Relationship between Expression of Chalcone Synthase Genes and Chromones in Artificial Agarwood induced by Formic Acid Stimulation Combined with Fusarium sp. A2 Inoculation
Abstract
:1. Introduction
2. Results
2.1. Analysis of the Accumulation of Secondary Metabolite Compounds
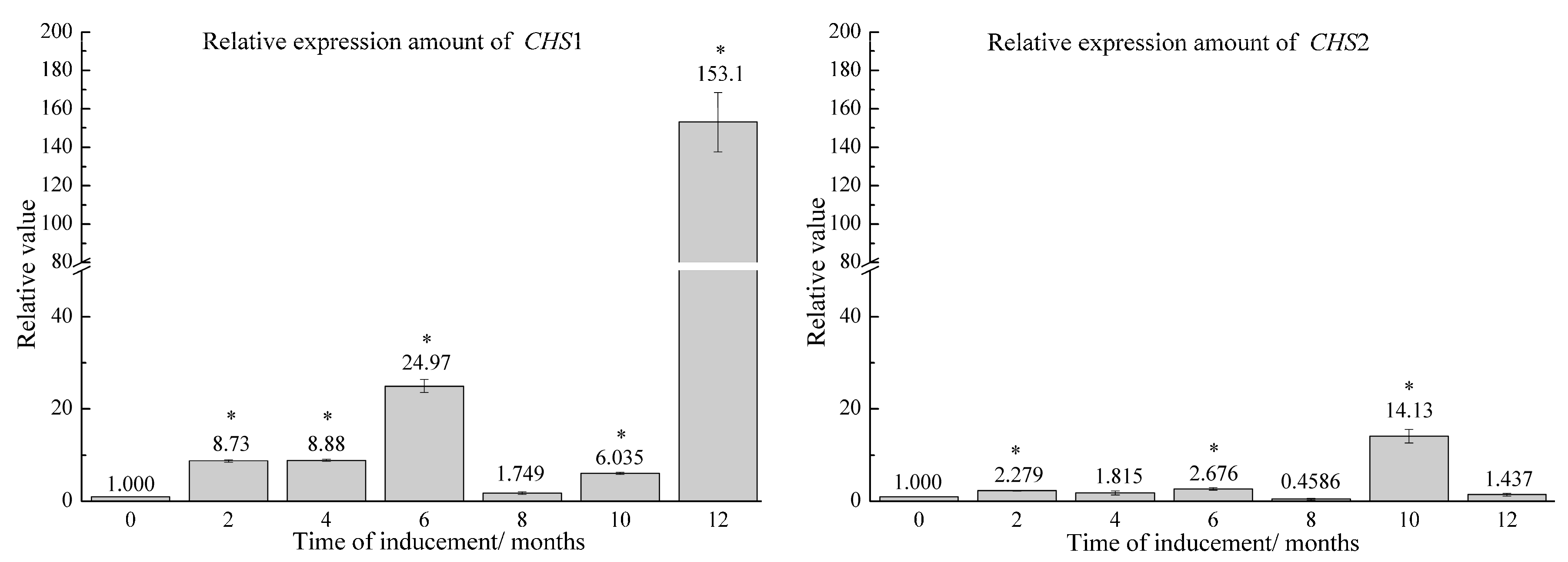
2.2. Relative Expression of Candidate Genes in Agarwood Samples Induced by FA Stimulation Combined with F. sp. A2 Inoculation
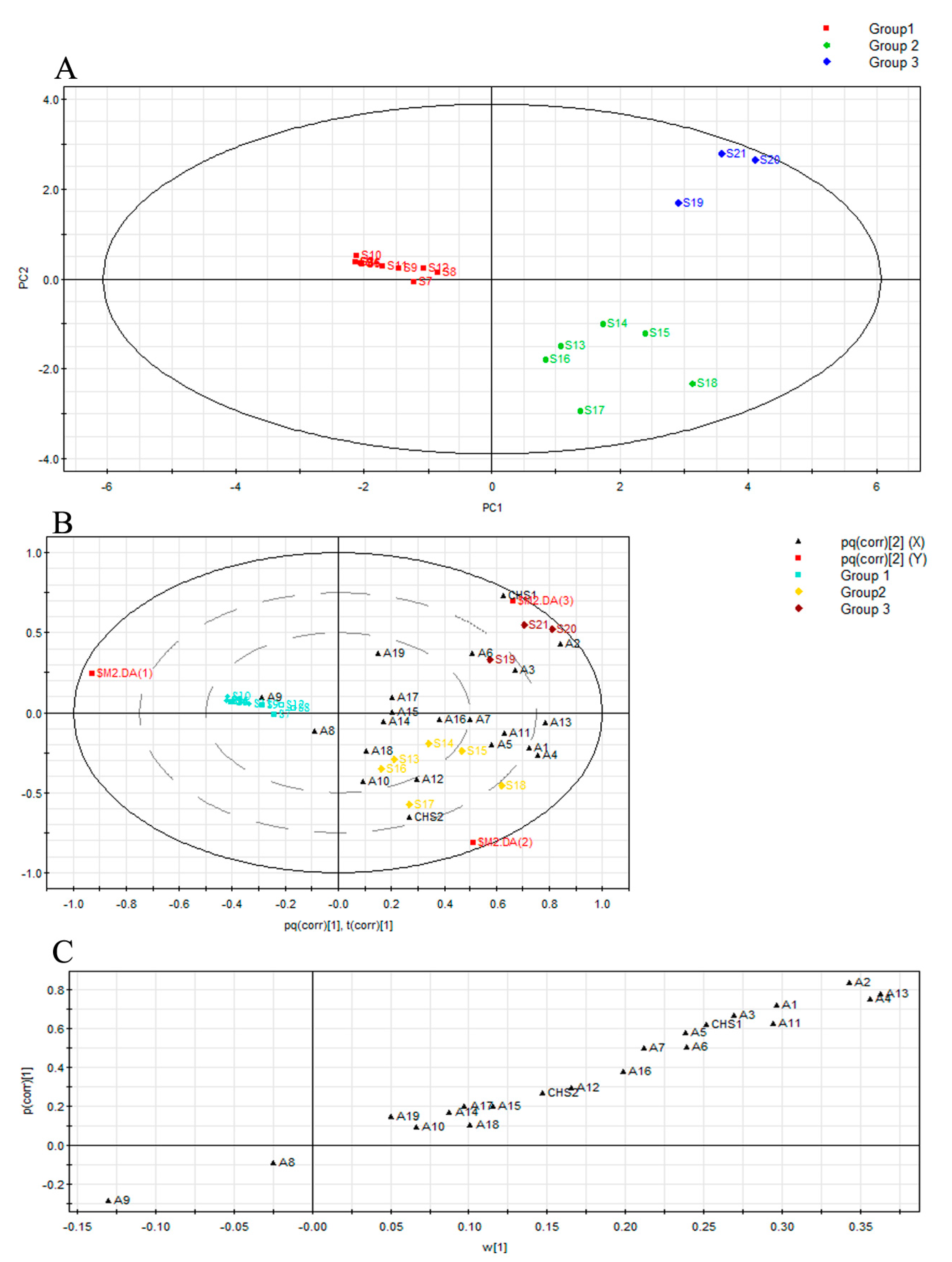
2.3. Multivariate Analysis of Agarwood by Their Mass Ions and Identification
3. Discussion
4. Materials and Methods
4.1. Source of Plant Materials
4.2. Gas Chromatography Mass Spectrometry Analysis
4.3. Quantitative Real-Time PCR (qRT-PCR)
4.4. Data Processing and Statistics Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, Y.F. The list of major national protected wild plant species in China. Plants 1999, 5, 3–11. [Google Scholar]
- Ng, L.T.; Chang, Y.S.; Azizol, A.K. A review on agar (gaharu) producing Aquilaria species. J. Trop. For. Prod. 1997, 2, 272–285. [Google Scholar]
- Bhattacharyya, B.; Datta, A.; Baruah, H.K. On the formation and development of agaru in Aquilaria agallocha. Sci. Cult. 1952, 18, 240–243. [Google Scholar]
- Jain, T.C.; Maheshwari, M.L.; Bhattacharyya, S.C. Terpenoids XXX The composition of the oil from uninfected agarwood (Aquilaria agallocha Roxb.). Perf. Essent. Oil Rec. 1962, 53, 294–298. [Google Scholar]
- Tamuli, P.; Bhuyan, D.; Boruah, P.; Nath, S.C. Seed-borne fungi of agarwood. Indian Phyto. 1999, 52, 312–315. [Google Scholar]
- Tamuli, P.; Boruah, P.; Saikia, R. Mycofloral study of the phyllosphere and soil of agarwood tree plantation. Plant Arch. 2006, 6, 695–697. [Google Scholar]
- Mohamed, R.; Jong, P.L.; Zali, M.S. Fungal diversity in wounded stems of Aquilaria malaccensis. Fungal Divers. 2010, 43, 67–74. [Google Scholar] [CrossRef]
- Qi, S.Y. Aquilaria species: In vitro culture and the production eaglewood (agarwood). In Medicinal and Aromatic Plants VIII; Springer: Berlin, Germany, 1995; Volume 33, pp. 36–46. [Google Scholar]
