Gamma-Aminobutyric Acid Increases the Production of Short-Chain Fatty Acids and Decreases pH Values in Mouse Colon
Abstract
:1. Introduction
2. Results
2.1. Weight and Health Status of Mice
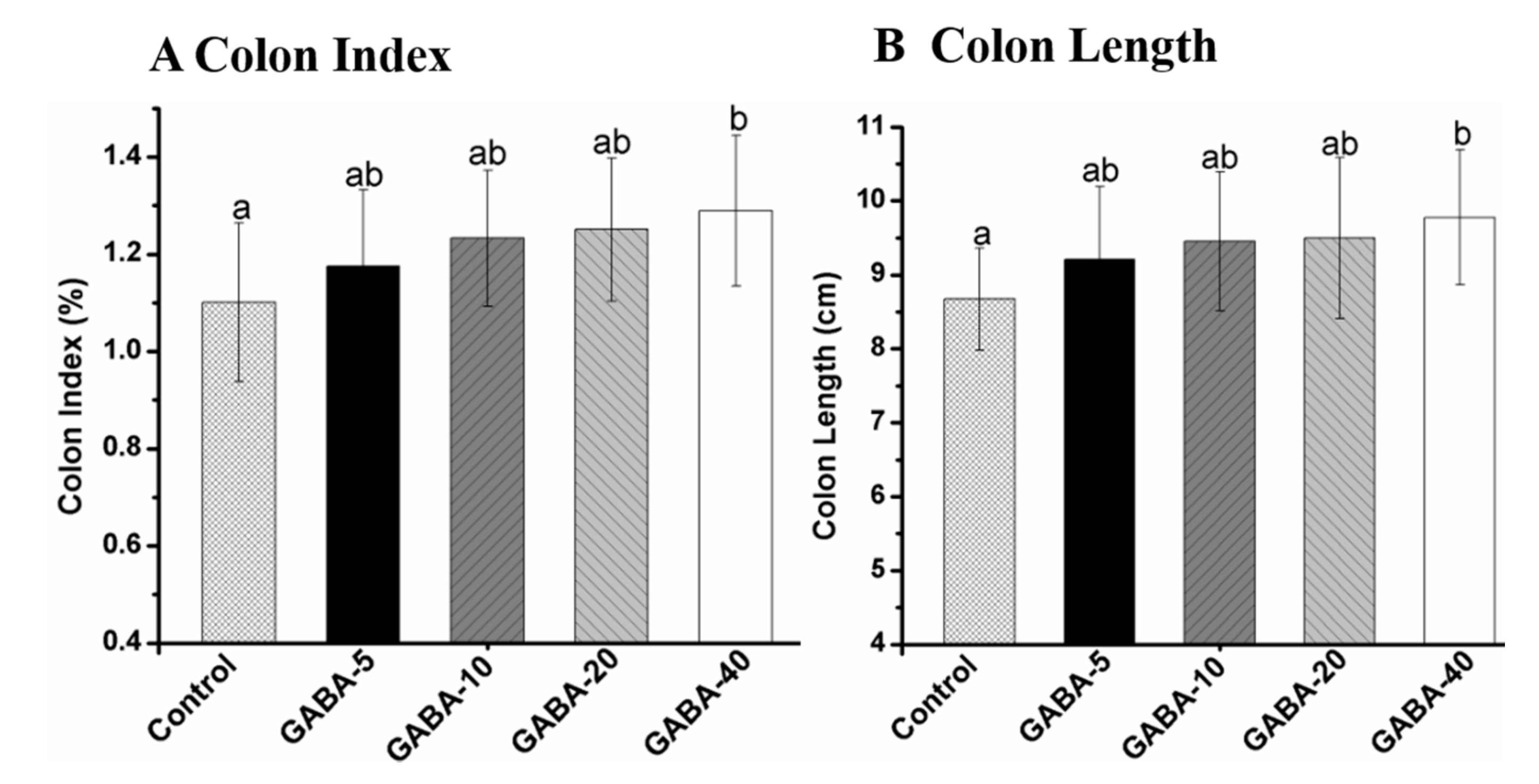
2.2. Colon Index and Length
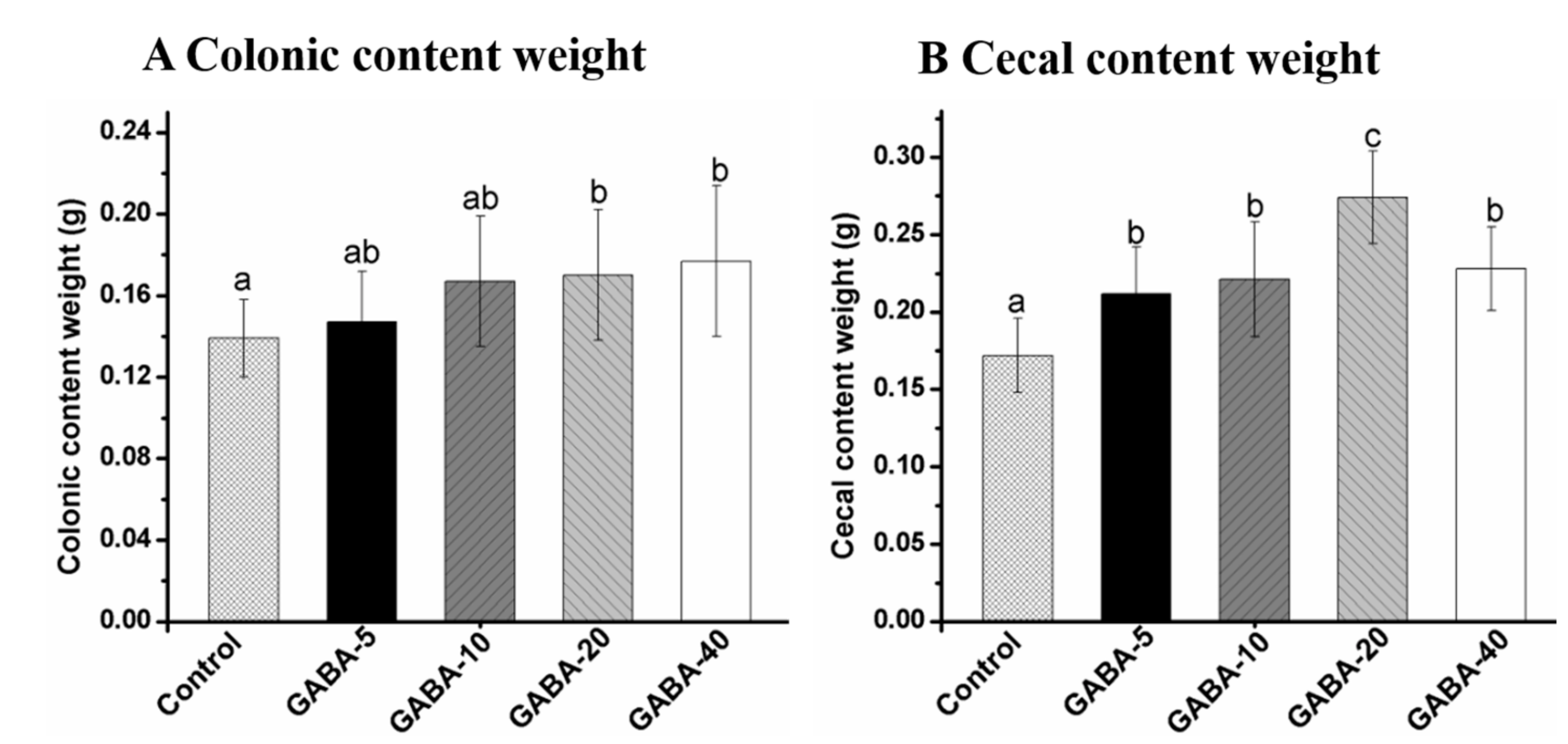
2.3. Effect of GABA on Weight of Colonic and Cecal Contents
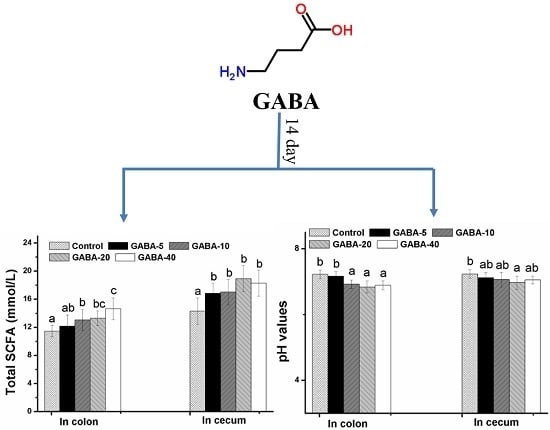
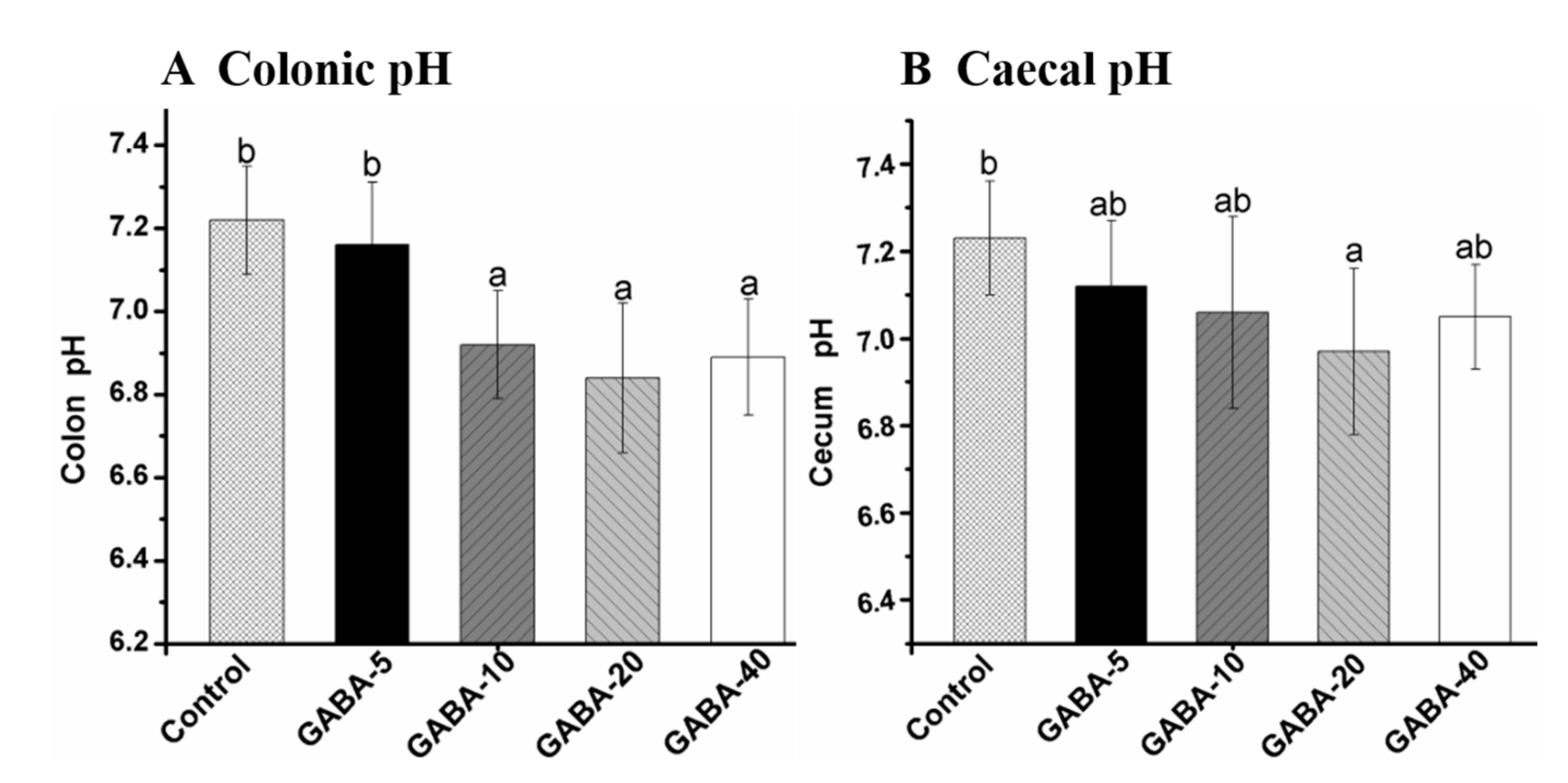
2.4. Effect of GABA on pH Value in the Colonic and Cecal Contents
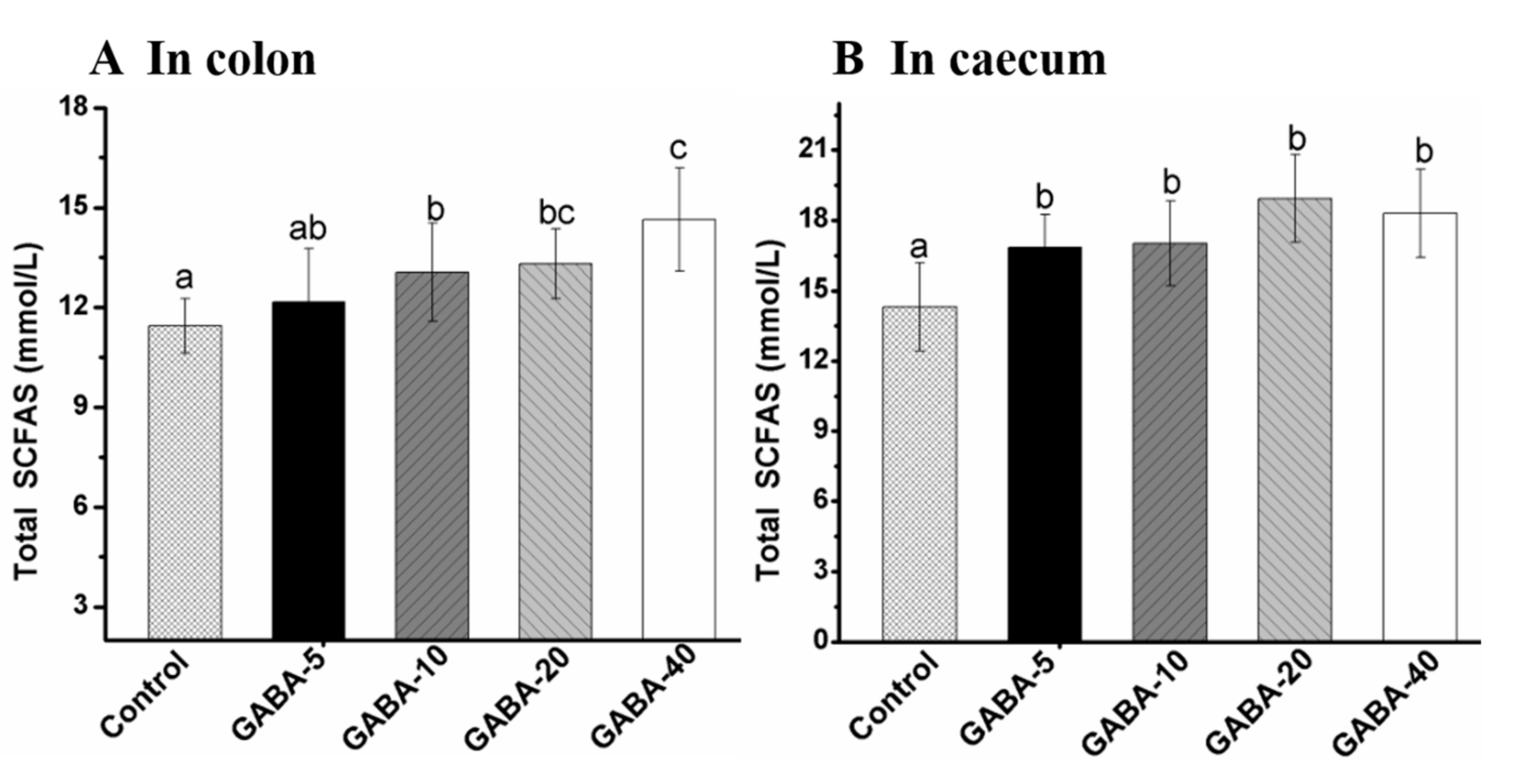
2.5. Effect of GABA on Total SCFA in the Colonic and Cecal Contents
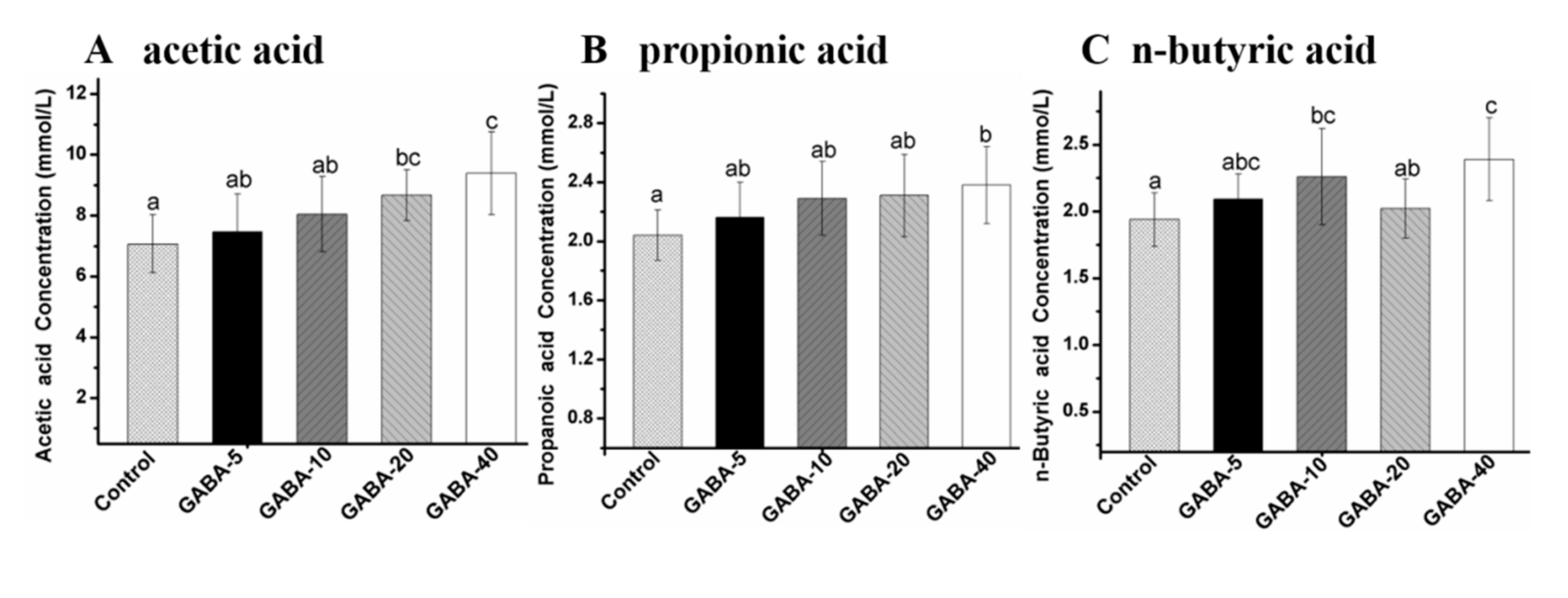
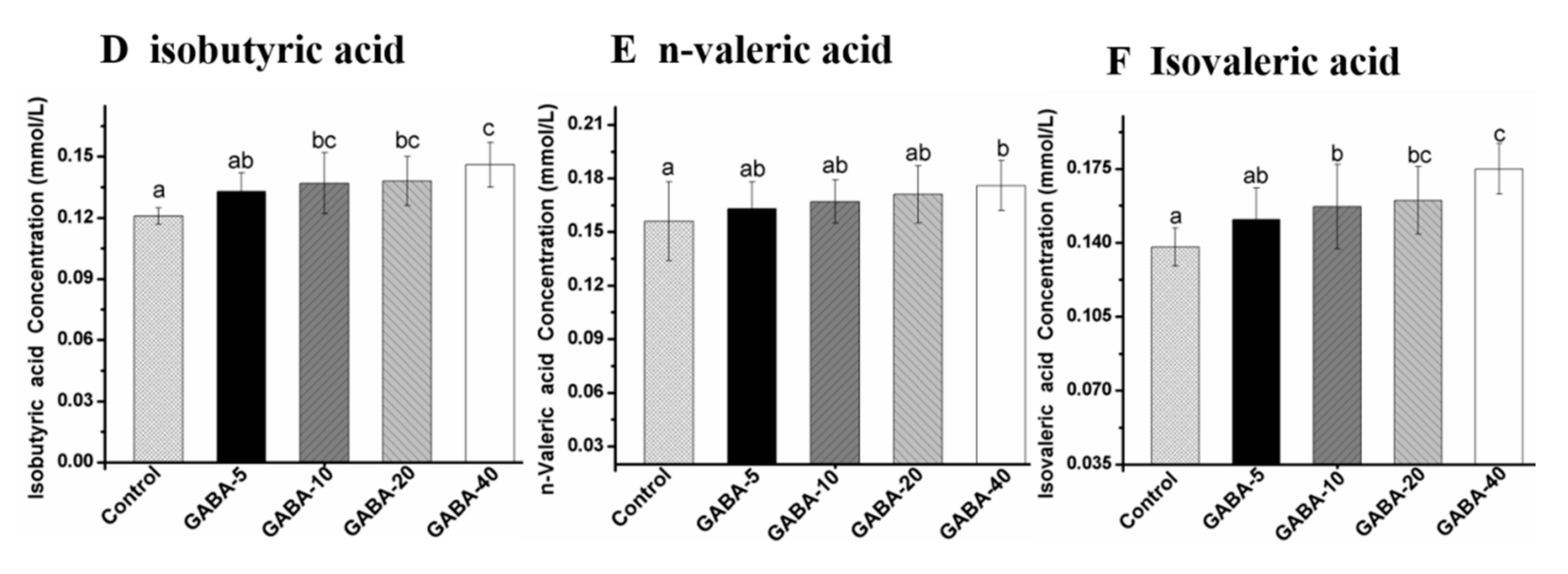
2.6. Effect of GABA on Individual SCFA in the Colonic Contents
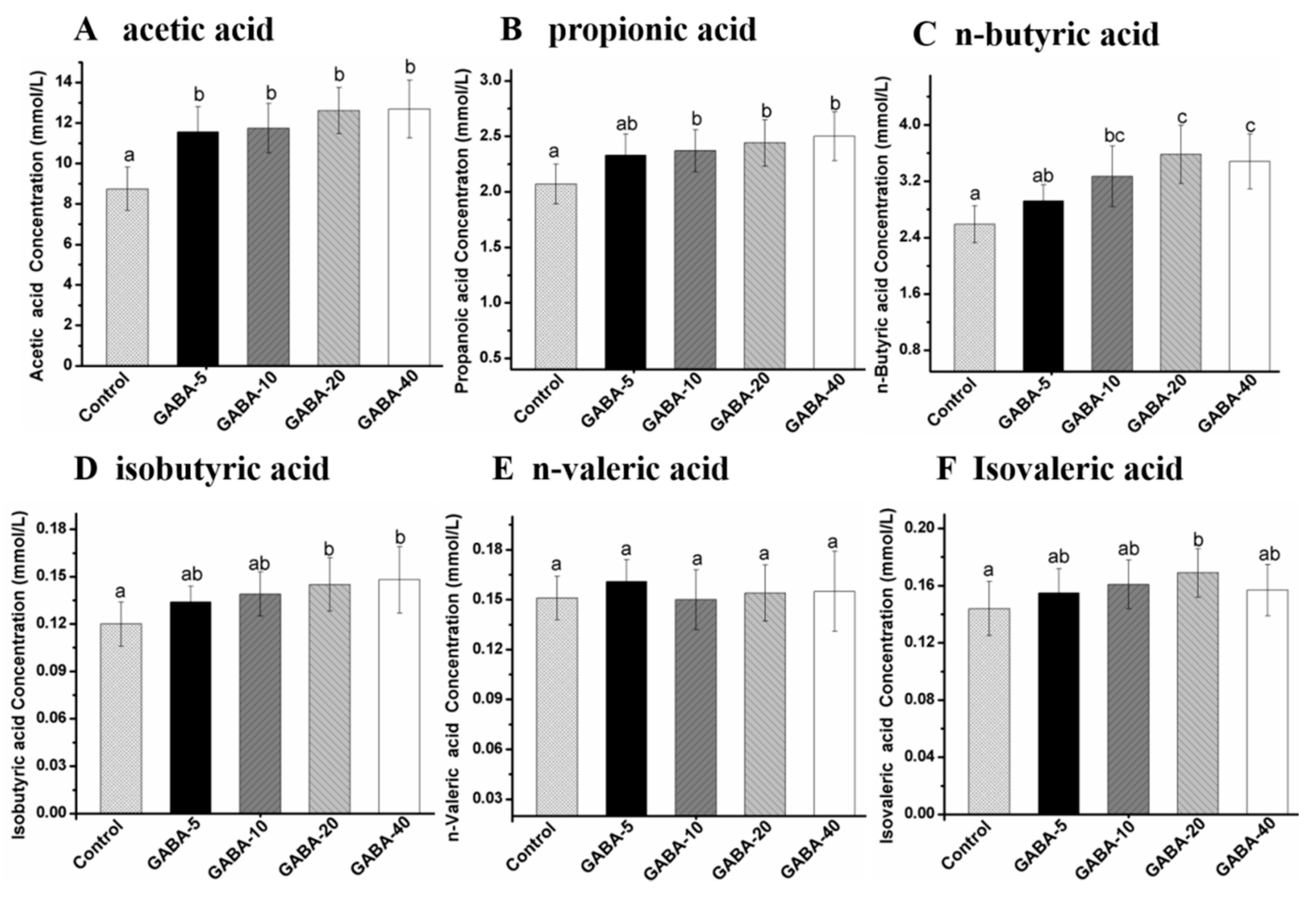
2.7. Effect of GABA on Individual SCFA in Cecal Contents
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Animal Experiment
4.4. Analysis of SCFAs
4.5. Determination of Colon Index and Length
4.6. Determination of pH Value
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bustos-Fernandez, L. Colon. Structure and Function; Plenum Medical Book Co.: New York, NY, USA, 1983; p. 691. [Google Scholar]
- Wong, J.M.; De, S.R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Fahey, G.C.; Wolf, B.W. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J. Nutr. 1997, 127, 130–136. [Google Scholar] [PubMed]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar] [PubMed]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [PubMed]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K. Induction of tissue transglutaminase expression by propionate and n-butyrate in colon cancer cell lines. J. Nutr. Biochem. 