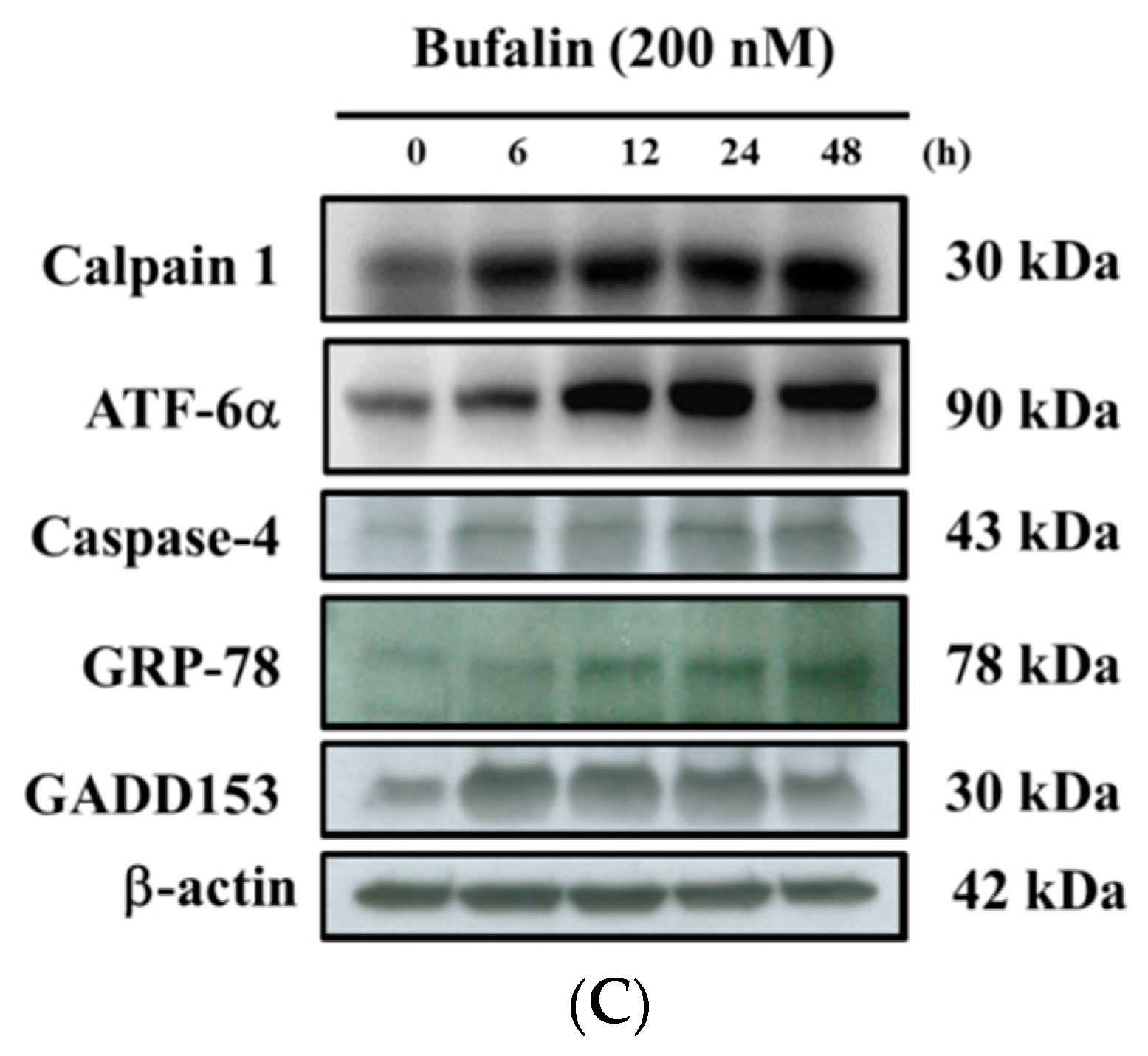
2.6. Bufalin Altered Apoptosis Associated Protein Expression in U-2 OS Cells
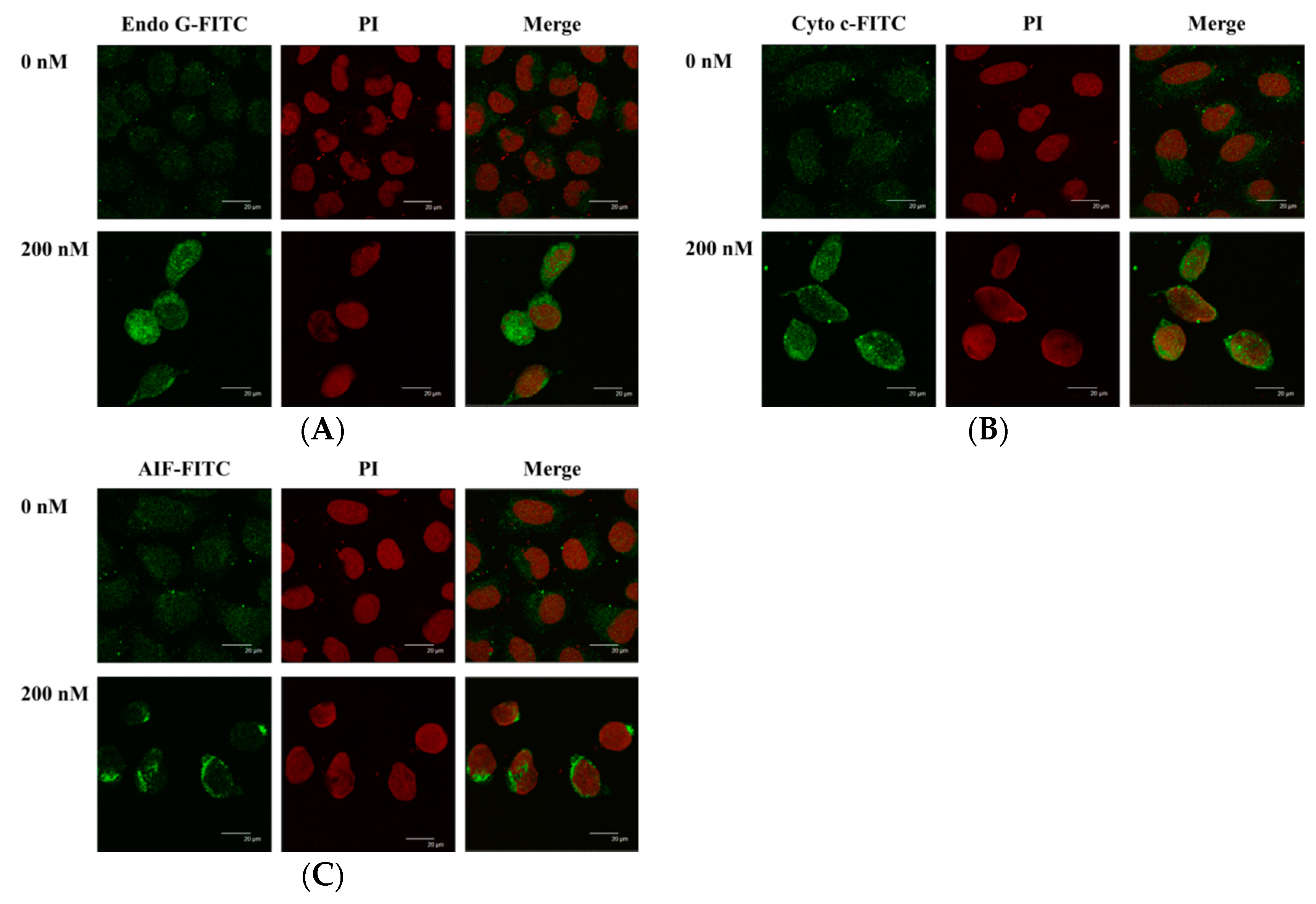
2.7. Bufalin Altered the Translocation of Apoptotic Associated Proteins in U-2 OS Cells
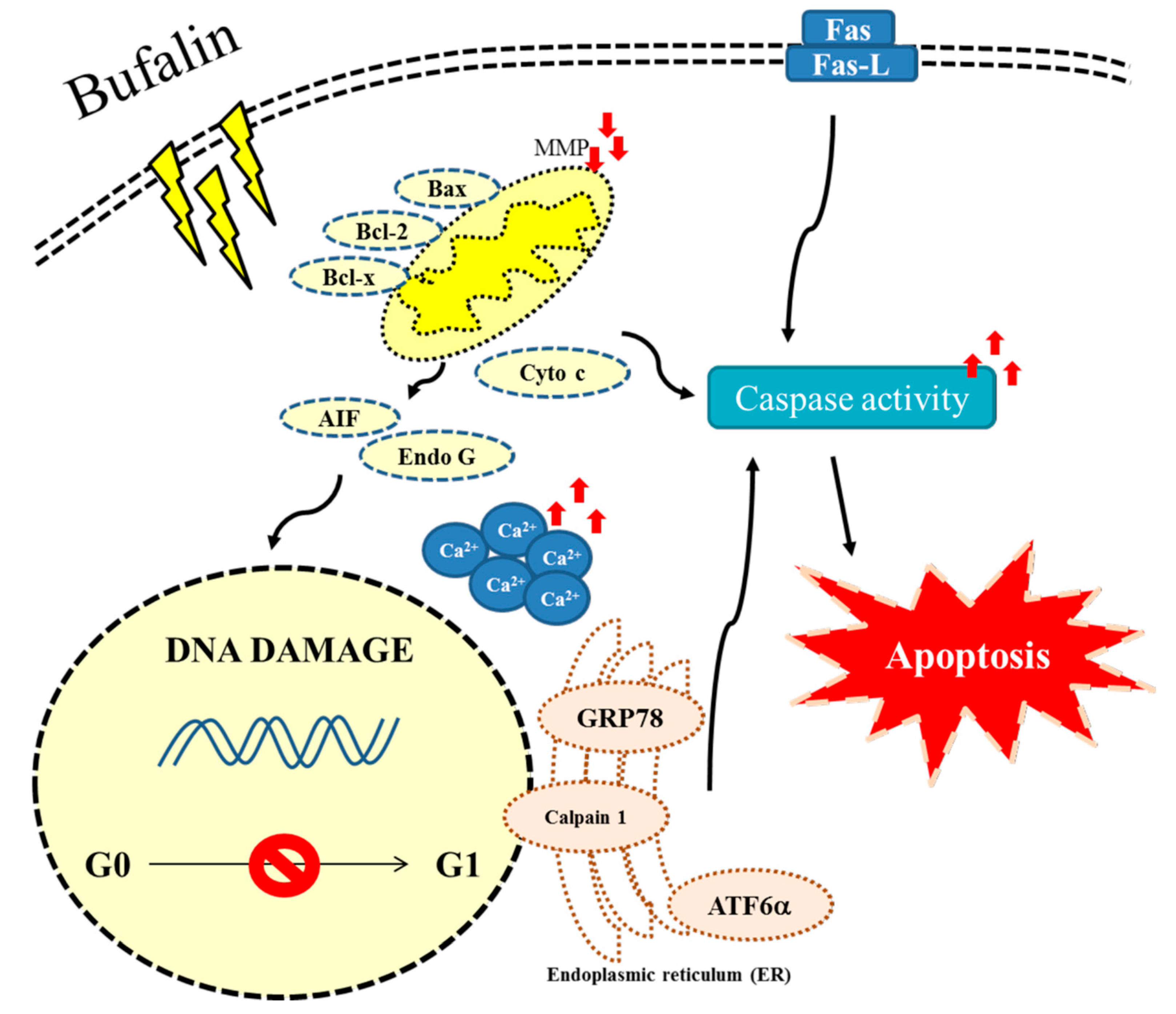
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell’s Morphology Examination and Total Viability Assays
4.4. Cell Cycle Distribution
4.5. Annexin V/PI Staining for Apoptotic Cell Death
4.6. TUNNEL Assay
4.7. Measurements of Reactive Oxygen Species (ROS), Intracellular Ca2+ and Mitochondrial Membrane Potential
4.8. Measurements of Caspase-3, Caspase-8 and Caspase-9 Activities
4.9. Western Blotting Analysis
4.10. Confocal Laser Scanning Microscopy Assay
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franchi, A. Epidemiology and classification of bone tumors. Clin. Cases Miner. Bone Metab. 2012, 9, 92–95. [Google Scholar] [PubMed]
- Fernandes, R.S.; Dos Santos Ferreira, D.; de Aguiar Ferreira, C.; Giammarile, F.; Rubello, D.; de Barros, A.L. Development of imaging probes for bone cancer in animal models. A systematic review. Biomed. Pharmacother. 2016, 83, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society Cancer Facts & Figures 2016. Available online: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf (accessed on 20 December 2016).
- Ministry of Health and Welfare. The Cancer Mortality Report of the Department of Health, Taiwan; Ministry of Health and Welfare: Taipei, Taiwan, 2016.
- Grewal, S.; Merchant, T.; Reymond, R.; McInerney, M.; Hodge, C.; Shearer, P. Auditory late effects of childhood cancer therapy: A report from the Children’s Oncology Group. Pediatrics 2010, 125, e938–e950. [Google Scholar] [CrossRef] [PubMed]
- Papagelopoulos, P.J.; Mavrogenis, A.F.; Badekas, A.; Sim, F.H. Foot malignancies: A multidisciplinary approach. Foot Ankle Clin. 2003, 8, 751–763. [Google Scholar] [CrossRef]