- Cui, J.L.; Xiao, P.G.; Guo, S.X.; Wang, M.L. Fungus inducing the formation of agilawood from Aquilaria sinensis. J. Chin. Pharm. Sci. 2012, 47, 335–338. [Google Scholar]
- Cui, J.L.; Xiao, P.G.; Guo, S.X. Field test and analysis for fungus inducing the formation of agilawood from Aquilaria sinensis. J. Chin. Pharm. Sci. 2012, 47, 1614–1617. [Google Scholar]
- Tabata, Y.; Widjaja, E.; Mulyaningsih, T.; Parman, I.; Wiriadinata, H.; Mandang, Y.I.; Itoh, T. Structural survey and artificial induction of aloeswood. Wood Res. 2003, 90, 11–12. [Google Scholar]
- Ma, H.M.; Liang, K.N.; Zhou, Z.Z.; Lin, M.P.; Yang, W. Isolation, identification and analysis of fungi of agarwood formation. J. Cent. South Univ. For. Technol. 2012, 7, 72–75. [Google Scholar]
- Subehan, U.J.; Ueda, J.Y.; Fujino, H.; Attamimi, F.; Kadota, S. A field survey of agarwood in Indonesia. J. Tradit. Med. 2005, 22, 244–251. [Google Scholar]
- Tian, J.J.; Gao, X.X.; Zhang, W.M.; Qu, L.H. Molecular identification of endophytic fungi from Aquilaria sinensis and artificial agarwood induced by pinholes-infusion technique. Afr. J. Biotechnol. 2013, 12, 3115–3131. [Google Scholar]
- Gao, X.X.; Zhou, W.P.; Zhong, Z.J.; Liu, X.J.; Zhang, W.M.; Wu, G.Q. The correlation analysis between benzyl-acetone and the alcohol soluble extract content from agarwood. J. Chin. Med. Mater. 2012, 35, 919–924. [Google Scholar]
- Feng, N.X. The Study of Endophytic Fungi in Agarwood; Nanchang University: Nanchang, China, 2008. [Google Scholar]
- Guo, S.X.; Gong, L.J.; Chen, X.M.; Wang, C.L.; Meng, Z.X. Method for Fungus Inducing the Formation of Agilawood from Aquilaria sinensis. Chinese Patent 101781623A, 21 July 2010. [Google Scholar]
- Ma, H.M.; Liang, K.N.; Zhou, Z.Z.; Huang, G.H.; Lin, M.P. Method for Rapid Formation of Agilawood of Using Fungi Fermentation. Chinese Patent 102550311A, 11 July 2010. [Google Scholar]
