Establishing Reliable Cu-64 Production Process: From Target Plating to Molecular Specific Tumor Micro-PET Imaging
Abstract
:1. Introduction
2. Results and Discussion
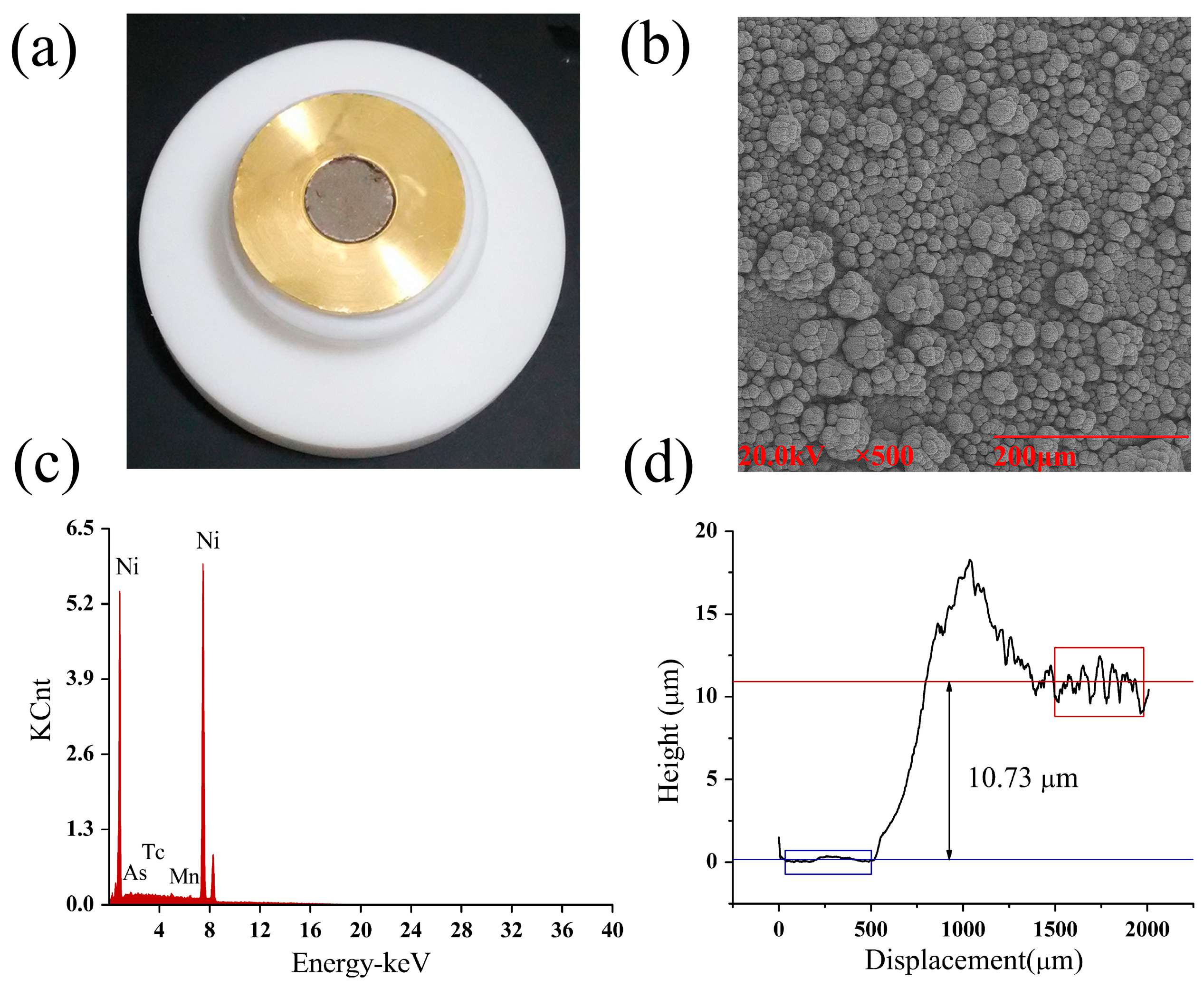
2.1. Preparation of 64Ni Target
2.2. Quality Control of 64Ni Target
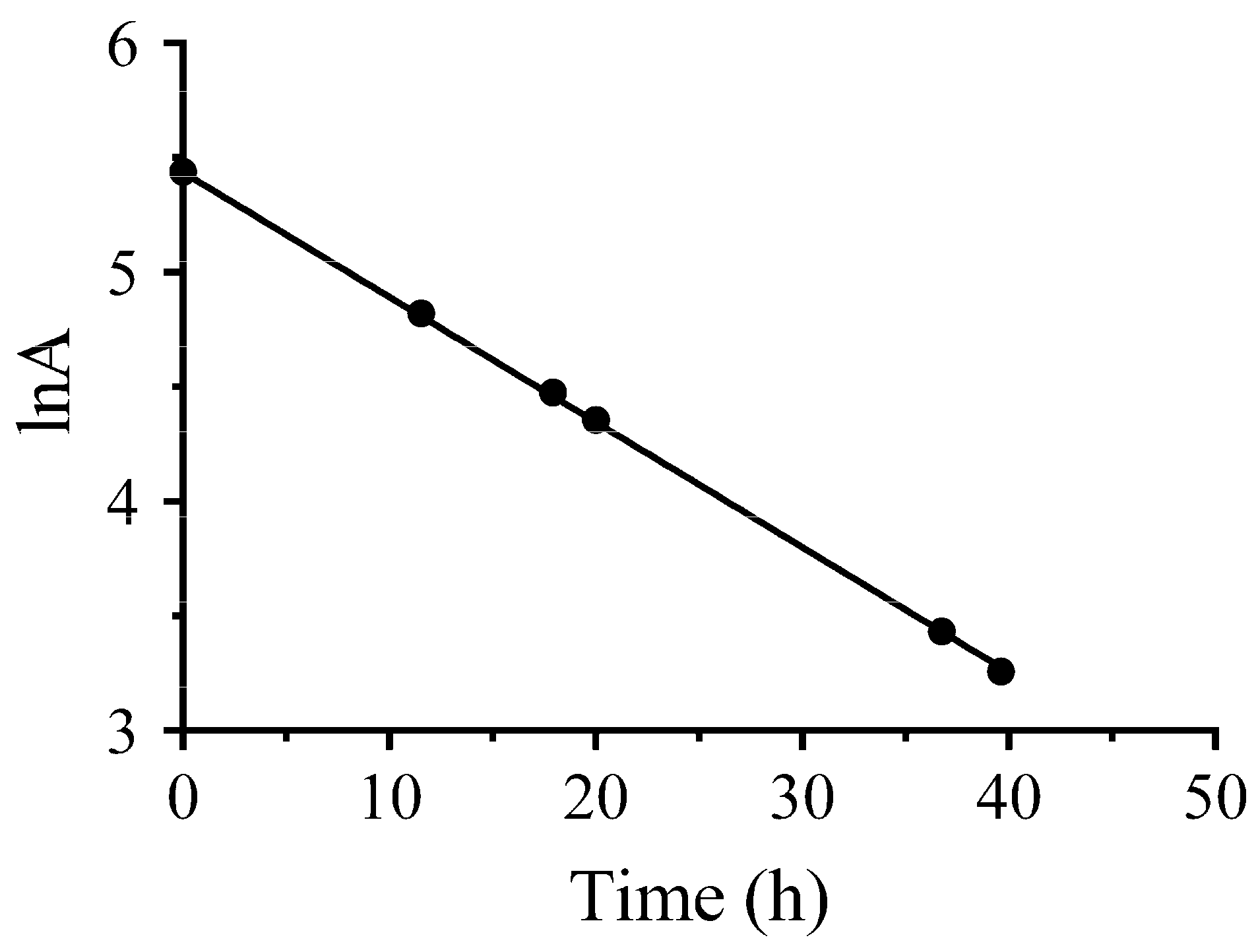
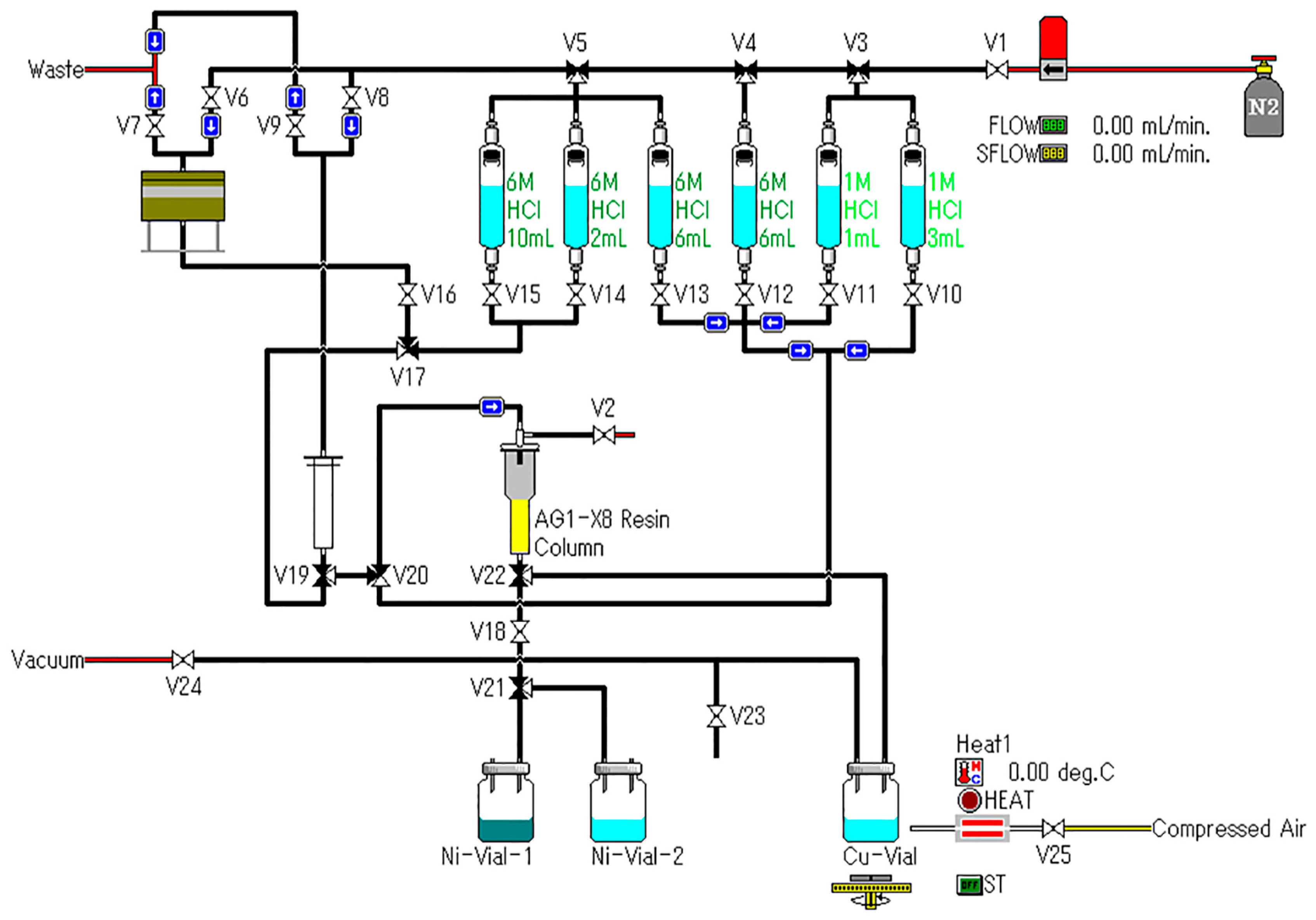
2.3. Preparation of 64Cu
2.4. Quality of 64Cu
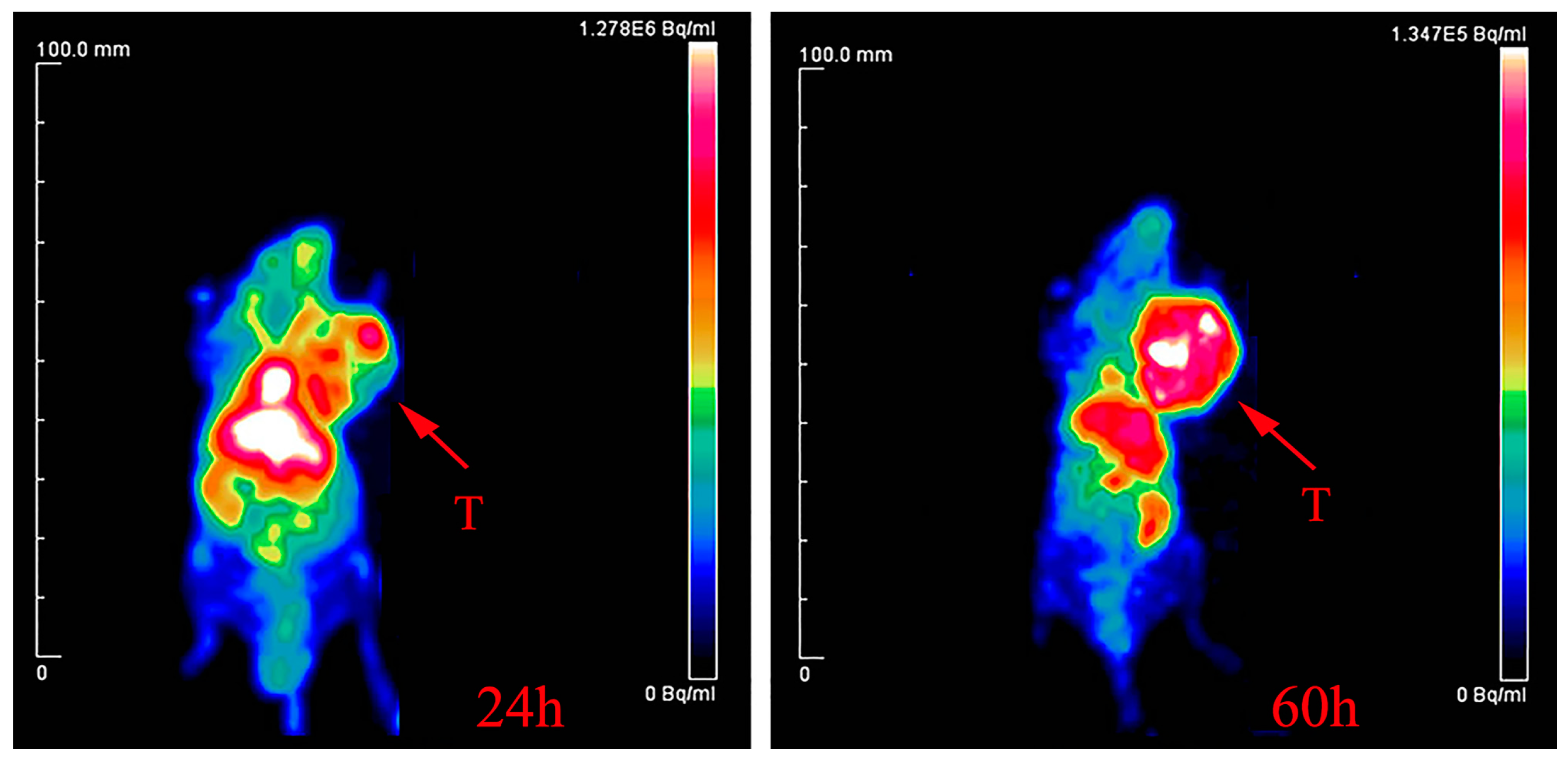
2.5. Radio-Synthesis of 64Cu-DOTA-Rituximab and Micro-PET Imaging
3. Materials and Methods
3.1. Materials and Reagents
3.2. Equipment
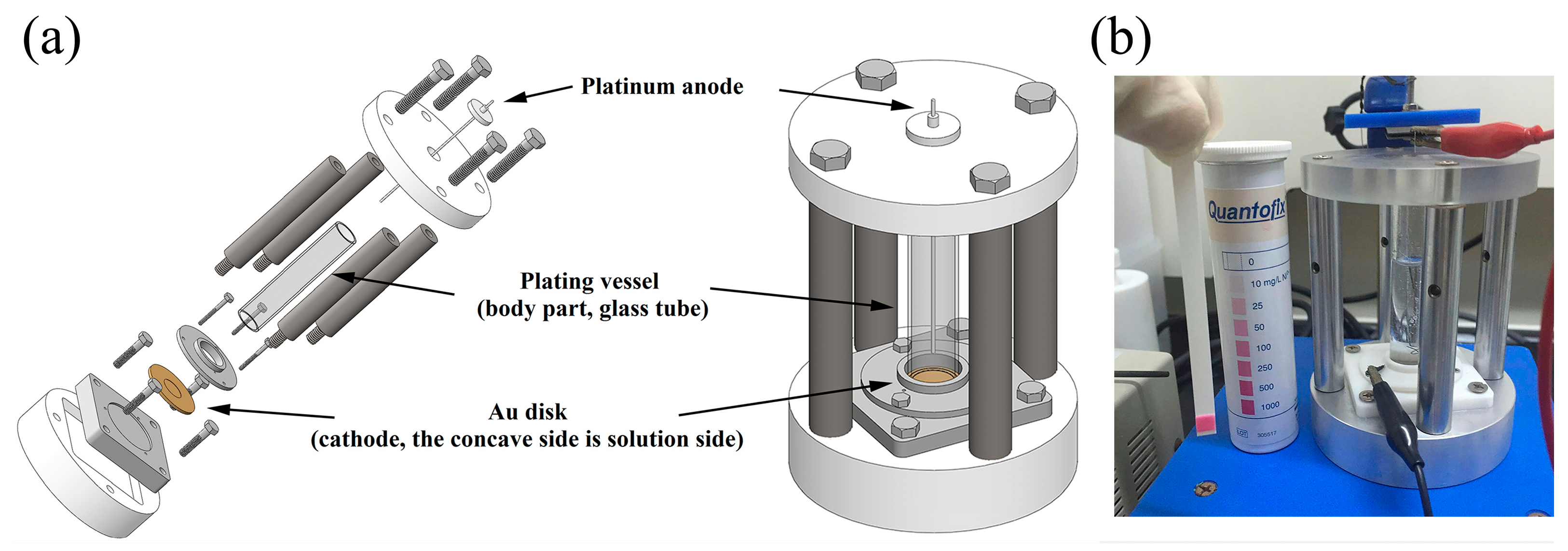
3.3. Plating Solution
3.4. Characterizations of Ni-64 Target
3.5. Preparation of 64Ni Target and Irradiation
3.6. Radiochemical Separation
3.7. Radio-nuclide Analysis
3.8. Radiolabeling of DOTA-Rituximab and Micro-PET Imaging
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nayak, T.K.; Brechbiel, M.W. Radioimmunoimaging with longer-lived positron-emitting radionuclides: Potentials and challenges. Bioconjug. Chem. 2009, 20, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Sheh, Y.; Lewis, J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009, 36, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Copper