Structural Analysis and Immuno-Stimulating Activity of an Acidic Polysaccharide from the Stems of Dendrobium nobile Lindl.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation, Purification and Composition Analysis of DNP-4W
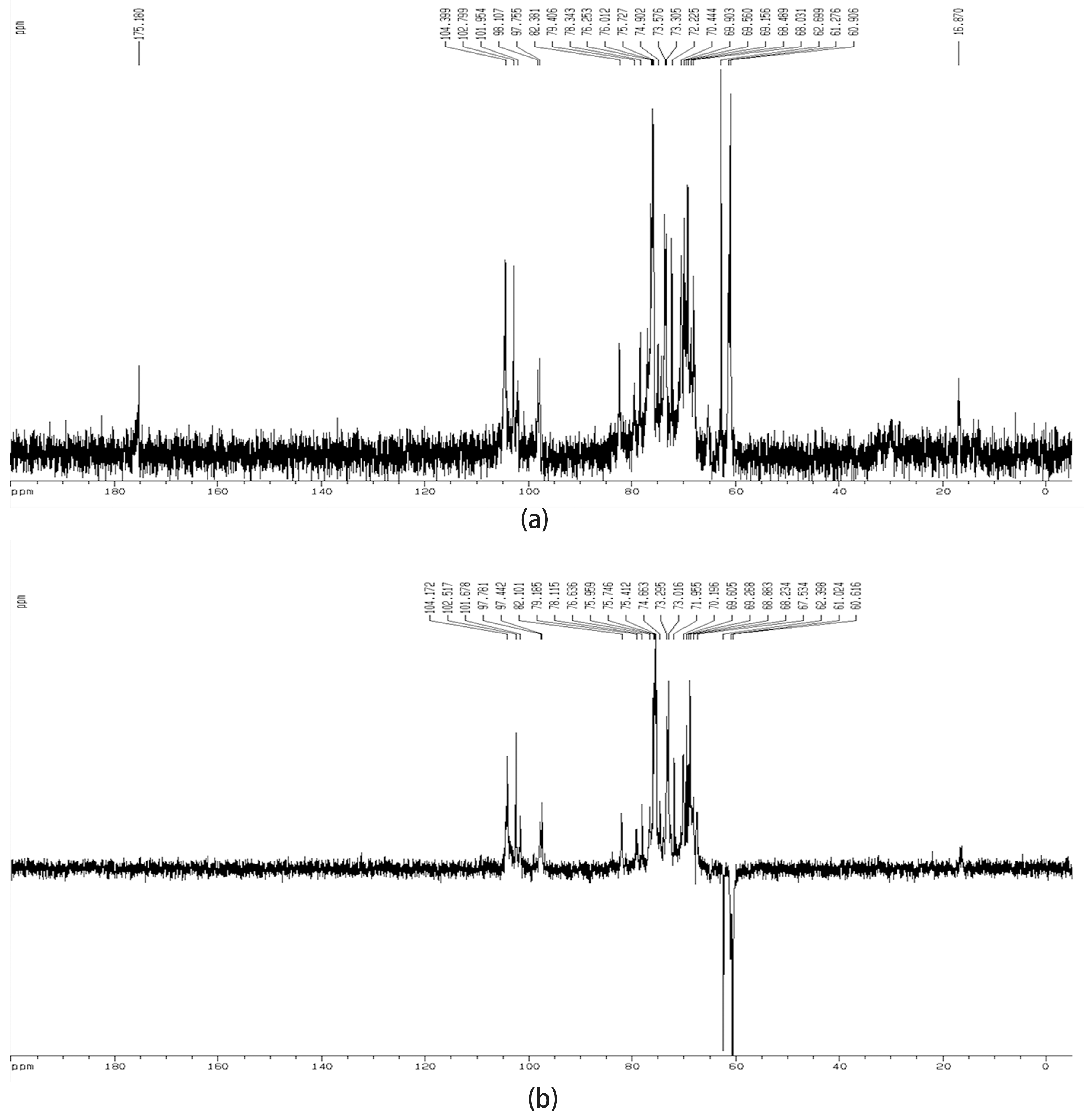
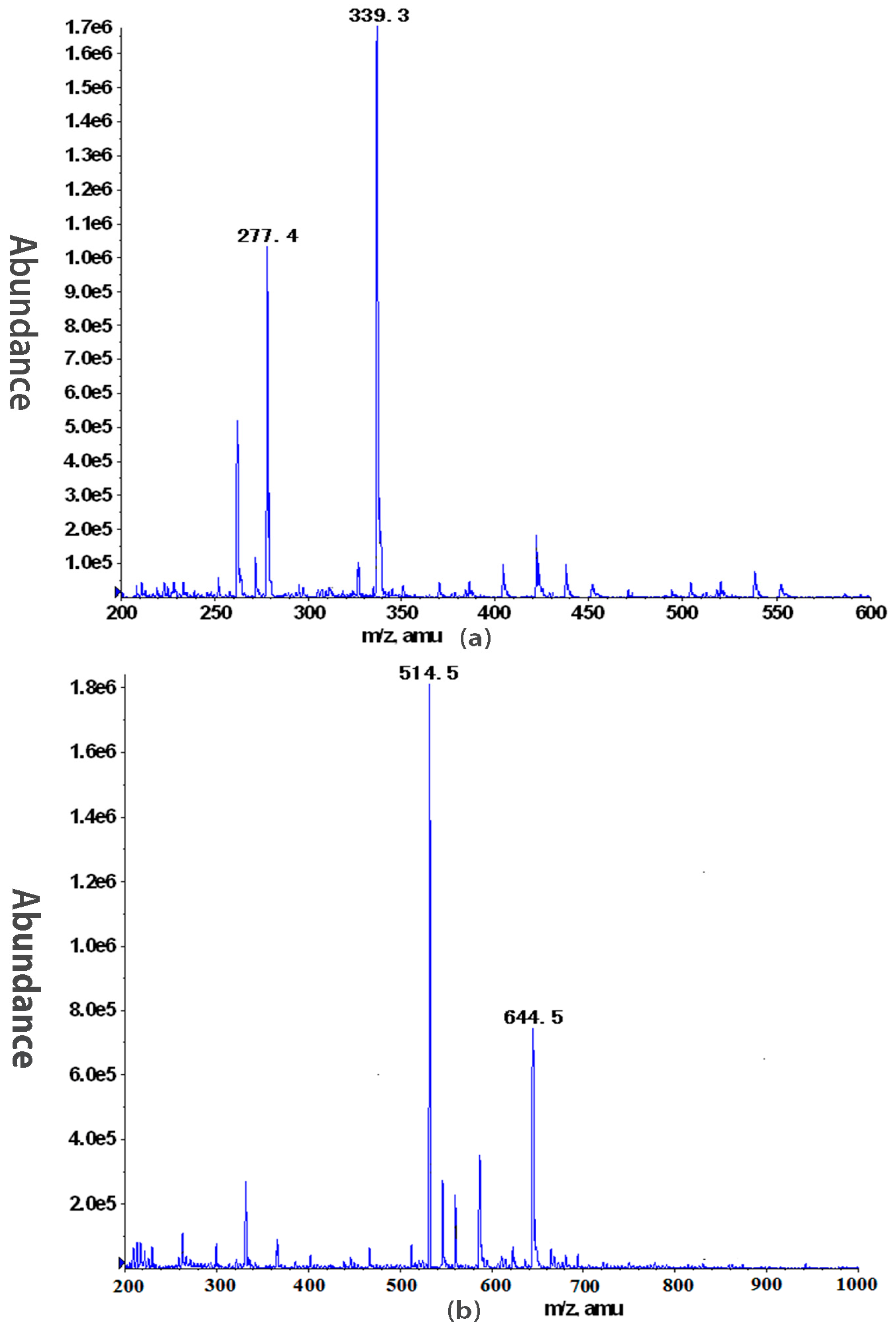
2.2. Structural Characterization of DNP-W4
2.3. Immunological Activity
3. Experimental Section
3.1. Plant Materials and Chemical Reagents
3.2. General Methods
3.3. Isolation and Purification
3.4. Gel-permeation Chromatography (GPC) and Molecular Mass
3.5. Chemical Analysis
3.6. Partial Hydrolysis with Acid
3.7. Periodate Oxidation–Smith Degradation
3.8. Enzymatic Hydrolysis
3.9. Immunobiological Activity Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Du, Y.Q.; Liu, Y.; Wang, J.H. Polysaccharides from Umbilicaria esculenta cultivated in huangshan mountain and immunomodulatory activity. Int. J. Biol. Macromol. 2015, 72, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.W.; Li, Y.R.; Tao, S.C.; Wei, G.; Huang, Y.C.; Chen, D.F.; Wu, C.F. Purification, Characterization and biological activity of polysaccharides from dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.P.; Cui, S.W.; Phillips, A.O.; Xie, M.Y.; Phillips, G.O.; Al-Assaf, S.; Zhang, X.L. Elucidation of the structure of a bioactive hydrophilic polysaccharide from Cordyceps sinensis by methylation analysis and NMR spectroscopy. Carbohydr. Polym. 2011, 84, 894–899. [Google Scholar] [CrossRef]
- Lo, S.F.; Mulabagal, V.; Chen, C.; Kuo, C.L.; Tsay, H.S. Bioguided fractionation and isolation of free radical scavenging components from in vitro propagated Chinese medicinal plants Dendrobium tosaense Makino and Dendrobium moniliforme SW. J. Agric. Food Chem. 2004, 52, 6916–6919. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.P.; Wang, Y.; Zha, X.Q.; Huang, L. Micropropagation of Dendrobium densiflorum Lindl. ex Wall. through protocorm-like bodies: Effects of plant growth regulators and lanthanoids. Plant Cell Tiss. Organ. Cult. 2008, 93, 333–340. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Committee. Chinese Pharmacopoeia; Chemical Industry Press: Beijing, China, 2010; pp. 85–87. [Google Scholar]
