Barbatic Acid Offers a New Possibility for Control of Biomphalaria Glabrata and Schistosomiasis
Abstract
:1. Introduction
2. Results
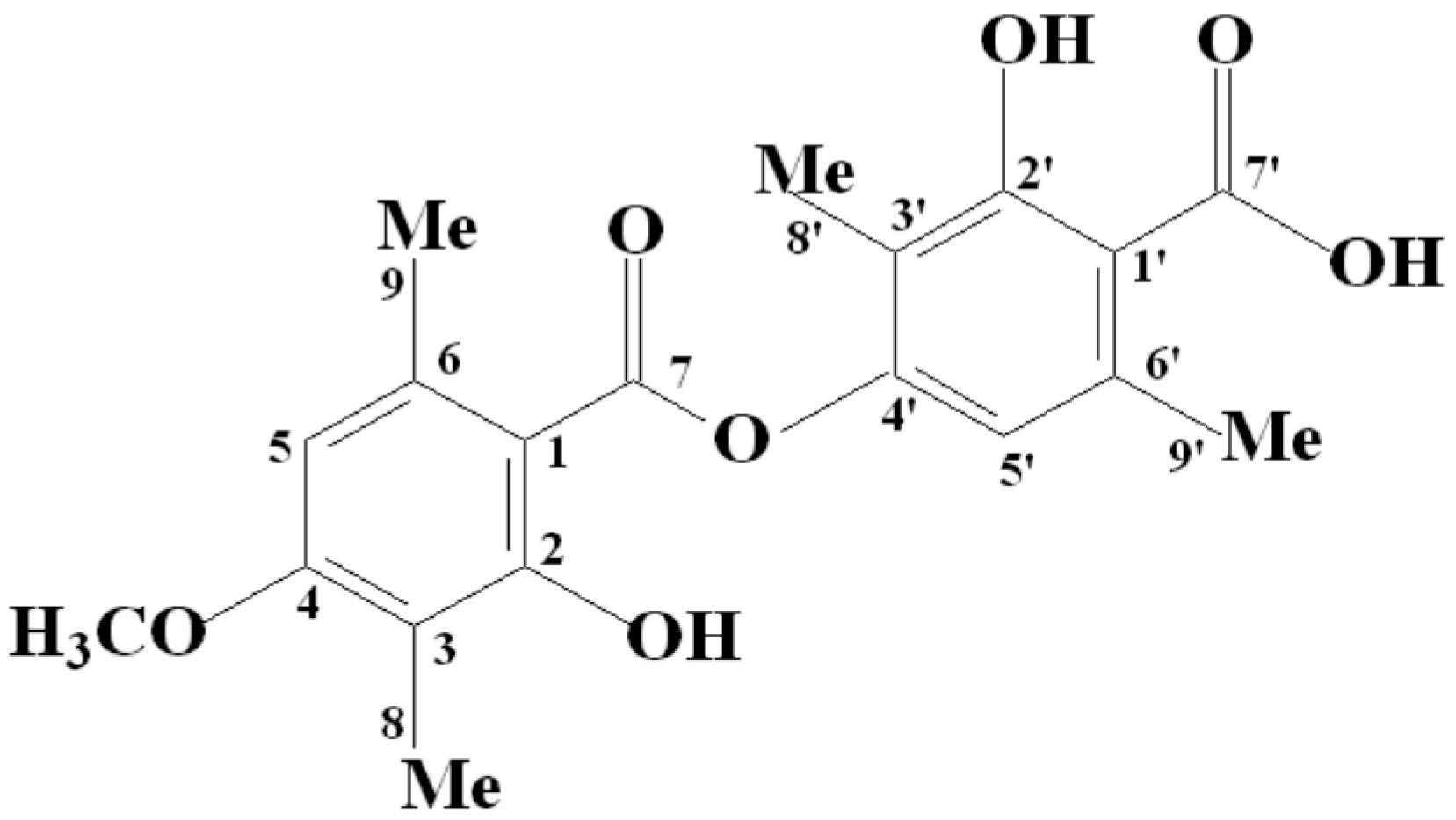
2.1. Chemical Analysis
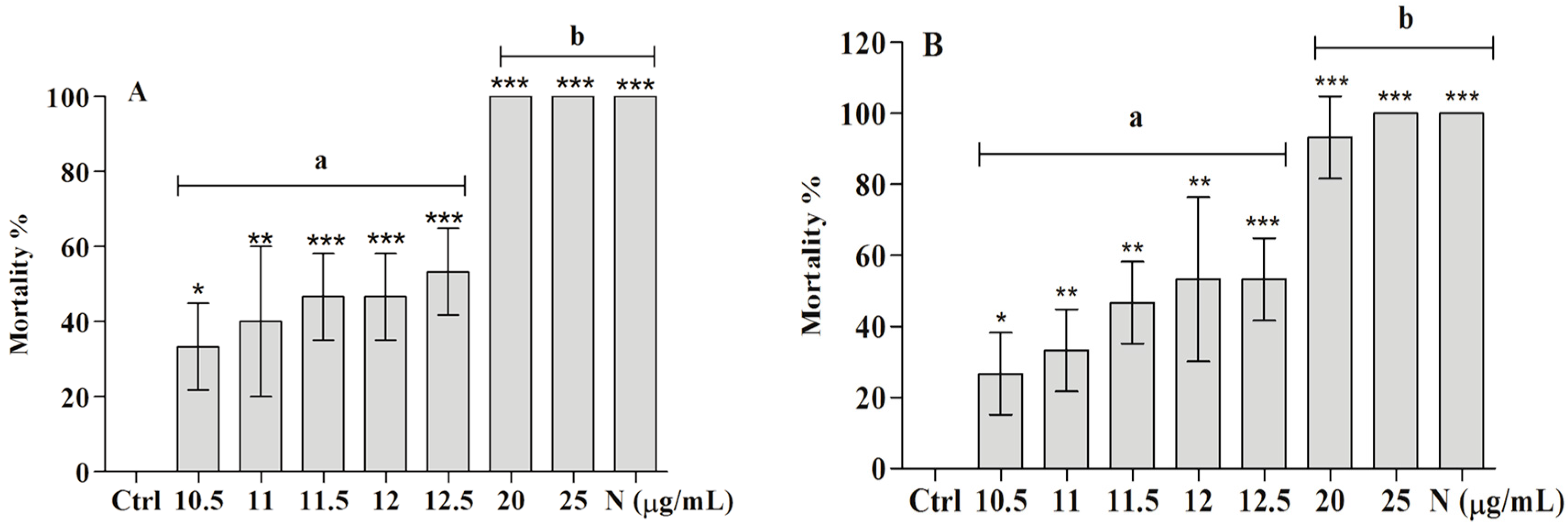
2.2. Toxicity of the Ether Extract of And BAR from C. aggregata on Embryo and Adult Mollusks
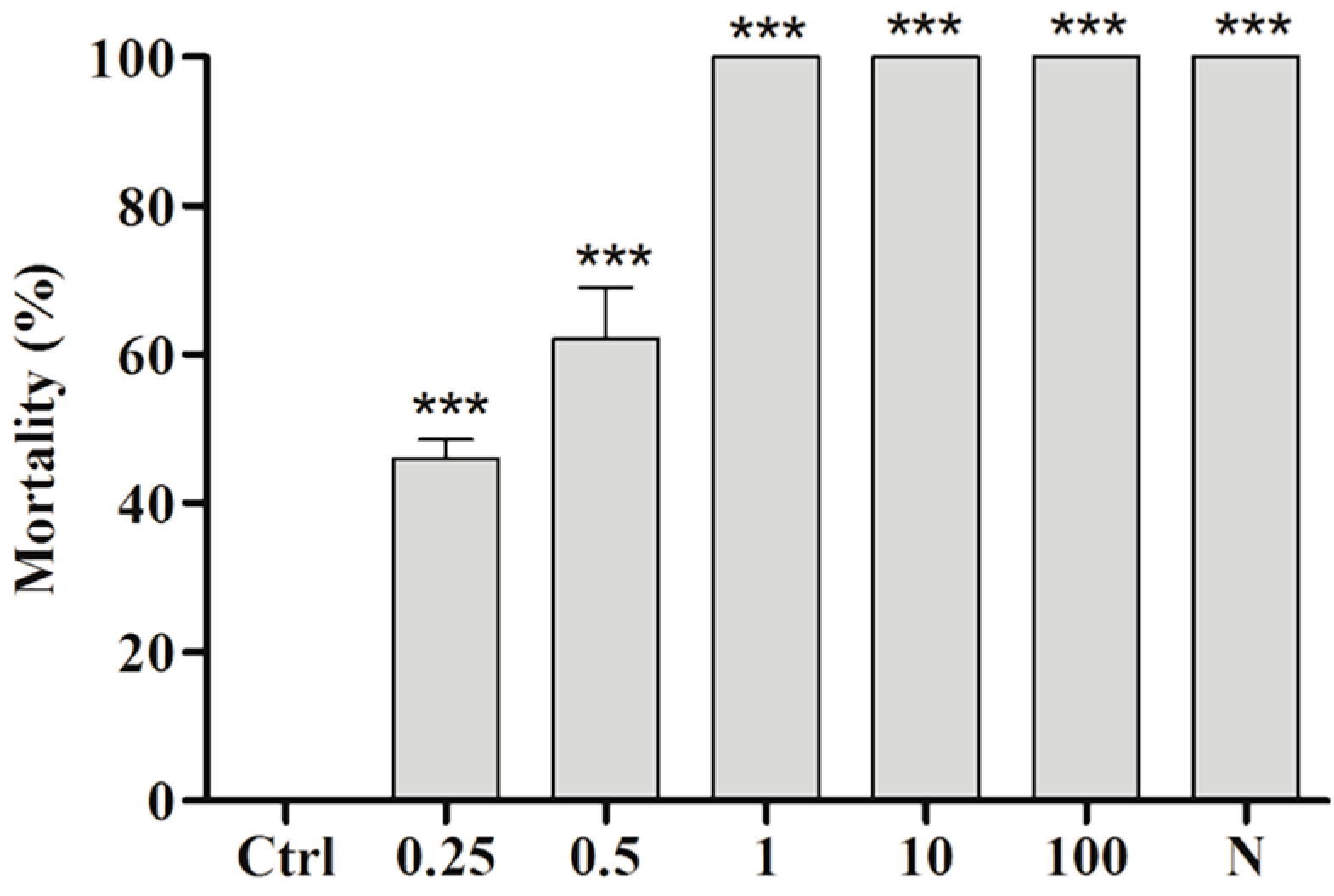
2.3. Toxicity of BAR from C. aggregata to S. mansoni Cercariae
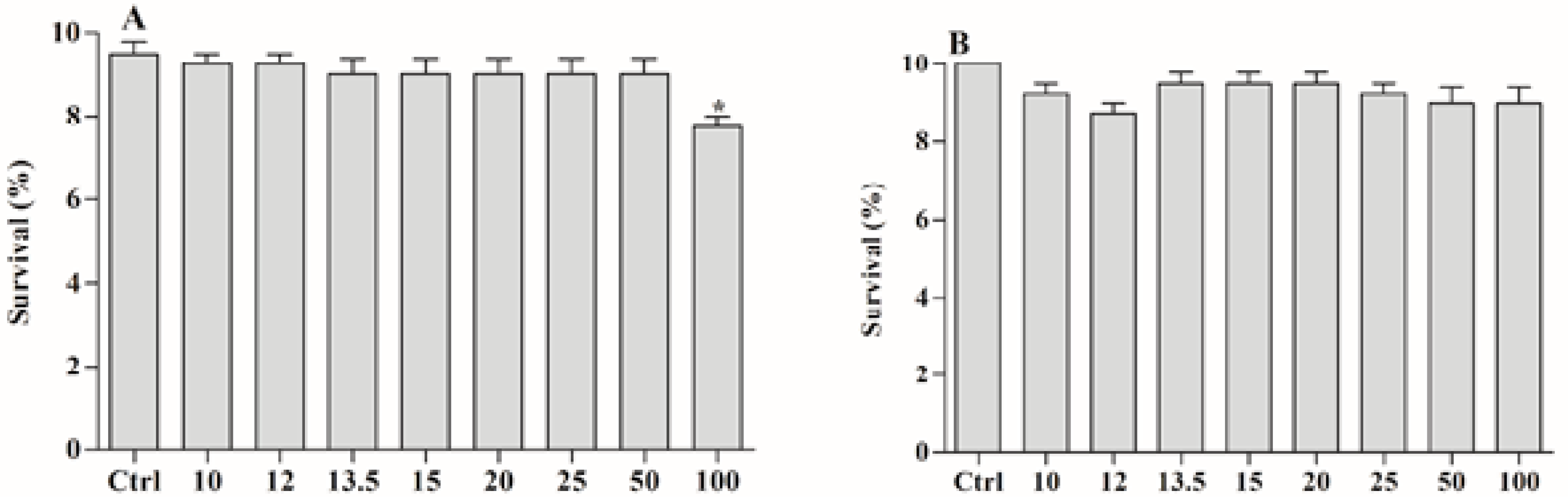
2.4. Toxicity of the Ether Extract of and BAR from C. aggregata against A. salina
3. Discussion
4. Materials and Methods
4.1. Extract Production and Purification of BAR from Cladia aggregata (Sw.) Nyl.
4.2. Mollusks
4.3. Embryotoxic Activity of the Ether Extract of and BAR from Cladia aggregata
4.4. Molluscicidal Activity of the Ether Extract of and BAR from Cladia aggregata
4.5. Cercaricidal Activity of the Ether Extract of and BAR from C. aggregata
4.6. Environmental Toxicity Assays with Artemia salina
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Media Centre Schistosomiais; World Health Organization: Geneva, Switzerland, 2015; Volume 15, pp. 1–8. [Google Scholar]
- Rapado, L.N.; Pinheiro, A.S.; Lopes, P.O.M.V.; Fokoue, H.H.; Scotti, M.T.; Marques, J.V.; Ohlweiler, F.P.; Borrely, S.I.; Pereira, C.A.B.; Kato, M.J.; et al. Schistosomiasis Control Using Pirplatine against Biomphalaria glabrata at Different Developmental Stages. PLoS Negl. Trop. Dis. 2013, 7, e2251. [Google Scholar] [CrossRef] [PubMed]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiais. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- World Health Organization. The Control of Schistosomiasis; WHO Technical Report; WHO: Geneva, Switzerland, 1993; p. 86. [Google Scholar]