- Wang, L.; Zhang, W.M.; Gao, X.X.; Wu, G.Q.; Li, H.H.; Chen, Q. An Artificial Induced Method of Aquilaria sinensis. Chinese Patent 102302041A, 1 July 2011. [Google Scholar]
- Naef, R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour Fragr. J. 2011, 26, 73–89. [Google Scholar] [CrossRef]
- Li, W.; Mei, W.L.; Wang, H.; Zuo, W.J.; Yang, D.L.; Dai, H.F. Chemical constituents from stems of Aquilaria sinensis. Chin. J. Chin. Mater. Med. 2013, 38, 2826–2831. [Google Scholar]
- Chen, D.; Bi, D.; Song, Y.L.; Tu, P.F. Flavanoids from the stems of Aquilaria sinensis. Chin. J. Nat. Med. 2012, 10, 287–291. [Google Scholar] [CrossRef]
- Peng, K.; Mei, W.L.; Wu, J.; Dai, H.F. Flavones from the stem of Aquilaria sinensis. J. Trop. Subtrop. Bot. 2010, 18, 97–100. [Google Scholar]
- Qi, S.Y.; Lin, L.D.; Hu, H.C. The formation of chromone in Aquilaria sinensis. Chin. Tradit. Herb. Drugs 2000, 31, 658–659. [Google Scholar]
- Chen, X.F. The Chalcone Synthase (CHS) Gene Cloning and Bioinformatics Analysis of Three Species of Ferns; Shanghai Normal University: Shanghai, China, 2013. [Google Scholar]
- Kenmotsu., Y.; Ogita, S.; Katoh, Y.; Yamamura, Y.; Takao, Y.; Tatsuo, Y.; Fujino, H.; Kadota, S.; Kurosaki, F. Methyl jasmonate-induced enhancement of expression activity of Am-FaPS-1, a putative farnesyl diphosphate synthase gene from Aquilaria microcarpa. J. Nat. Med. 2011, 65, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Kenmotsu, Y.; Yamamura, Y.; Ogita, S.; Katoh, Y.; Kurosaki, F. Transcriptional activation of putative calmodulin genes Am-cam-1 and Am-cam-2 from Aquilaria microcarpa, in response to external stimuli. Biol. Pharm. Bull. 2010, 33, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Zhang, Z.; Wang, M.X.; Wei, J.H.; Chen, H.; Gao, Z.H.; Sui, C.; Luo, H.; Zhang, X.; Yang, Y.; et al. Identification of genes related to agarwood formation: Transcriptome analysis of healthy and wounded tissues of Aquilaria sinensis. BMC Genom. 2013, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.X.; Li, W.L.; Zhang, Z.; Wei, J.H.; Yang, Y.; Xu, Y.H. Cloning and bioinformatics analysis of chalcone synthase (AsCHS1) gene in Aquilaria sinensis. Chin. J. Chin. Mater. Med. 2013, 38, 149–152. [Google Scholar]