- Luo, J.P.; Deng, Y.Y.; Zha, X.Q. Mechanism of polysaccharides from Dendrobium huoshanense on streptozotocin-induced diabetic cataract. Pharm. Biol. 2008, 46, 243–249. [Google Scholar] [CrossRef]
- Xie, S.Z.; Hao, R.; Zha, X.Q.; Pan, L.H.; Liu, J.; Luo, J.P. Polysaccharide of dendrobium huoshanense activates macrophages via toll-like receptor 4-mediated signaling pathways. Carbohydr. Polym. 2016, 146, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Luo, J.P.; Zha, X.Q.; Feng, B.J. Comparison of antitumor activities of different polysaccharide fractions from the stems of Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 114–118. [Google Scholar] [CrossRef]
- Zha, X.Q.; Deng, Y.Y.; Li, X.L.; Wang, J.F.; Pan, L.H.; Luo, J.P. The core structure of a Dendrobium huoshanense polysaccharide required for the inhibition of human lens epithelial cell apoptosis. Carbohydr. Polym. 2017, 155, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, Y.D.; Fu, X.Q.; Wang, Y.S.; Liu, Y.; Huo, B.; Sheng, J.; Xu, X. Dendrobium candidum inhibits MCF-7 cells proliferation by inducing cell cycle arrest at G2/M phase and regulating key biomarkers. OncoTargets Ther. 2016, 9, 21–30. [Google Scholar]
- Yang, D.; Liu, L.Y.; Cheng, Z.Q.; Xu, F.Q.; Fan, W.W.; Zi, C.T.; Dong, F.W.; Zhou, J.; Ding, Z.T.; Hu, J.M. Five new phenolic compounds from dendrobium aphyllum. Fitoterapia 2015, 100, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nie, S.; Cai, H.; Cui, S.W.; Xie, M.; Phillips, G.O. Study on Dendrobium officinale O-acetyl-glucomannan (dendronan): Part VII. The immunomodulatory and antioxidant activity. J. Funct. Foods 2016, 15, 525–532. [Google Scholar] [CrossRef]
- Luo, A.X.; Ge, Z.G.; Fan, Y.J.; Luo, A.S.; Chun, Z.; He, X.J. In vitro and In vivo antioxidant activity of a water-Soluble polysaccharide from Dendrobium denneanum. Molecules 2011, 16, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhang, B.W.; Luo, J.P. Molecular weight, chain conformation and antioxidant activities of sulfated beta-d-galactan derivatives from Dendrobium nobile Lindl. Curr. Top. Nutraceut. Res. 2015, 13, 205–212. [Google Scholar]