- Chen, Y.Q.X.; Qiong, M.; Liu, Y.L.; Li, X.R.; Yang, S.L.; Zhuge, H.X. Laboratory evaluation of the molluscicidal Activity of Pulsatilla Chinensis (Bunge) Regel Saponins against the Snail Oncomelania Hupensis. Biomed. Environ. Sci. 2012, 25, 224–229. [Google Scholar] [PubMed]
- Lima, N.M.F.; Santos, A.F.; Porfírio, Z.; Goulart, M.O.F.; Sant’Ana, A.E.G. Toxicity of lapachol and isolapachol and their potassium salts against Biomphalaria glabrata, Schistosoma mansoni carcarie, Artemia salina and Tilapia nilotica. Acta Trop. 2002, 83, 43–47. [Google Scholar] [CrossRef]
- Guo, D.; Chen, J.; Du, X.; Han, B. Screening of molluscicidal strain against Oncomelania hupensis from the rhizosphere of medicinal plant Phytolacca acinosa Roxb. Pharmacogn. Mag. 2010, 6, 159–165. [Google Scholar] [PubMed]
- Miyasato, P.A.; Kawano, T.; Freitas, J.C.; Berlinck, R.G.S.; Nakano, E.; Tallarico, L.F. Molluscicidal activity of some marine substances against the snail Biomphalaria glabrata (Mollusca, Planorbidae). Parasitol. Res. 2012, 110, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L.; Hill, D.J. The Lichen-Forming Fungi, 1st ed.; Glasgov, Blackie: Glasgov, UK, 1984; p. 158. [Google Scholar]
- Martins, M.C.B.; Lima, M.J.G.; Silva, F.P.; Azevedo-Ximenes, E.; Silva, N.H.; Pereira, E.C. Cladia aggregata (lichen) from Brazilian Northeast: Chemical characterization and antimicrobial activity. Braz. Arch. Biol. Tecnol. 2010, 53, 115–122. [Google Scholar] [CrossRef]
- Segatore, B.; Bellio, P.; Setacci, D.; Brisdelli, F.; Piovano, M.; Garbarino, J.A.; Nicoletti, M.; Amicosante, G.; Perilli, M.; Celenza, G. In vitro interaction of usnic acid in combination with antimicrobial agents against methicillin-resistant Staphylococcus aureus clinical isolates determined by FICI and E model methods. Phytomedicine 2012, 19, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Caggia, S.; Piovano, M.; Garbarino, J.; Cardile, V. Effect of vicanicin and protolichesterinic acid on human prostate cancer cells: Role of Hsp70 protein. Chem. Biol. Interact. 