- Cao, T.J.; Dai, H.F.; Li, H.L.; Guo, D.; Mei, W.L.; Peng, S.Q. Cloning and function analysis of the chalcone synthase gene (AsCHS1) promoter in Aquilaria sinensis. Chin. J. Trop. Crop. 2014, 35, 1950–1956. [Google Scholar]
- Wang, X.H.; Gao, B.W.; Liu, X.; Dong, X.J.; Zhang, Z.X.; Fan, H.Y.; Zhang, L.; Wang, H.; Shi, S.P.; Tu, P.F. Salinity stress induces the production of 2-(2-phenylethyl)chromones and regulates novel classes of responsive genes involved in signal transduction in Aquilaria sinensis calli. BMC Plant Biol. 2016, 16, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Wu, H.Q.; He, X.; Wang, L.; Zhang, W.M.; Li, H.H.; Fan, Y.F.; Tan, G.H.; Liu, T.M.; Gao, X.X. Transcriptome sequencing of chemically induced Aquilaria sinensis to identify genes related to agarwood formation. PLoS ONE 2016, 11, e0155505. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.X.; Xie, M.R.; Liu, S.F.; Guo, X.L.; Chen, X.Y.; Zhong, Z.J.; Wang, L.; Zhang, W.M. Chromatographic fingerprint analysis of metabolites in natural and artificial agarwood using gas chromatography-mass spectrometry combined with chemometric methods. J. Chromatogr. B 2014, 967, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Dai, H.F.; Wang, H.; Mei, W.L. Analysis of chemical constituents of essential oil between two batches of agarwood produced by inoculation method using gas chromatography-mass spectrometry. Lishizhen Med. Mater. Med. Res. 2010, 21, 1901–1902. [Google Scholar]
- Xu, F.; Cheng, S.Y.; Cheng, S.H.; Wang, Y.; Du, H.W. Time course of expression of chalcone synthase gene in Ginko biloba. J. Plant Physiol. Mol. Biol. 2007, 33, 309–317. [Google Scholar]
- Liu, T.; Mu, L.; Liang, Y.L.; Wang, J.J.; Yang, S.C. Relationship between expression of chalcone synthase (CHS) and scutellarin content in Erigeron breviscapus. Chin. J. Chin. Mater. Med. 2013, 33, 2241–2244. [Google Scholar]