- Petrilli, A.S.; de Camargo, B.; Filho, V.O.; Bruniera, P.; Brunetto, A.L.; Jesus-Garcia, R.; Camargo, O.P.; Pena, W.; Pericles, P.; Davi, A.; et al. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: Prognostic factors and impact on survival. J. Clin. Oncol. 2006, 24, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Cao, Z.; Yu, D.; Fu, S.; Zhang, G.; Yang, P.; Pan, Y.; Yang, B.; Han, H.; Zhou, Q. Columbamine suppresses the proliferation and neovascularization of metastatic osteosarcoma U2OS cells with low cytotoxicity. Toxicol. Lett. 2012, 215, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Verheij, M.; Vens, C.; van Triest, B. Novel therapeutics in combination with radiotherapy to improve cancer treatment: Rationale, mechanisms of action and clinical perspective. Drug Resist. Update 2010, 13, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Mak, T.W. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat. Rev. Cancer 2004, 4, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Philchenkov, A. Caspases: Potential targets for regulating cell death. J. Cell. Mol. Med. 2004, 8, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.M.; Eslami, H.; Larijani, B.; Davoodi, J. Involvement of caspase-8, -9, and -3 in high glucose-induced apoptosis in PC12 cells. Neurosci. Lett. 2009, 459, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Lien, J.C.; Chen, Y.Y.; Hsu, S.C.; Chang, S.J.; Huang, A.C.; Amagaya, S.; Funayana, S.; Wood, W.G.; Kuo, C.L.; et al. Crude extract of Euphorbia formosana induces apoptosis of DU145 human prostate cancer cells acts through the caspase-dependent and independent signaling pathway. Environ. Toxicol. 2016, 31, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.P.; Chung, H.W.; Liu, K.C.; Wu, R.S.; Yang, J.S.; Tang, N.Y.; Lo, C.; Hsia, T.C.; Yu, C.C.; Chueh, F.S.; et al. Diallyl sulfide induces cell cycle arrest and apoptosis in HeLa human cervical cancer cells through the p53, caspase- and mitochondria-dependent pathways. Int. J. Oncol. 2011, 38, 1605–1613. [Google Scholar] [PubMed]