2012, 195, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, V.; Stojanović, I.; Jadranin, M.; Vajs, V.; Djordjević, I.; Smelcerović, A.G. Effect of four lichen acids isolated from Hypogymnia physodes on viability of rat thymocytes. Food Chem. Toxicol. 2013, 51, 60–164. [Google Scholar]
- Kohlhardt-Floehr, C.; Boehm, F.; Troppens, S.; Lademann, T.; Truscott, G. Prooxidant and antioxidant behaviour of usnic acid from lichens under UVB-light irradiation—Studies on human cells. J. Photochem. Photobiol. B Biol. 2010, 101, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, T.K.; Chuliá-Zeidán, F.; Vasques, L.M.; Santos, J.P.A.; Rocha, R.F.; Pasquali, M.A.B. Redox characterization of usnic acid and its cytotoxic effect on human neuron-like cells (SH-SY5Y). Toxicol. In Vitro 2012, 26, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, K.A.; Lind, M.; Nybakken, L.; Gauslaa, I. Possible functional roles of cortical depsides and medullary depsidonas in the foliose lichen Hypogymnia phydodes. Flora 2009, 204, 40–48. [Google Scholar] [CrossRef]
- Silva, M.D.C.; Sá, R.A.; Napoleão, T.H.; Gomes, F.S.; Santos, N.D.L.; Albuquerque, A.C.; Xavier, H.S.; Paiva, P.M.G.; Correia, M.T.S.; Coelho, L.C.B.B. Purified Cladonia verticillaris lichen lectin: Insecticidal activity on Nasutitermes corniger (Isoptera: Termitidae). Int. Biodeterior. Biodegrad. 2009, 63, 334–340. [Google Scholar] [CrossRef]
- Martins, M.C.B.; Silva, M.C.; Silva, L.R.S.; Lima, V.L.M.; Pereira, E.C.; Falcao, E.P.S.; Melo, A.M.M.A.; Silva, N.H. Usnic Acid Potassium Salt: An Alternative for the Control of Biomphalaria glabrata (Say, 1818). PLoS ONE 2014, 9, e111102. [Google Scholar] [CrossRef] [PubMed]