- Wang, J.H.; Luo, J.P.; Yang, X.F.; Zha, X.Q. Structural analysis of a rhamnoarabinogalactan from the stems of Dendrobium nobile Lindl. Food Chem. 2010, 122, 572–576. [Google Scholar] [CrossRef]
- Wang, J.H.; Zha, X.Q.; Luo, J.P.; Yang, X.F. An acetylated galactomannoglucan from the stems of Dendrobium nobile Lindl. Carbohydr. Res. 2010, 345, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Shimamura, H.; Nakamura, S.I.; Kameoka, H. Antimutagenic activity of gigantol from Dendrobium nobile. J. Agric. Food Chem. 1997, 45, 2849–2853. [Google Scholar] [CrossRef]
- Luo, A.X.; He, X.J.; Zhou, S.D.; Luo, A.S.; Chun, Z. In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. extracts. Int. J. Biol. Macromol. 2009, 45, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.K.; Chen, A.L. The alkaloid of Chin-shih-hu. J. Biol. Chem. 1935, 111, 653–665. [Google Scholar]
- Hedman, K.; Leander, K. Studies on Orchidaceae alkaloids. Quaternary salts of the dendrobine type from Dendrobium nobile Lindl. Acta Chem. Scand. 1972, 26, 3177–3180. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Shimamura, H.; Nakamura, S. Moscatilin from Dendrobium. Nobile, a naturally occurring bibenzyl compound with potential antimutagenic activity. J. Agric. Food Chem. 1999, 47, 2163–2167. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Fujiwara, M.; Yoshida, N.; Kobayashi, J. New picrotoxinin-type and dendrobine-type sesquiterpenoids from Dendrobium snowflake ‘Red Star’. Tetrahedron 2000, 56, 5801–5805. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Kumaria, S. Molecular characterization of Dendrobium nobile Lindl. an endangered medicinal orchid, based on randomly amplified polymorphic DNA. Plant Syst. Evol. 2015, 301, 201–210. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Kumaria, S.; Diengdoh, R.; Tandon, P. Genetic stability and phytochemical analysis of the in vitro regenerated plants of Dendrobium nobile Lindl. an endangered medicinal orchid. Meta Gene 2014, 2, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.X.; He, X.J.; Zhou, S.D.; Fan, Y.J.; Luo, A.S.; Chun, Z. Purification composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Gerwig, G.J.; Kamerling, J.P.; Vliegenthart, J.F.G. Determination of the D and L configuration of neutral monosaccharides by high-solution capillary GLC. Carbohydr. Res. 1978, 62, 349–357. [Google Scholar] [CrossRef]