- Takai, N.; Kira, N.; Ishii, T.; Yoshida, T.; Nishida, M.; Nishida, Y.; Nasu, K.; Narahara, H. Bufalin, a traditional oriental medicine, induces apoptosis in human cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.H.; Xu, Z.Y.; Gong, Y.B.; Wang, L.F.; Wang, Z.Q.; Xu, L.; Cao, F.; Liao, M.J. Bufalin Reverses HGF-Induced Resistance to EGFR-TKIs in EGFR Mutant Lung Cancer Cells via Blockage of Met/PI3k/Akt Pathway and Induction of Apoptosis. Evid. Based Complement. Altern. Med. 2013, 2013, 243859. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.P.; Yu, C.S.; Yu, C.C.; Yang, J.S.; Chiang, J.H.; Lu, C.C.; Huang, H.Y.; Tang, N.Y.; Yang, J.H.; Huang, A.C.; et al. Triggering apoptotic death of human malignant melanoma a375.s2 cells by bufalin: Involvement of caspase cascade-dependent and independent mitochondrial signaling pathways. Evid. Based Complement. Altern. Med. 2012, 2012, 591241. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Qu, X.; Xu, C.; Zhu, Z.; Zhang, L.; Xu, L.; Song, N.; Teng, Y.; Liu, Y. Down-regulation of Cbl-b by bufalin results in up-regulation of DR4/DR5 and sensitization of TRAIL-induced apoptosis in breast cancer cells. J. Cancer Res. Clin. Oncol. 2012, 138, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.C.; Yang, J.S.; Peng, S.F.; Lu, C.C.; Chiang, J.H.; Chung, J.G.; Lin, M.W.; Lin, J.K.; Amagaya, S.; Wai-Shan Chung, C.; et al. Bufalin increases sensitivity to AKT/mTOR-induced autophagic cell death in SK-HEP-1 human hepatocellular carcinoma cells. Int. J. Oncol. 2012, 41, 1431–1442. [Google Scholar] [PubMed]
- Li, D.; Qu, X.; Hou, K.; Zhang, Y.; Dong, Q.; Teng, Y.; Zhang, J.; Liu, Y. PI3K/Akt is involved in bufalin-induced apoptosis in gastric cancer cells. Anti-Cancer Drugs 2009, 20, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, Y.; Wang, Z.; Liu, R.; Gong, X. Bufalin induces the interplay between apoptosis and autophagy in glioma cells through endoplasmic reticulum stress. Int. J. Biol. Sci. 2014, 10, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Zhao, Y.F.; Cao, Y.L.; Gu, W.; Pang, J.; Zhan, H.S. Bufalin exerts inhibitory effects on IL-1beta-mediated proliferation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation 2014, 37, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Wu, T.Y.; Hsiao, Y.T.; Lin, J.H.; Hsu, S.C.; Hsia, T.C.; Yang, S.T.; Hsu, W.H.; Chung, J.G. Bufalin induces cell death in human lung cancer cells through disruption of DNA damage response pathways. Am. J. Chin. Med. 2014, 42, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Q.; Shen, J.N.; Su, W.W.; Wang, J.; Huang, G.; Jin, S.; Guo, Q.C.; Zou, C.Y.; Li, H.M.; Li, F.B. Bufalin induces apoptosis in human osteosarcoma U-2 OS and U-2 OS methotrexate300-resistant cell lines. Acta Pharmacol. Sin. 2007, 28, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, M.; Li, Z.; Gao, P.; Zhou, X.; Zhang, J. Bufalin induces apoptosis in the U-2 OS human osteosarcoma cell line via triggering the mitochondrial pathway. Mol. Med. Rep. 2016, 13, 817–822. [Google Scholar] [PubMed]
- Zhang, J.; Sha, J.; Zhou, Y.; Han, K.; Wang, Y.; Su, Y.; Yin, X.; Hu, H.; Yao, Y. Bufalin Inhibits Proliferation and Induces Apoptosis in Osteosarcoma Cells by Downregulating MicroRNA-221. Evid. Based Complement. Altern. Med. 2016, 2016, 7319464. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bi, Z. Bufalin inhibited the growth of human osteosarcoma MG-63 cells via down-regulation of Bcl-2/Bax and triggering of the mitochondrial pathway. Tumour Biol. 2014, 35, 4885–4890. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, J.Q.; Jia, Q.; Shen, J.N.; Huang, G.; Xie, X.B.; Zou, C.Y. Bufalin induces apoptosis in osteosarcoma U-2 OS and U-2 OS methotrexate 300-resistant cell lines in vitro. Zhonghua Zhong Liu Za Zhi 2010, 32, 734–738. [Google Scholar] [PubMed]
- Xie, X.B.; Yin, J.Q.; Wen, L.L.; Gao, Z.H.; Zou, C.Y.; Wang, J.; Huang, G.; Tang, Q.L.; Colombo, C.; He, W.L.; et al. Critical role of heat shock protein 27 in bufalin-induced apoptosis in human osteosarcomas: A proteomic-based research. PLoS ONE 2012, 7, e47375. [Google Scholar] [CrossRef] [PubMed]