1999, 10, 397–404. [Google Scholar] [CrossRef]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Kimura, M.; Hayakawa, K.; Sansawa, H. Involvement of gamma-aminobutyric acid (GABA) B receptors in the hypotensive effect of systemically administered GABA in spontaneously hypertensive rats. Jpn. J. Pharmacol. 2002, 89, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Yokoi, K.; Jennings, N.B.; Li, Y.; Rebhun, R.B.; Rousseau, D.L., Jr.; Fan, D.; Sood, A.K. Inhibition of experimental colon cancer metastasis by the GABA-receptor agonist nembutal. Cancer Biol. Ther. 2005, 4, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Krantis, A. GABA in the mammalian enteric nervous system. Physiology 2000, 15, 284–290. [Google Scholar]
- Li, Y.; Xiang, Y.Y.; Lu, W.Y.; Liu, C.; Li, J. A novel role of intestine epithelial GABAergic signaling in regulating intestinal fluid secretion. Am. J. Physiol. 2012, 303, G453–G460. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.G.; Mulè, F.; Serio, R. Functional evidence for GABA as modulator of the contractility of the longitudinal muscle in mouse duodenum: Role of GABA A and GABA C receptors. Neuropharmacology 2007, 52, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA receptors in the gastrointestinal tract: From motility to inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Auteri, M.; Zizzo, M.G.; Mastropaolo, M.; Serio, R. Opposite role played by GABA A and GABA B receptors in the modulation of peristaltic activity in mouse distal colon. Eur. J. Pharmacol. 2014, 731, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Du, A.; Xiong, Y.; Jiang, J.; Zhang, Y.; Tian, Z.; Yan, H. γ-Aminobutyric acid inhibits the proliferation and increases oxaliplatin sensitivity in human colon cancer cells. Tumor Biol. 2016, 37, 14885–14894. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Hsu, C.K.; Ko, C.Y.; Chan, Y.C. Dietary intake of xylooligosaccharides improves the intestinal microbiota, fecal moisture, and pH value in the elderly. Nutr. Res. 2007, 27, 756–761. [Google Scholar] [CrossRef]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35, S35–S38. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 1980, 21, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Harig, J.M.; Soergel, K.H.; Komorowski, R.A.; Wood, C.M. Treatment of diversion colitis with short-chain-fatty acid irrigation. N. Engl. J. Med. 1989, 320, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 1982, 83, 424–429. [Google Scholar] [PubMed]
- Cummings, J.H. Short chain fatty acids in the human colon. Gut 1981, 22, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Remesy, C.; Demigne, C. Partition and absorption of volatile fatty acids in the alimentary canal of the rat. Ann. Rech. Vet. 1976, 7, 39–55. [Google Scholar] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Min, F.F.; Wan, Y.J.; Nie, S.P.; Xie, M.Y. Study on colon health benefit of polysaccharide from Cyclocarya paliurus leaves in vivo. J. Funct. Foods 2014, 11, 203–209. [Google Scholar] [CrossRef]
- Henningsson, I.; Bjiirck, I.; Nyman, M. Short-chain fatty acid formation at fermentation of indigestible carbohydrates. Food Nutr. Res. 2001, 45, 165–168. [Google Scholar] [CrossRef]
- Meynell, G. Antibacterial mechanisms of the mouse gut: II. The role of Eh and volatile fatty acids in the normal gut. Br. J. Exp. Pathol. 1963, 44, 209–219. [Google Scholar] [PubMed]
