Optimization of Ultrasound-Assisted Extraction of Antioxidants from the Mung Bean Coat
Abstract
:1. Introduction
2. Results and Discussion
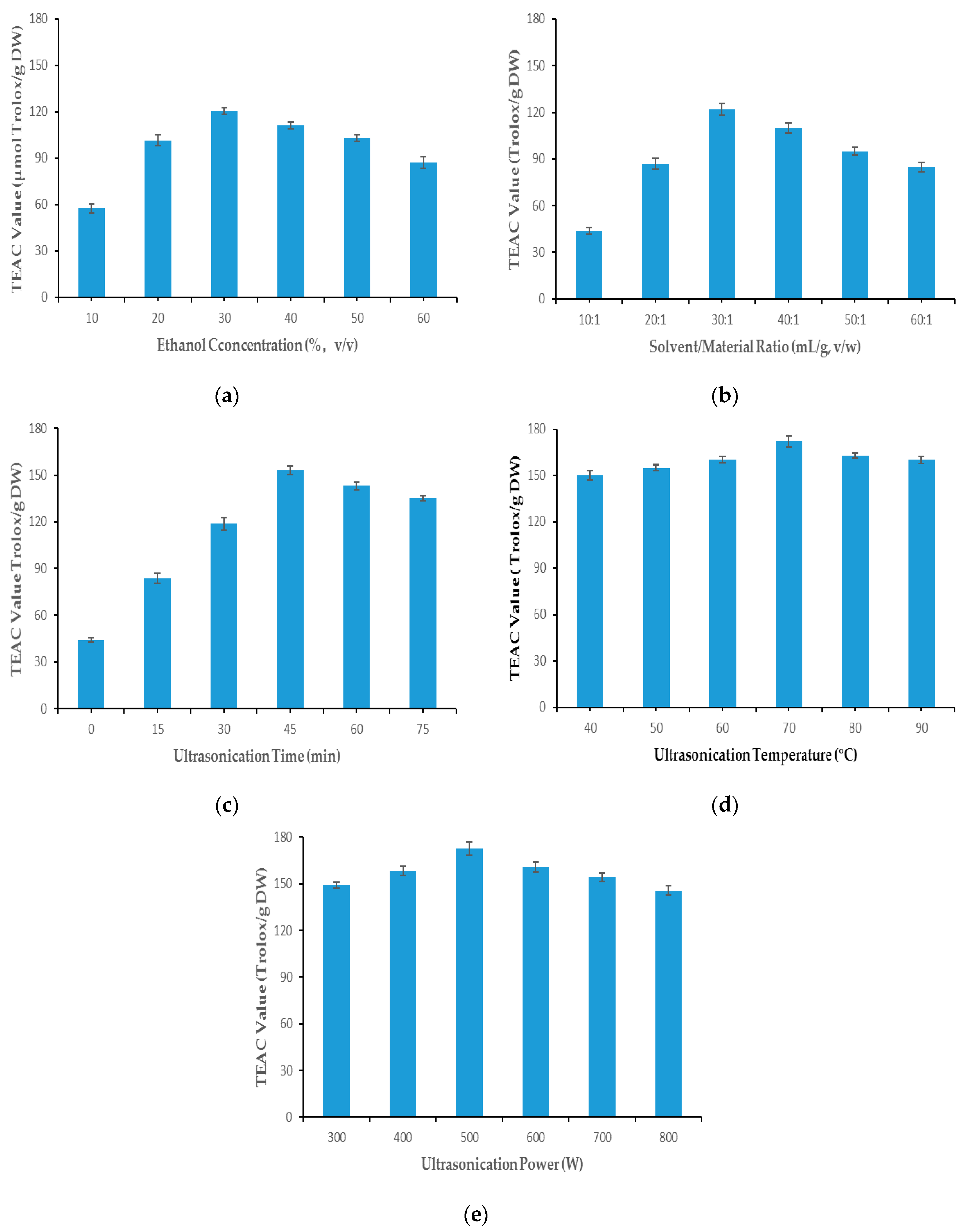
2.1. Single-Factor Experiments
2.1.1. Ethanol Concentration
2.1.2. Solvent/Material Ratio
2.1.3. Ultrasonication Time
2.1.4. Ultrasonication Temperature
2.1.5. Ultrasonication Power
2.2. Results of Response Surface Methodology Experiment
2.2.1. Central Composite Rotatable Design
2.2.2. Fitting Model
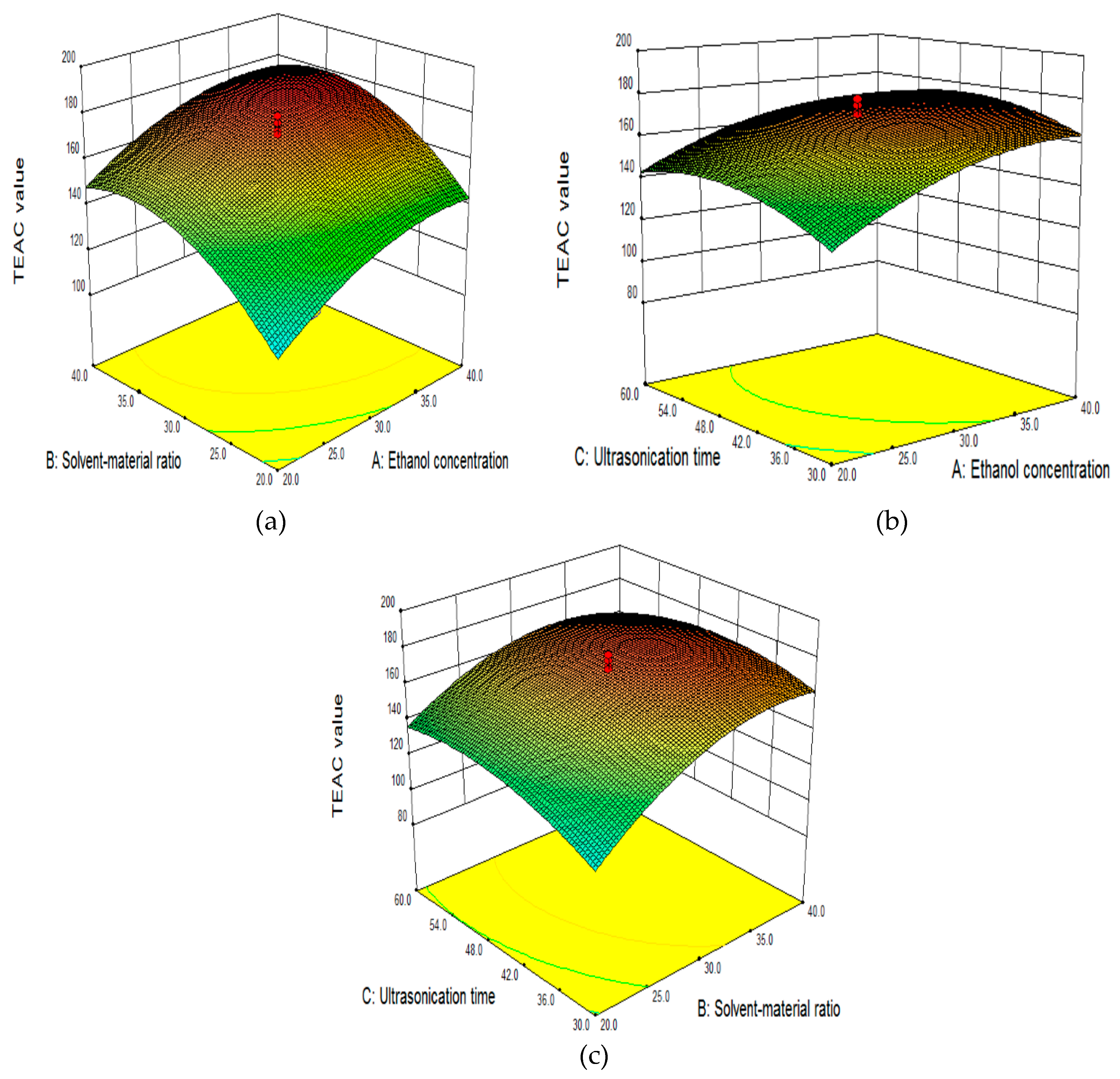
2.2.3. Effect of Independent Variables on TEAC Value in the RSM Model
2.2.4. Verification Experiments and Polyphenolic Compound Profile
2.2.5. Comparison of UAE with Conventional Methods
3. Materials and Methods
3.1. Reagents and Sample Preparation
3.2. Extraction Section
3.2.1. UAE Procedure
3.2.2. Conventional Methods
3.3. Determination of the Yield of Antioxidants
3.4. High-Performance Liquid Chromatography Analysis of Antioxidant Components
3.5. Design of the Experiment
3.5.1. Single-Factor Experiments
3.5.2. Response Surface Methodology
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar]
- Gan, R.Y.; Shah, N.P.; Wang, M.; Lui, W.; Corke, H. Fermentation alters antioxidant capacity and polyphenol distribution in selected edible legumes. Int. J. Food Sci. Technol. 2016, 51, 875–884. [Google Scholar]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Food. 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Deng, G.F.; Xu, X.R.; Zhang, Y.; Li, D.; Gan, R.Y.; Li, H.B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Food. 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Deng, G.F.; Xu, X.R.; Guo, Y.J.; Xia, E.Q.; Li, S.; Wu, S.; Chen, F.; Ling, W.H.; Li, H.B. Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. J. Funct. Food. 2012, 4, 906–914. [Google Scholar] [CrossRef]
- Li, S.; Li, S.K.; Gan, R.Y.; Song, F.L.; Kuang, L.; Li, H.B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crop. Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Dahiya, P.K.; Linnemann, A.R.; Van Boekel, M.A.J.S.; Khetarpaul, N.; Grewal, R.B.; Nout, M.J.R. Mung bean: Technological and nutritional potential. Crit. Rev. Food Sci. Nutr. 2015, 55, 670–688. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Y.; Dong, Y.M.; Ren, H.K.; Li, L.; He, C.F. A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata). Chem. Cent. J. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Wang, M.; Lui, W.; Wu, K.; Corke, H. Dynamic changes in phytochemical composition and antioxidant capacity in green and black mung bean (Vigna radiata) sprouts. Int. J. Food Sci. Technol. 2016, 51, 2090–2098. [Google Scholar] [CrossRef]
- Gan, R.Y.; Deng, Z.; Yan, A.; Shah, N.P.; Lui, W.; Chan, C.; Corke, H. Pigmented edible bean coats as natural sources of polyphenols with antioxidant and antibacterial effects. LWT-Food Sci. Technol. 2016, 73, 168–177. [Google Scholar] [CrossRef]
- Li, A.P.; Li, Z.Y.; Jia, J.P.; Qin, X.M. Chemical comparison of coat and kernel of mung bean by nuclear magnetic resonance-based metabolic fingerprinting approach. Spectr. Lett. 2016, 49, 217–224. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran Jeganathan, P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.L.; Meullemiestre, A.; Fabiano-Tixier, A.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [PubMed]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Xu, D.P.; Li, S.; Li, H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from sugar apple (Annona squamosa L.) peel using response surface methodology. Molecules 2015, 20, 20448–20459. [Google Scholar] [PubMed]
- Li, A.N.; Li, S.; Li, Y.; Xu, D.P.; Li, H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the Osmanthus fragrans flower. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Xu, D.P.; Xu, X.R.; Chen, Y.M.; Ling, W.H.; Chen, F.; Li, H.B. Optimization of ultrasound-assisted extraction of lycopene from papaya processing waste by response surface methodology. Food Anal. Meth. 2015, 8, 1207–1214. [Google Scholar] [CrossRef]
- Xia, E.Q.; Ai, X.X.; Zang, S.Y.; Guan, T.T.; Xu, X.R.; Li, H.B. Ultrasound-assisted extraction of phillyrin from Forsythia suspensa. Ultrason. Sonochem. 2011, 18, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Pan, S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.). Ultrason. Sonochem. 