- Tian, L.; Li, B.; Zhang, J.Y.; Fan, S.X.; Yan, S.R.; Sun, J.M. Correlation analysis between gene expression of isoflavone synthesis relative enzymes and isoflavone accumulation during the seed development in soybean. J. Plant Genet. Resour. 2014, 15, 1295–1304. [Google Scholar]
- Zhang, L.; Zhu, L.X.; Xu, C.; Cui, C.M.; Sheng, H.Y.; Li, R.; Wang, H.Q. The effect of silencing chalcone synthase on anthocyanin metabolism in peach. Acta Hortic. Sin. 2015, 42, 31–37. [Google Scholar]
- Hoffmann, T.; Kalinowski, G.; Schwab, W. RNAi-induced silencing of gene expression in strawberry fruit (Fragaria × ananassa) by agroinfiltration: A rapid assay for gene function analysis. Plant J. 2006, 48, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, W.M.; Pan, Q.L.; Li, H.H.; Tao, M.H.; Gao, X.X. Isolation and molecular identification of endophytic fungi from Aquilaria sinensis. J. Fungal Res. 2009, 7, 37–42. [Google Scholar]
- Chen, X.D.; Zhou, W.P.; Liu, S.F.; Chen, X.Y.; Zhong, Z.J.; Zhang, W.M.; Gao, X.X. A research of different method for isolating total RNA from artificial agarwood induced by Fusarium sp. A2. Lishizhen Med. Mater. Med. Res. 2015, 26, 993–995. [Google Scholar]
- Chen, X.D.; Xie, M.R.; Liu, S.F.; Zhou, W.P.; Zhang, W.M.; Gao, X.X. Relationship between expression of sesquiterpene synthase gene and sesquiterpene content in artificial agarwood induced by Fusarium sp. A2. J. Chin. Pharm. 2014, 39, 197–203. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, F.S. Herbivore-induced volatiles and their roles in plant defence. Acta Entomol. Sin. 1998, 41, 204–214. [Google Scholar]
Sample Availability: Samples of the agarwood are available from the authors. |
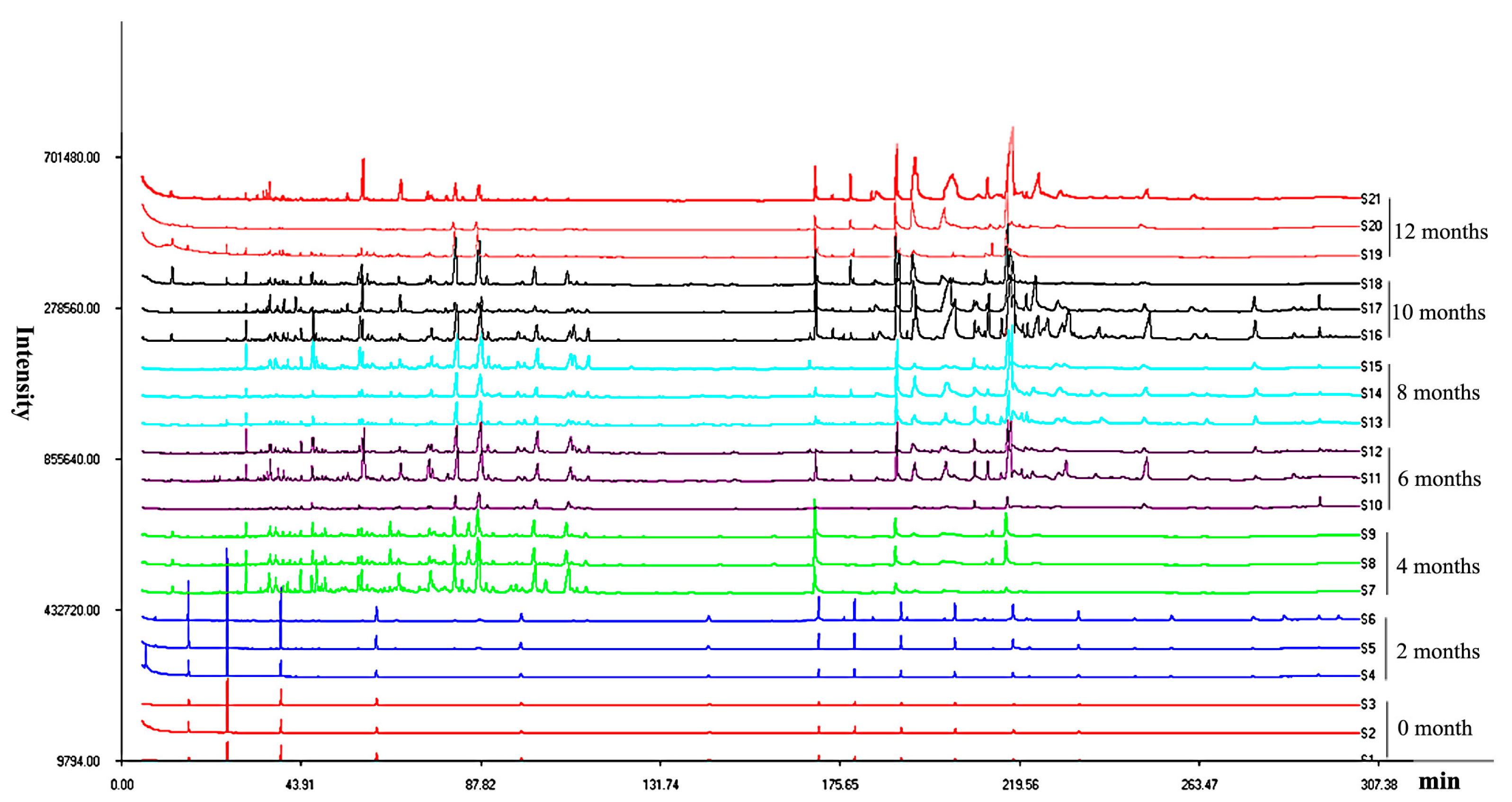
| No. | RT b | RI c | Chemical Name | Formula | Relative Percentage Content/% (Sample Number of Each Compound Can Be Retrieved) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Month | 2 Months | 4 Months | 6 Months | 8 Months | 10 Months | 12 Months | |||||