- Hentges, D.J. Inhibition of Shigella flexneri by the normal intestinal flora I. Mechanisms of inhibition by Klebsiella. J. Bacteriol. 1967, 93, 1369–1373. [Google Scholar] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: A possible explanation for trophic effects of fermentable fibre, gut microbes and luminal trophic factors. Br. J. Nutr. 1987, 58, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W.; Pomare, E.; Elia, M.; Cummings, J. The contribution of the large intestine to blood acetate in man. Clin. Sci. 1991, 80, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Wakabayashi, S.; Takeda, H. Effects of intravenous injection and intraperitoneal continual administration of sodium propionate on serum cholesterol levels in rats. J. Nutr. Sci. Vitaminol. 1995, 41, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Sharma, R.; Frost, G. Propionate. Anti-obesity and satiety enhancing factor? Appetite 2011, 56, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Haga, S.; Kasai, T.; Kiriyama, S. Fermentation products of sugar-beet fiber by cecal bacteria lower plasma cholesterol concentration in rats. J. Nutr. 1998, 128, 688–693. [Google Scholar] [PubMed]
- Whitehead, R.; Young, G.; Bhathal, P. Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215). Gut 1986, 27, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Leonel, A.J.; Alvarez-Leite, J.I. Butyrate: Implications for intestinal function. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Qian, J.; Lu, Q.; Tian, Y.; Chen, Q.; Zhang, Y. Protective effects of butyrate on intestinal ischemia—Reperfusion injury in rats. J. Surg. Res. 2015, 197, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, S.; Wang, C.; Jiang, R.; Wan, J. Histone Deacetylase Inhibitors Attenuate Acute Lung Injury During Cecal Ligation and Puncture-Induced Polymicrobial Sepsis. World J. Surg. 2010, 34, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Pu, C.; Xia, L.; Yu, X.; Zhu, B.; Cheng, R.; Xu, L.; Zhang, J. Salecan diet increases short chain fatty acids and enriches beneficial microbiota in the mouse cecum. Carbohydr. Polym. 