- Wang, X.S.; Zheng, Y.; Zuo, J.P.; Fang, J.N. Structural features of an immunoactive acidic arabinogalactan from Centella asiatica. Carbohydr. Polym. 2005, 59, 281–288. [Google Scholar] [CrossRef]
- Bao, J.Y.; Wang, X.S.; Fang, J.N.; Li, X.Y. Structural features of a pectic arabinogalactan with immunological activity from the leaves of Diospyros kaki. Carbohydr. Res. 2003, 338, 1291–1297. [Google Scholar]
- Martinez, M.; Pinto, G.L.D.; Alvarez, S.; Troconis, N.G.D.; Ocando, E.; Rivas, C. Composition and properties of Albizia lebbeck gum exudate. Biochem. Syst. Ecol. 1995, 23, 843–848. [Google Scholar] [CrossRef]
- Reis, A.; Domingues, R.M.; Domingues, P.; Ferrer-Correia, A.J.; Coimbra, M.A. Positive and negative electrospray ionization tandem mass spectrometry as a tool for structural characterization of acid released oligosaccharides from olive pulp glucuronoxylans. Carbohydr. Res. 2003, 338, 1497–1505. [Google Scholar] [CrossRef]
- Staub, A.M. Removal of protein-sevag method. Methods Carbohydr. Chem. 1965, 5, 5–6. [Google Scholar]
- Taylor, R.L.; Conrad, H.E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry 1972, 11, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Needs, P.W.; Selvendran, R.R. Avoiding oxidative degradation during sodium hydroxydimethyloidine-mediated carbohydrate methylation in dimethyl sulfoxide. Carbohydr. Res. 1993, 245, 1–10. [Google Scholar] [CrossRef]
- Deters, A.; Dauer, A.; Schnetz, E.; Fartasch, M.; Hensel, A. High molecular compounds (polysaccharides and proanthocyanidins) from Hamamelis virginiana bark: Influence on human skin keratinocyte proliferation and differentiation and influence on irritated skin. Phytochemistry 2001, 58, 949–958. [Google Scholar] [CrossRef]
- Heeg, K.; Reimann, J.; Kabelitz, D.; Hardt, C.; Wagner, H. A rapid colorimetric assay for the determination of IL-2-producing helper T cell frequencies. J. Immunol. Methods 1985, 77, 237–246. [Google Scholar] [CrossRef]
Sample Availability: Not Available. |

| Items | DNP-W4 | DNP-W4E | HDNP-W4 |
|---|---|---|---|
| Man | 1.0 | 1.0 | 0.1 |
| Glc | 4.9 | 5.2 | 4.1 |