- Boustie, J.; Tomasi, S.; Grube, M. Bioactive lichen metabolites: Alpine habitats as an untapped source. Phytochem. Rev. 2010, 10, 287–307. [Google Scholar] [CrossRef]
- Asplund, J.; Solhaug, K.A.; Gauslaa, Y. Fungal depsidonas—An inducible or constitutive defence against herbivores in the lichen Lobaria pulmonaria? Basic Appl. Ecol. 2009, 10, 273–278. [Google Scholar] [CrossRef]
- Lawrey, J.D. Correlations between lichen secondary chemistry and grazing activity by Pallifera varia. Bryologist 1980, 23, 128–134. [Google Scholar] [CrossRef]
- Endo, T.; Takahagi, T.; Kinoshita, Y.; Yamamoto, Y.; Sato, F. Inhibition of photosystem II of spinach by lichen-derived depsides. Biosci. Biotechol. Biochem. 1998, 62, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.C; Müller, K. Depsides as non-redox inhibitors of leucotriene B4 biosyntesis and HaCaT cell growth. Novel analogues of barbatic and diffractaic acid. Eur. J. Med. Chem. 1999, 34, 1035–1042. [Google Scholar] [CrossRef]
- Manojlović, N.T.; Vasiljević, P.J.; Gritsanapan, W.; Supabphol, R.; Manojlovic, I. Phytochemical and antioxidant studies of Laurera benguelensis growing in Thailand. Biol. Res. 2010, 43, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.C.B.; Rocha, T.A.; Silva, T.D.S.; Cavlacanti-Neto, M.P.; Santos, N.P.S.; Silva, T.G.; Aguiar-Jumior, F.C.A.; Falcão, E.P.S.; Pereira, E.C.; Silva, N.H. In Vitro and in Vivo Antineoplastic Activity of Barbatic Acid. Int. Arch. Med. 2016, 9. [Google Scholar] [CrossRef]
- Corthout, J.; Pieters, L.; Claeys, M.; Geerts, S.T.; Berghe, D.V.; Vlietinck, A. Antibacterial and Molluscicidal Phenolic Acids from Spondias mombin. Planta Med. 1994, 60, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Yadav, R.P; Singh, A. Molluscicidal from some common medicinal plants of eastern Uttar Pradesh, India. J. Appl. Toxicol. 