2014, 102, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.; O’Toole, P.; Fitzgerald, G.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, R.; Stone, T.W. The Gut-Brain Axis, BDNF, NMDA and CNS Disorders. Neurochem. Res. 2016, 41, 2819–2835. [Google Scholar] [CrossRef] [PubMed]
- Oriach, C.S.; Robertson, R.C.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Food for thought: The role of nutrition in the microbiota-gut-brain axis. Clin. Nutr. Exp. 2016, 6, 25–38. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Nie, S.P.; Huang, X.J.; Hu, J.J.; Cui, S.W.; Xie, M.Y.; Phillips, G.O. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part VII. improving effects on colonic health of mice. J. Agric. Food Chem. 2015, 64, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Knapp, B.K.; Bauer, L.L.; Swanson, K.S.; Tappenden, K.A.; Fahey, G.C.; Godoy, M.R.C.D. Soluble Fiber Dextrin and Soluble Corn Fiber Supplementation Modify Indices of Health in Cecum and Colon of Sprague-Dawley Rats. Nutrients 2013, 5, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Koecher, K.J.; Noack, J.A.; Timm, D.A.; Klosterbuer, A.S.; Thomas, W.; Slavin, J.L. Estimation and interpretation of fermentation in the gut: Coupling results from a 24 h batch in vitro system with fecal measurements from a human intervention feeding study using fructo-oligosaccharides, inulin, gum acacia, and pea fiber. J. Agric. Food Chem. 2014, 62, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Macfarlane, G.T. The control and consequences of bacterial fermentation in the human colon. J. Appl. Microbiol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Grandison, L.; Guidotti, A. Stimulation of food intake by muscimol and beta endorphin. Neuropharmacology 1977, 16, 533–536. [Google Scholar] [CrossRef]