- Numazawa, S.; Shinoki, M.A.; Ito, H.; Yoshida, T.; Kuroiwa, Y. Involvement of Na+,K(+)-ATPase inhibition in K562 cell differentiation induced by bufalin. J. Cell. Physiol. 1994, 160, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Watabe, M.; Hashimoto, S.; Nakajo, S.; Nakaya, K. Cell cycle arrest and protein kinase modulating effect of bufalin on human leukemia ML1 cells. Anticancer Res. 1994, 14, 1193–1198. [Google Scholar] [PubMed]
- Hong, S.H.; Choi, Y.H. Bufalin induces apoptosis through activation of both the intrinsic and extrinsic pathways in human bladder cancer cells. Oncol. Rep. 2012, 27, 114–120. [Google Scholar] [PubMed]
- Hsia, T.C.; Yu, C.C.; Hsu, S.C.; Tang, N.Y.; Lu, H.F.; Huang, Y.P.; Wu, S.H.; Lin, J.G.; Chung, J.G. Cantharidin induces apoptosis of H460 human lung cancer cells through mitochondria-dependent pathways. Int. J. Oncol. 2014, 45, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Chang, M.Y.; Wang, M.J.; Liu, H.C.; Chang, S.J.; Harnod, T.; Hung, C.H.; Lee, H.T.; Shen, C.C.; Chung, J.G. Phenethyl isothiocyanate alters the gene expression and the levels of protein associated with cell cycle regulation in human glioblastoma GBM 8401 cells. Environ. Toxicol. 2017, 32, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS Metabolism in Cancer Cells: The Potential Role of Quercetin. Cancers 2010, 2, 1288–1311. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Update 2004, 7, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Azad, M.B.; Gibson, S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009, 16, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.S.; Boyman, L.; Chikando, A.C.; Khairallah, R.J.; Lederer, W.J. Mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA 2013, 110, 10479–10486. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kaufman, R.J. The impact of the unfolded protein response on human disease. J. Cell Biol. 2012, 197, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.W.; Yang, J.S.; Pai, S.J.; Wu, P.P.; Chang, S.J.; Chueh, F.S.; Fan, M.J.; Chiou, S.M.; Kuo, H.M.; Yeh, C.C.; et al. Bufalin induces G0/G1 phase arrest through inhibiting the levels of cyclin D, cyclin E, CDK2 and CDK4, and triggers apoptosis via mitochondrial signaling pathway in T24 human bladder cancer cells. Mutat. Res. 