2010, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bakry, F.A.; Mohamed, R.T.; El-Hommossany, K. Biological and biochemical responses of Biomphalaria alexandrina to some extracts of the plants Solanum siniacum and Artemisia judaica L. Pestic. Biochem. Physiol. 2011, 99, 174–180. [Google Scholar] [CrossRef]
- Fröberg, L.; Baur, A.; Baur, B. Differential herbivore damage to calcicolous lichens by snails. Lichenologist 1993, 25, 83–95. [Google Scholar] [CrossRef]
- Benesperi, R.; Tretiach, M. Differential land snail damage to selected species of the lichen genus Peltigera. Biochem. System. Ecol. 2004, 32, 127–138. [Google Scholar] [CrossRef]
- Gauslaa, Y. Lichen palatability depends on investment in herbivore defense. Oecology 2005, 143, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Fonseca, S.A.; César, F.A.; Albuquerque, M.C.P.A.; Santana, J.V.; Santana, A. A penta-substituted pyridine alkaloid from the rhizome of Jatropha elliptica (Pohl) Muell. Arg. is active against Schistosoma mansoni and Biomphalaria glabrata. Parasitol. Res. 2014, 113, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.G.; Souza, J.M.; Wakabayashi, K.A.L.; Laurentiz, R.S.; Vinhólis, A.H.C.; Rezende, K.C.S. In vitro efficacy of the essencial oil of Piper cubeba L. (Piperaceae) against Schistosoma mansoni. Parasitol. Res. 2012, 110, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Kiros, G.; Erko, B.; Giday, M.; Mekonnen, Y. Laboratory assessment of molluscicidal and cercariacidal effects of Glinus lotoides fruits. BMC Res. Notes 2014, 7, 3–7. [Google Scholar] [CrossRef] [PubMed]