chelation chemistry and its role in copper radiopharmaceuticals. Curr. Pharm. Des. 2007, 13, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Arksey, N.; Iagaru, A.; Chin, F.T.; Gambhir, S.S. Validation of 64Cu-dota-rituximab injection preparation under good manufacturing practices: A Pet tracer for imaging of B-cell non-Hodgkin lymphoma. Mol. Imaging 2015, 14, 1–11. [Google Scholar]
- Kurihara, H.; Hamada, A.; Yoshida, M.; Shimma, S.; Hashimoto, J.; Yonemori, K.; Tani, H.; Miyakita, Y.; Kanayama, Y.; Wada, Y.; et al. 64Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res. 2015, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Sanghera, B.; Wood, K.; Sonoda, L.I.; Gogbashian, A.; Lowe, G.; Nunes, A.; Stirling, J.; Shepherd, C.; Beynon, G.; Wong, W.L. Pre-clinical positron emission tomography reconstruction algorithm effect on Cu-64 ATSM lesion hypoxia. Mol. Imaging Radionucl. Ther. 2016, 25, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Grassi, I.; Nanni, C.; Cicoria, G.; Blasi, C.; Bunkheila, F.; Lopci, E.; Colletti, P.M.; Rubello, D.; Fanti, S. Usefulness of 64Cu-ATSM in head and neck cancer: A preliminary prospective study. Clin. Nucl. Med. 2014, 39, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Handley, M.G.; Medina, R.A.; Mariotti, E.; Kenny, G.D.; Shaw, K.P.; Yan, R.; Eykyn, T.R.; Blower, P.J.; Southworth, R. Cardiac hypoxia imaging: Second-generation analogues of 64Cu-ATSM. J. Nucl. Med. 2014, 55, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.; Knigge, U.; Binderup, T.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; Rasmussen, P.; Elema, D.; et al. 64Cu-DOTATATE PET for neuroendocrine tumors: A prospective head-to-head comparison with 111In-DTPA-octreotide in 112 patients. J. Nucl. Med. 2015, 56, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Dehdashti, F.; Cutler, P.D.; Schwarz, S.W.; Laforest, R.; Bass, L.A.; Lewis, J.S.; Mccarthy, D.W. 64Cu-Teta-octreotide as a pet imaging agent for patients with neuroendocrine tumors. J. Nucl. Med. 2001, 42, 213–221. [Google Scholar] [PubMed]
- Chang, A.J.; Grigsby, P.W. Differences in uptake and distribution of 64Cu-Atsm and 18 F-FDG in primary tumors of patients with squamous cell carcinoma of the cervix. Fuel Energy Abstr. 2010, 78, S144. [Google Scholar]
- Chang, A.J. Intratumoral heterogeneity of 64Cu-ATSM uptake is a prognostic indicator in patients with cervical cancer. Omics J. Radiol. 2013, 2, 545–553. [Google Scholar] [CrossRef]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.; Knigge, U.; Mortensen, J.; Oturai, P.; Berthelsen, A.K.; Loft, A.; Binderup, T.; Rasmussen, P.; Elema, D.; Klausen, T.L.; et al. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: First-in-humans study. J. Nucl. Med. 2012, 53, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Grubmüller, B.; Baum, R.P.; Capasso, E.; Singh, A.; Ahmadi, Y.; Knoll, P.; Floth, A.; Righi, S.; Zandieh, S.; Meleddu, C.; et al. 64Cu-PSMA-617 PET/CT imaging of prostate adenocarcinoma: First in-human studies. Cancer Biother. Radiopharm. 2016, 31, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, M.; Bedeschi, P.; Scardaoni, R.; Sudati, F.; Savi, A.; Pepe, A.; Masiello, V.; Todde, S.; Gianolli, L.; Messa, C.; et al. Automated production of copper radioisotopes and preparation of high specific activity [(64)Cu]Cu-ATSM for PET studies. Appl. Radiat. Isot. 2010, 68, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Burke, P.; Golovko, O.; Clark, J.C.; Aigbirhio, F.I. An automated method for regular productions of copper-64 for PET radiopharmaceuticals. Inorg. Chim. Acta 2010, 363, 1316–1319. [Google Scholar] [CrossRef]