2013, 20, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Han, X.; Li, J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Safaryan, M.J.; Ganjloo, A.; Bimakr, M.; Zarringhalami, S. Optimization of ultrasound-assisted extraction, preliminary characterization and in vitro antioxidant activity of polysaccharides from green pea pods. Foods 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, X.; Yang, W.; Liu, S. Response surface methodology for optimization of the ultrasonic extraction of polysaccharides from Codonopsis pilosula Nannf. var. modesta L.T. Shen. Carbohydr. Polym. 2011, 84, 503–508. [Google Scholar] [CrossRef]
- Li, Q.; Yu, N.; Wang, Y.; Sun, Y.; Lu, K.; Guan, W. Extraction optimization of Bruguiera gymnorrhiza polysaccharides with radical scavenging activities. Carbohydr. Polym. 2013, 96, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, Y.; Liang, Z.; Wang, W. Investigation on ultrasound-assisted extraction of salvianolic acid B from Salvia miltiorrhiza root. Ultrason. Sonochem. 2010, 17, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Murthy, H.N.; Hahn, E.; Lee, H.L.; Paek, K. Parameters affecting the extraction of ginsenosides from the adventitious roots of ginseng (Panax ginseng CA Meyer). Sep. Purif. Technol. 2007, 56, 401–406. [Google Scholar] [CrossRef]
- Hemwimol, S.; Pavasant, P.; Shotipruk, A. Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia. Ultrason. Sonochem. 2006, 13, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, Q.; Fei, S.; Gu, H.; Yang, L. Optimization of ultrasonic circulating extraction of samara oil from Acer saccharum using combination of Plackett-Burman design and Box-Behnken design. Ultrason. Sonochem. 2017, 35, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.B. Extraction of natural antioxidants from the Thelephora ganbajun mushroom by an ultrasound-assisted extraction technique and evaluation of antiproliferative activity of the extract against human cancer cells. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.H.; Fu, C.L. Application of response surface methodology for extraction optimization of germinant pumpkin seeds protein. Food Chem. 2005, 92, 701–706. [Google Scholar]