2012, 732, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Abdelwahab, S.I.; Kamalidehghan, B.; Syam, S.; May, K.S.; Harmal, N.S.; Shafifiyaz, N.; Hadi, A.H.; Hashim, N.M.; Rahmani, M.; et al. Involvement of NF-kappaB and Bcl2/Bax signaling pathways in the apoptosis of MCF7 cells induced by a xanthone compound Pyranocycloartobiloxanthone A. Phytomedicine 2012, 19, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, L.; Wei, T.; Zhao, Y.; Chen, C. The inhibition of death receptor mediated apoptosis through lysosome stabilization following internalization of carboxyfullerene nanoparticles. Biomaterials 2011, 32, 4030–4041. [Google Scholar] [CrossRef] [PubMed]
- Grunnet, L.G.; Aikin, R.; Tonnesen, M.F.; Paraskevas, S.; Blaabjerg, L.; Storling, J.; Rosenberg, L.; Billestrup, N.; Maysinger, D.; Mandrup-Poulsen, T. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes 2009, 58, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Santin, I.; Moore, F.; Colli, M.L.; Gurzov, E.N.; Marselli, L.; Marchetti, P.; Eizirik, D.L. PTPN2, a candidate gene for type 1 diabetes, modulates pancreatic beta-cell apoptosis via regulation of the BH3-only protein Bim. Diabetes 2011, 60, 3279–3288. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, Y.B.; Sun, J.Y.; Xiang, Y.; Yang, J.; Dai, S.Y.; Zhang, X. Neuroglobin Attenuates Beta Amyloid-Induced Apoptosis Through Inhibiting Caspases Activity by Activating PI3K/Akt Signaling Pathway. J. Mol. Neurosci. 2016, 58, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.W.; Chiu, Y.J.; Fan, M.J.; Lu, H.F.; Yeh, H.F.; Li, K.H.; Chen, P.Y.; Chung, J.G.; Yang, J.S. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol. Nutr. Food Res. 2010, 54, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chuang, Y.J.; Yu, C.C.; Yang, J.S.; Lu, C.C.; Chiang, J.H.; Lin, J.P.; Tang, N.Y.; Huang, A.C.; Chung, J.G. Apigenin induces apoptosis through mitochondrial dysfunction in U-2 OS human osteosarcoma cells and inhibits osteosarcoma xenograft tumor growth in vivo. J. Agric. Food Chem. 2012, 60, 11395–11402. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.; Hsu, S.C.; Chueh, F.S.; Chen, Y.Y.; Yang, J.S.; Lin, J.P.; Lien, J.C.; Tsai, C.H.; Chung, J.G. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-kappaB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013, 33, 1941–1950. [Google Scholar] [PubMed]