- Topping, D.L.; Gooden, J.M.; Brown, I.L.; Biebrick, D.A.; Mcgrath, L.; Trimble, R.P.; Choct, M.; Illman, R.J. A high amylose (amylomaize) starch raises proximal large bowel starch and increases colon length in pigs. J. Nutr. 1997, 127, 615–622. [Google Scholar] [PubMed]
- Jädert, C.; Phillipson, M.; Holm, L.; Lundberg, J.O.; Borniquel, S. Preventive and therapeutic effects of nitrite supplementation in experimental inflammatory bowel disease. Redox Biol. 2014, 2, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, J.; Hu, M.; Tang, J.; Shao, Z.; Li, M. Protective effects of γ-aminobutyric acid (GABA) on the small intestinal mucosa in heat-stressed Wenchang chicken. J. Anim. Plant Sci. 2015, 25, 78–87. [Google Scholar]
- Carvalho, L.; Brait, D.; Vaz, M.; Lollo, P.; Morato, P.; Oesterreich, S.; Raposo, J.; Freitas, K. Partially Hydrolyzed Guar Gum Increases Ferroportin Expression in the Colon of Anemic Growing Rats. Nutrients 2017, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Pattananandecha, T.; Sirilun, S.; Duangjitcharoen, Y.; Sivamaruthi, B.S.; Suwannalert, P.; Peerajan, S.; Chaiyasut, C. Hydrolysed inulin alleviates the azoxymethane-induced preneoplastic aberrant crypt foci by altering selected intestinal microbiota in Sprague-Dawley rats. Pharm. Biol. 2016, 54, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.A.; Singh, G.; Mahony, D.E.; Meier, C.E. Effect of pH on bile salt degradation by mixed fecal cultures. Steroids 1978, 32, 245–256. [Google Scholar] [CrossRef]