- Raza, A.; Li, F.; Xu, X.; Tang, J. Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int. J. Biol. Macromol. 2017, 94, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Zhang, Q.; Li, Y.F.; Dong, L.L.; Liu, S.L. Optimization of ultrasound extraction of Alisma orientalis polysaccharides by response surface methodology and their antioxidant activities. Carbohydr. Polym. 2015, 119, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Szydlowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szlyk, E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Hayta, M.; Imen, E.M. Optimization of ultrasound-assisted antioxidant compounds extraction from germinated chickpea using response surface methodology. LWT-Food Sci. Technol. 2017, 77, 208–216. [Google Scholar] [CrossRef]
- Song, H.; Yang, R.; Zhao, W.; Katiyo, W.; Hua, X.; Zhang, W. Innovative assistant extraction of flavonoids from pine (Larix olgensis Henry) needles by high-density steam flash-explosion. J. Agric. Food Chem. 2014, 62, 3806–3812. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.N.; Li, H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of Jatropha integerrima by response surface methodology. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Run | X1 (Ethanol Concentration, %, v/v) | X2 (Solvent/Material Ratio, mL/g) | X3 (Ultrasonication Extraction Time, min) | Response Y (TEAC Value, µmol Trolox/g DW) |
|---|---|---|---|---|
| 1 | 30 | 13.2 | 45 | 96.917 |
| 2 | 20 | 20 | 60 | 115.408 |
| 3 * | 30 | 30 | 45 | 168.774 |
| 4 | 30 | 30 | 70.2 | 146.164 |
| 5 | 20 | 40 | 60 | 143.642 |
| 6 * | 30 | 30 | 45 | 159.208 |
| 7 * | 30 | 30 | 45 | 170.878 |
| 8 | 30 | 30 | 19.8 | 132.018 |
| 9 | 20 | 20 | 30 | 93.305 |
| 10 | 40 | 20 | 60 | 139.380 |
| 11 | 13.2 | 30 | 45 | 114.183 |
| 12 | 40 | 40 | 60 | 162.812 |
| 13 | 30 | 46.8 | 45 | 148.213 |
| 14 | 46.8 | 30 | 45 | 168.825 |
| 15 | 40 | 40 | 30 | 170.658 |
| 16 * | 30 | 30 | 45 | 172.434 |
| 17 | 40 | 20 | 30 | 125.065 |
| 18 * | 30 | 30 | 45 | 175.756 |
| 19 * | 30 | 30 | 45 | 178.869 |
| 20 | 20 | 40 | 30 | 140.013 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value | Significant |
|---|---|---|---|---|---|---|
| Model | 12903.46 | 9 | 1433.72 | 41.87 | < 0.0001 | significant |
| X1 | 2854.53 | 1 | 2854.53 | 83.36 | < 0.0001 | |
| X2 | 3881.47 | 1 | 3881.47 | 113.35 | < 0.0001 | |
| X3 | 229.56 | 1 | 229.56 | 6.70 | 0.0270 | |
| X1X2 | 4.38 | 1 | 4.38 | 0.13 | 0.7282 | |
| X1X3 | 46.38 | 1 | 46.38 | 1.35 | 0.2715 | |
| X2X3 | 206.40 | 1 | 206.40 | 6.03 | 0.0340 | |
| X12 | 1315.57 | 1 | 1315.57 | 38.42 | 0.0001 | |