- Chueh, F.S.; Chen, Y.L.; Hsu, S.C.; Yang, J.S.; Hsueh, S.C.; Ji, B.C.; Lu, H.F.; Chung, J.G. Triptolide induced DNA damage in A375.S2 human malignant melanoma cells is mediated via reduction of DNA repair genes. Oncol. Rep. 2013, 29, 613–618. [Google Scholar] [PubMed]
- Ho, C.C.; Huang, A.C.; Yu, C.S.; Lien, J.C.; Wu, S.H.; Huang, Y.P.; Huang, H.Y.; Kuo, J.H.; Liao, W.Y.; Yang, J.S.; et al. Ellagic acid induces apoptosis in TSGH8301 human bladder cancer cells through the endoplasmic reticulum stress- and mitochondria-dependent signaling pathways. Environ. Toxicol. 2014, 29, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Ghosh, S.; Sil, P.C. Amelioration of aspirin induced oxidative impairment and apoptotic cell death by a novel antioxidant protein molecule isolated from the herb Phyllanthus niruri. PLoS ONE 2014, 9, e89026. [Google Scholar]
- Liu, K.C.; Yen, C.Y.; Wu, R.S.; Yang, J.S.; Lu, H.F.; Lu, K.W.; Lo, C.; Chen, H.Y.; Tang, N.Y.; Wu, C.C.; et al. The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells. Environ. Toxicol. 2014, 29, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Wu, L.W.; Huang, A.C.; Yu, C.C.; Lien, J.C.; Huang, Y.P.; Yang, J.S.; Yang, J.H.; Hsiao, Y.P.; Wood, W.G.; et al. Benzyl isothiocyanate (BITC) induces G2/M phase arrest and apoptosis in human melanoma A375.S2 cells through reactive oxygen species (ROS) and both mitochondria-dependent and death receptor-mediated multiple signaling pathways. J. Agric. Food Chem. 2012, 60, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Ho, T.-J.; Lin, T.-H.; Hsu, W.-Y.; Huang, S.-M.; Liao, C.-C.; Lai, C.-H.; Liu, X.; Tsang, H.; Lai, C.