- Uraipan, S.; Brigidi, P.; Hongpattarakere, T. Antagonistic mechanisms of synbiosis between Lactobacillus plantarum CIF17AN2 and green banana starch in the proximal colon model challenged with Salmonella Typhimurium. Anaerobe 2014, 28, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Fan, S.T.; Nie, S.P.; Yu, Q.; Xiong, T.; Gong, D.; Xie, M.Y. Lactobacillus plantarum NCU116 attenuates cyclophosphamide-induced intestinal mucosal injury, metabolism and intestinal microbiota disorders in mice. Food Funct. 2016, 7, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.; Clark, M.; Senior, P.; Furness, J. Gene expression and localization of GABA C receptors in neurons of the rat gastrointestinal tract. Neuroscience 2001, 107, 181–189. [Google Scholar] [CrossRef]
- Uezono, Y.; Kaibara, M.; Hayashi, H.; Kawakami, S.; Enjoji, A.; Kanematsu, T.; Taniyama, K. Characterization of GABAB receptor in the human colon. J. Pharmacol. Sci. 2004, 94, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.H.; Oliveira, L.B.; Colombari, D.S.A.; De Luca, L.A.; Menani, J.V.; Callera, J.C. Sodium intake by hyperosmotic rats treated with a GABA(A) receptor agonist into the lateral parabrachial nucleus. Brain Res. 2008, 1190, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sandle, G.I. Salt and water absorption in the human colon: A modern appraisal. Gut 1998, 43, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.B.; Clausen, M.R. Short-Chain Fatty Acids in the Human Colon: Relation to Gastrointestinal Health and Disease. Scand. J. Gastroenterol. 1996, 216, 132–148. [Google Scholar] [CrossRef]
- Ruppin, H.; Barmeir, S.; Soergel, K.H.; Wood, C.M.; Schmitt, M.G. Absorption of short-chain fatty acids by the colon. Gastroenterology 1980, 78, 1500–1507. [Google Scholar] [PubMed]