- Rajec, P.; Csiba, V.; Leporis, M.; Štefečka, M.; Pataky, E.L.; Reich, M.; Ometáková, J. Preparation and characterization of nickel targets for cyclotron production of 64Cu. J. Radioanal. Nucl. Chem. 2010, 286, 665–670. [Google Scholar] [CrossRef]
- Thisgaard, H.; Jensen, M.; Elema, D.R. Medium to large scale radioisotope production for targeted radiotherapy using a small PET cyclotron. Appl. Radiat. Isot. 2011, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kume, M.; Carey, P.C.; Gaehle, G.; Madrid, E.; Voller, T.; Margenau, W.; Welch, M.J.; Lapi, S.E. A semi-automated system for the routine production of copper-64. Appl. Radiat. Isot. 2012, 70, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Ohya, T.; Nagatsu, K.; Suzuki, H.; Fukada, M.; Minegishi, K.; Hanyu, M.; Fukumura, T.; Zhang, M.R. Efficient preparation of high-quality 64Cu for routine use. Nucl. Med. Biol. 2016, 43, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, D.W.; Shefer, R.E.; Klinkowstein, R.E.; Bass, L.A.; Margeneau, W.H.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997, 24, 35–43. [Google Scholar] [CrossRef]
- Elomaa, V.V.; Jurttila, J.; Rajander, J.; Solin, O. Automation of 64Cu production at turku PET centre. Appl. Radiat. Isot. 2014, 89, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Rajander, J.; Schlesinger, J.; Avila-Rodriguez, M.A.; Solin, O. Increasing specific activity in Cu-64 production by reprocessing the Ni-64 target material. J. Label. Compd. Radiopharm. 2009, 52, S234. [Google Scholar]
- Avila-Rodriguez, M.A.; Nye, J.A.; Nickles, R.J. Simultaneous production of high specific activity 64Cu and 61Co with 11.4 MeV protons on enriched 64Ni nuclei. Appl. Radiat. Isot. 2007, 65, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Ometáková, J.; Rajec, P.; Csiba, V.; Leporis, M. Automated production of 64Cu prepared by 18 MeV cyclotron. J. Radioanal. Nucl. Chem. 2012, 293, 217–222. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, H.; Lee, J.C.; Kim, K.M.; Lee, K.C.; Ha, H.J.; Choi, T.H.; An, G.I.; Cheon, G.J. A simple Cu-64 production and its application of Cu-64 ATSM. Appl. Radiat. Isot. 2009, 67, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Hagemeister, F. Rituximab for the treatment of non-hodgkin′s lymphoma and chronic lymphocytic leukaemia. Drugs 2010, 70, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, H.; Zhang, B.; Liu, F.; Chen, J.; Wang, Y.; Wang, Y.; Zhang, Z.; Wu, L.; Si, L.; et