- El-Beshbishi, S.N.; El Bardicy, S.; Tadros, M.; Ayoub, M.; Taman, A. Spotlight on the in vitro effect of atremisinin-naphthoquine phosphate on Schistosoma mansoni and its snail host Biomphalaria alexandrina. Acta Trop. 2015, 141, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.P.; Mattos, A.C.A.; Pereira, N.A.; Anchieta, N.F.; Silva, M.S.; Dias, D.F. Potential Schistosomicidal Constituents from Garcinia brasiliensis. Planta Med. 2015, 81, 733–741. [Google Scholar] [PubMed]
- Liang, Y.S; Wang, W.; Dai, J.R.; Li, H.J.; Tao, Y.H.; Zhang, J.F. Susceptibility to praziquantel of male and female cercariae of praziquantel-resistant and susceptible isolates of Schistosoma mansoni. J. Helminthol. 2010, 84, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, L.M.; Gonçalves, K.; OYama, N.M.; Coelho, R.G.; Spielmann, A.A.; Honda, N.K. In vitro radical-scavenging activity, toxicity against A. salina, and NMR profiles of extracts lichens collected from Brazil and Antarctica. Quim. Nova 2014, 37, 1015–1021. [Google Scholar]
- Ahti, T. The lichen family Cladoniaceae in Paraiba, Pernambuco and Sergipe, Northeast Brazil. Bryophyt. Divers. Evol. 1993, 7, 55–70. [Google Scholar] [CrossRef]
- Oliveira-Filho, E.C.; Geraldino, B.R.; Coelho, D.R.; De-Carvalho, R.R.; Paumgartten, F.J. Comparative toxicity of Euphorbia milii latex and synthetic molluscicides to Biomphalaria glabrata embryos. Chemosphere 2010, 2, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.F.S.; Cogo, J.; Wiepieski, C.C.P.; Laverde, J.R. Bioensaio de atividade moluscicida adaptado para a avaliação de extratos de plantas medicinais. Arq. Ciênc. Vet. Zool. Unipar 2008, 11, 179–181. [Google Scholar]
- Santos, A.F.; Cavada, B.S.; Rocha, B.A.M.; Nascimento, K.S.; Sant’Ana, A. Toxicity of some glucose/mannose-binding lectins to Biomphalaria glabrata and Artemia salina. Bioresour. Technol. 2010, 101, 794–798. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Substance | Concentration (µg/mL) | No. of Fecund Embryos * | Viability ♦ (%) | Inviability ■ (%) |