- Hu, J.L.; Nie, S.P.; Min, F.F.; Xie, M.Y. Polysaccharide from seeds of Plantago asiatica L. increases short-chain fatty acid production and fecal moisture along with lowering pH in mouse colon. J. Agric. Food Chem. 2012, 60, 11525–11532. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |
| Group | 0 Day | 7 Day | 14 Day |
|---|---|---|---|
| Control | 22.98 ± 1.72 a | 27.46 ± 1.77 b | 30.74 ± 2.08 c |
| GABA-5 | 22.40 ± 1.47 a | 27.15 ± 2.57 b | 30.68 ± 1.93 c |
| GABA-10 | 22.96 ± 1.25 a | 27.45 ± 1.62 b | 30.71 ± 1.75 c |
| GABA-20 | 23.13 ± 1.32 a | 28.14 ± 1.51 b | 31.62 ± 1.54 c |
| GABA-40 | 22.89 ± 2.06 a | 27.71 ± 1.89 b | 31.11 ± 1.84 c |
| Condition | Running Parameter |
|---|---|
| Chromatographic column | HP-INNOWAX (30 m × 0.32 mm × 0.5 μm) |
| Detector and temperature | FID, 240 °C |
| Carrier gas and flow rate | N2, 1.2 mL/min |
| Injection volume | 0.2 μL |
| Air velocity | 300 mL/min |
| Hydrogen flow rate | 30 mL/min |
| Split ratio | 0 |
| Temperature-rising procedure | 100 °C (0.5 min, 4 °C/min) − 170 °C; 170 °C (20 °C/min) − 230 °C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Chen, H.-H.; Nie, S.-P.; Yin, J.-Y.; Xie, M.-Y. Gamma-Aminobutyric Acid Increases the Production of Short-Chain Fatty Acids and Decreases pH Values in Mouse Colon. Molecules 2017, 22, 653. https://doi.org/10.3390/molecules22040653
Xie M, Chen H-H, Nie S-P, Yin J-Y, Xie M-Y. Gamma-Aminobutyric Acid Increases the Production of Short-Chain Fatty Acids and Decreases pH Values in Mouse Colon. Molecules. 2017; 22(4):653. https://doi.org/10.3390/molecules22040653
Chicago/Turabian StyleXie, Min, Hai-Hong Chen, Shao-Ping Nie, Jun-Yi Yin, and Ming-Yong Xie. 2017. "Gamma-Aminobutyric Acid Increases the Production of Short-Chain Fatty Acids and Decreases pH Values in Mouse Colon" Molecules 22, no. 4: 653. https://doi.org/10.3390/molecules22040653