-C.; et al. P-coumaric acid regulates exon 12 splicing of the ATP7B gene by modulating hnRNP A1 protein expressions. Biomedicine 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.M.; Velmurugan, B.K.; Kuo, W.W.; Chen, Y.S.; Ho, T.J.; Tsai, C.T.; Ye, C.X.; Tsai, C.H.; Tsai, F.J.; Huang, C.Y. Inhibitory effect of alpinate Oxyphyllae fructus extracts on Ang II-induced cardiac pathological remodeling-related pathways in H9c2 cardiomyoblast cells. Biomedicine 2013, 3, 148–152. [Google Scholar] [CrossRef]
- Ma, Y.S.; Weng, S.W.; Lin, M.W.; Lu, C.C.; Chiang, J.H.; Yang, J.S.; Lai, K.C.; Lin, J.P.; Tang, N.Y.; Lin, J.G.; et al. Antitumor effects of emodin on LS1034 human colon cancer cells in vitro and in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts model. Food Chem. Toxicol. 2012, 50, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds Bufalin are not available from the authors.

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-H.; Shih, Y.-L.; Lee, M.-H.; Au, M.-K.; Chen, Y.-L.; Lu, H.-F.; Chung, J.-G. Bufalin Induces Apoptosis of Human Osteosarcoma U-2 OS Cells through Endoplasmic Reticulum Stress, Caspase- and Mitochondria-Dependent Signaling Pathways. Molecules 2017, 22, 437. https://doi.org/10.3390/molecules22030437
Lee C-H, Shih Y-L, Lee M-H, Au M-K, Chen Y-L, Lu H-F, Chung J-G. Bufalin Induces Apoptosis of Human Osteosarcoma U-2 OS Cells through Endoplasmic Reticulum Stress, Caspase- and Mitochondria-Dependent Signaling Pathways. Molecules. 2017; 22(3):437. https://doi.org/10.3390/molecules22030437
Chicago/Turabian StyleLee, Ching-Hsiao, Yung-Luen Shih, Mei-Hui Lee, Man-Kuan Au, Yung-Liang Chen, Hsu-Feng Lu, and Jing-Gung Chung. 2017. "Bufalin Induces Apoptosis of Human Osteosarcoma U-2 OS Cells through Endoplasmic Reticulum Stress, Caspase- and Mitochondria-Dependent Signaling Pathways" Molecules 22, no. 3: 437. https://doi.org/10.3390/molecules22030437
APA StyleLee, C.-H., Shih, Y.-L., Lee, M.-H., Au, M.-K., Chen, Y.-L., Lu, H.-F., & Chung, J.-G. (2017). Bufalin Induces Apoptosis of Human Osteosarcoma U-2 OS Cells through Endoplasmic Reticulum Stress, Caspase- and Mitochondria-Dependent Signaling Pathways. Molecules, 22(3), 437. https://doi.org/10.3390/molecules22030437