al. Synthesis of site-specific radiolabeled antibodies for radioimmunotherapy via genetic code expansion. Bioconjug. Chem. 2016, 27, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the rituximab is available from the authors. |


| Element | Wt % | At % |
|---|---|---|
| AsL | 00.00 | 00.00 |
| TcL | 00.15 | 00.09 |
| MnK | 00.07 | 00.07 |
| NiK | 99.78 | 99.84 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Zhu, H.; Wang, F.; Meng, X.; Ren, Q.; Xia, C.; Yang, Z. Establishing Reliable Cu-64 Production Process: From Target Plating to Molecular Specific Tumor Micro-PET Imaging. Molecules 2017, 22, 641. https://doi.org/10.3390/molecules22040641
Xie Q, Zhu H, Wang F, Meng X, Ren Q, Xia C, Yang Z. Establishing Reliable Cu-64 Production Process: From Target Plating to Molecular Specific Tumor Micro-PET Imaging. Molecules. 2017; 22(4):641. https://doi.org/10.3390/molecules22040641
Chicago/Turabian StyleXie, Qinghua, Hua Zhu, Feng Wang, Xiangxi Meng, Qiushi Ren, Chuanqin Xia, and Zhi Yang. 2017. "Establishing Reliable Cu-64 Production Process: From Target Plating to Molecular Specific Tumor Micro-PET Imaging" Molecules 22, no. 4: 641. https://doi.org/10.3390/molecules22040641