| Gal | 2.5 | 2.5 | 2.4 |
| Xyl | 0.5 | n.d. | 0.1 |
| Rha | 1.0 | n.d. | 0.1 |
| GalA | 0.9 | n.d. | 0.2 |
| Methylated Sugar | Linkages Types | DNP-W4 | HDNP-W4 | DP1 |
|---|---|---|---|---|
| 2,3,4,6-Me4-Glcp | β-d-Glcp-(1→ | 0.5 | Trace | 0.1 |
| 2,3,6-Me3-Glcp | →4)-β-d-Glcp-(1→ | 1.0 | 1.6 | n.d. |
| 2,3-Me2-Glcp | →4,6)-β-d-Glcp-(1→ | 1.0 | 0.1 | n.d. |
| 2,3,4-Me3-Glcp | →6)-β-d-Glcp-(1→ | 1.9 | 2.6 | n.d. |
| 2,4,6-Me3-Glcp | →3)-β-d-Glcp-(1→ | 0.5 | n.d. | 0.2 |
| 2,3,4-Me3-Galp | →6)-β-d-Galp-(1→ | 2.0 | 2.4 | n.d. |
| 2, 4-Me3-Galp | →3,6)-β-d-Galp-(1→ | 0.5 | 0.1 | n.d. |
| 2,3,4,6-Me3-Manp | β-d-Manp-(1→ | 0.5 | 0.1 | 0.2 |
| 2,3,6-Me3-Manp | →6)-β-d-Manp-(1→ | 0.6 | Trace | 0.2 |
| 2,3,4-Me3-Xylp | Xylp-(1→ | 0.5 | 0.1 | 0.4 |
| 3,4-Me3-Rhap | →2)-α-l-Rhap-(1→ | 1.0 | 0.1 | 1.0 |
| 2,3,6-Me3-GalAp | →4)-α-d-GalAp-(1→ | 0.9 | 0.2 | 1.0 |
| Items | DNP-W4 (OD557) | DNP-W4E (OD557) | HDNP-W4 (OD557) | |||
|---|---|---|---|---|---|---|
| ConA | LPS | ConA | LPS | ConA | LPS | |
| Control | 0.487 ± 0.06 | 0.540 ± 0.04 | 0.487 ± 0.06 | 0.540 ± 0.04 | 0.487 ± 0.06 | 0.540 ± 0.04 |
| 25 (μg/mL) | 0.468 ± 0.01 | 0.527 ± 0.02 | 0.520 ± 0.05 | 0.553 ± 0.05 | 0.521 ± 0.07 | 0.559 ± 0.04 |
| 50 (μg/mL) | 0.510 ± 0.05 | 0.577 ± 0.03 | 0.600 ± 0.02 * | 0.600 ± 0.04 | 0.518 ± 0.04 | 0.581 ± 0.02 |
| 100 (μg/mL) | 0.518 ± 0.08 | 0.573 ± 0.05 | 0.661 ± 0.05 ** | 0.686 ± 0.07 * | 0.614 ± 0.03 * | 0.635 ± 0.05 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-H.; Zuo, S.-R.; Luo, J.-P. Structural Analysis and Immuno-Stimulating Activity of an Acidic Polysaccharide from the Stems of Dendrobium nobile Lindl. Molecules 2017, 22, 611. https://doi.org/10.3390/molecules22040611
Wang J-H, Zuo S-R, Luo J-P. Structural Analysis and Immuno-Stimulating Activity of an Acidic Polysaccharide from the Stems of Dendrobium nobile Lindl. Molecules. 2017; 22(4):611. https://doi.org/10.3390/molecules22040611
Chicago/Turabian StyleWang, Jun-Hui, Shu-Rong Zuo, and Jian-Ping Luo. 2017. "Structural Analysis and Immuno-Stimulating Activity of an Acidic Polysaccharide from the Stems of Dendrobium nobile Lindl." Molecules 22, no. 4: 611. https://doi.org/10.3390/molecules22040611