|---|---|---|---|---|
| Ctrl † | – | 379 | 0.5 | 99.5 |
| Ctrl ‡ | 0.5% | 300 | 1.5 | 98.5 |
| 10.5 | – | – | – | |
| Ether extract | 11 | 44 | 6.8 | 93.2 |
| 12.5 | 26 | 0 | 100 | |
| 10 | 38 | 0 | 0 | |
| 11 | – | – | – | |
| BAR | 11.5 | – | – | – |
| 12 | 25 | 4 | 96 | |
| 12.5 | – | – | – | |
| 20 | – | – | – |
| Concentration (µg/mL) | 15 min | 30 min | 60 min | 120 min |
|---|---|---|---|---|
| Ctrl | − | − | − | − |
| 0.25 | − | + | + | + |
| 0.5 | − | + | ++ | ++ |
| 1 | − | ++ | +++ | +++ |
| 10 | ++ | +++ | +++ | +++ |
| 100 | +++ | +++ | +++ | +++ |
| N | +++ | +++ | +++ | +++ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, M.C.B.; Silva, M.C.; Silva, H.A.M.F.; Silva, L.R.S.; Albuquerque, M.C.P.d.A.; Aires, A.L.; Falcão, E.P.d.S.; Pereira, E.C.; De Melo, A.M.M.A.; Da Silva, N.H. Barbatic Acid Offers a New Possibility for Control of Biomphalaria Glabrata and Schistosomiasis. Molecules 2017, 22, 568. https://doi.org/10.3390/molecules22040568
Martins MCB, Silva MC, Silva HAMF, Silva LRS, Albuquerque MCPdA, Aires AL, Falcão EPdS, Pereira EC, De Melo AMMA, Da Silva NH. Barbatic Acid Offers a New Possibility for Control of Biomphalaria Glabrata and Schistosomiasis. Molecules. 2017; 22(4):568. https://doi.org/10.3390/molecules22040568
Chicago/Turabian StyleMartins, Mônica Cristina Barroso, Monique Costa Silva, Hianna Arely Milca Fagundes Silva, Luanna Ribeiro Santos Silva, Mônica Camelo Pessoa de Azevedo Albuquerque, André Lima Aires, Emerson Peter da Silva Falcão, Eugênia C. Pereira, Ana Maria Mendonça Albuquerque De Melo, and Nicácio Henrique Da Silva. 2017. "Barbatic Acid Offers a New Possibility for Control of Biomphalaria Glabrata and Schistosomiasis" Molecules 22, no. 4: 568. https://doi.org/10.3390/molecules22040568