| X22 | 3805.67 | 1 | 3805.67 | 111.13 | < 0.0001 | |
| X32 | 1561.00 | 1 | 1561.00 | 45.58 | < 0.0001 | |
| Residual | 342.45 | 10 | 34.24 | |||
| Lack of fit | 111.83 | 5 | 22.37 | 0.48 | 0.7771 | not significant |
| Pure error | 230.62 | 5 | 46.12 | |||
| Cor total | 13245.91 | 19 | ||||
| R2 = 0.9741 | ||||||
| Adj. R2 = 0.9509 | ||||||
| Optimal Condition | TEAC Value (µmol Trolox/g DW) | |||
|---|---|---|---|---|
| Ethanol Concentration | Solvent/Material Ratio | Extraction Time | Experimental Result | Predicted Value |
| 37.6% | 35.1 mL/g | 46.1 min | 178.28 ± 7.39 | 180.75 |
| Extraction Method | Ethanol Concentration (%) | Extraction Temperature (°C) | Extraction Time | TEAC Value (µmol Trolox/g DW) | TPC Value (mg GAE/g DW) | TFC (mg CE/g DW) |
|---|---|---|---|---|---|---|
| UAE | 37.6% | 70 | 46.1 min | 178.28 ± 7.39 | 33.91 ± 1.06 | 15.06 ± 1.11 |
| Maceration | 37.6% | 25 | 24 h | 158.66 ± 4.73 | 23.64 ± 1.28 | 6.67 ± 0.26 |
| Soxhlet | 37.6% | 95 | 4 h | 138.42 ± 3.63 | 19.96 ± 1.37 | 4.02 ± 0.18 |
| Independent Variables | Coded Levels | ||||
|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | |
| X1 (ethanol concentration, %, v/v) | 13.2 | 20 | 30 | 40 | 46.8 |
| X2 (solvent/material ratio, mL/g) | 13.2 | 20 | 30 | 40 | 46.8 |
| X3 (ultrasonication time, min) | 19.8 | 30 | 45 | 60 | 70.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zheng, J.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Optimization of Ultrasound-Assisted Extraction of Antioxidants from the Mung Bean Coat. Molecules 2017, 22, 638. https://doi.org/10.3390/molecules22040638
Zhou Y, Zheng J, Gan R-Y, Zhou T, Xu D-P, Li H-B. Optimization of Ultrasound-Assisted Extraction of Antioxidants from the Mung Bean Coat. Molecules. 2017; 22(4):638. https://doi.org/10.3390/molecules22040638
Chicago/Turabian StyleZhou, Yue, Jie Zheng, Ren-You Gan, Tong Zhou, Dong-Ping Xu, and Hua-Bin Li. 2017. "Optimization of Ultrasound-Assisted Extraction of Antioxidants from the Mung Bean Coat" Molecules 22, no. 4: 638. https://doi.org/10.3390/molecules22040638
APA StyleZhou, Y., Zheng, J., Gan, R.-Y., Zhou, T., Xu, D.-P., & Li, H.-B. (2017). Optimization of Ultrasound-Assisted Extraction of Antioxidants from the Mung Bean Coat. Molecules, 22(4), 638. https://doi.org/10.3390/molecules22040638