| A1 | 169.693 | 2297 | 2-(2-phenylethyl)chromone | C17H14O2 | - | - | 2.2000 | 1.0533 | 2.6300 | 4.1600 | 3.6667 |
| A2 | 178.191 | 2423.6 | 6-hydroxy-2-(2-phenylethyl)chromone | C17H14O3 | - | - | 0.0433 | 0.3300 | 0.3533 | 0.4733 | 1.3867 |
| A3 | 184.57 | 2513.5 | 6-hydroxy-2-(2-phenylethyl)chromone | C17H14O3 | - | - | - | 0.3167 | 0.2700 | 0.6267 | 1.3100 |
| A4 | 189.177 | 2578.4 | 6-methoxy-2-(2-phenylethyl)chromone | C17H14O3 | - | - | 1.8467 | 1.6767 | 6.9333 | 6.4700 | 5.8067 |
| A5 | 193.68 | 2641.8 | 6-methoxy-2-(2-phenylethyl)chromone | C17H14O3 | - | - | 0.3267 | 2.2867 | 2.6067 | 3.8633 | 3.1433 |
| A6 | 193.908 | 2641.6 | 6-hydroxy-7-methoxy-2-(2-phenylethyl)chromone | C18H16O3 | - | - | 0.1200 | - | 1.9733 | 0.2900 | 4.5767 |
| A7 | 200.424 | 2736.9 | 6-hydroxy-2-(2-phenylethyl)chromone | C17H14O3 | - | - | - | 1.3733 | 5.0600 | 3.2133 | 4.8733 |
| A8 | 201.506 | 2752.1 | 6-hydroxy-2-[2-(4'-methoxyphenyl)ethyl]chromone | C18H16O4 | - | - | - | 2.0500 | 1.3167 | - | - |
| A9 | 203.394 | 2778.7 | 6-hydroxy-2-(2-phenylethyl)chromone | C17H14O3 | - | - | - | 0.6533 | - | - | - |
| A10 | 203.424 | 2779.1 | 5-hydroxy-6-methoxy-2-(2-phenylethyl)chromone | C17H14O3 | - | - | - | 0.2433 | - | 0.6500 | - |
| A11 | 211.355 | 2890.8 | 6-methoxy-2-[2-(3-methoxyphenyl)ethyl]chromone | C19H18O4 | - | - | 0.2500 | 0.6200 | 1.2767 | 0.8100 | 1.1600 |
| A12 | 214.793 | 2939.2 | 6-methoxy-2-[2-(3-methoxyphenyl)ethyl]chromone | C19H18O4 | - | - | - | - | 0.6500 | 0.1833 | - |
| A13 | 216.212 | 2959.2 | 6,7-dimethoxy-2-(2-phenylethyl)chromone | C19H18O4 | - | - | 1.9500 | 6.0767 | 12.6967 | 11.6833 | 13.2467 |
| A14 | 218.373 | 2989.7 | 5,8-dihydroxy-2-[2-(4′-met hoxyphenethyl)]chromone | C18H16O5 | - | - | - | 2.9933 | 1.7667 | 1.0500 | 1.4100 |
| A15 | 220.064 | 3013.5 | 6,8-dihydroxy-2-[2-(3′-methoxy-4′-hydroxyl phenylethyl)]chromone | C18H16O5 | - | - | - | 0.4733 | 0.9467 | - | 0.4833 |
| A16 | 222.57 | 3048.8 | 6-hydroxy-7-methoxy-2-(2-phenylethyl)chromone | C18H16O3 | - | - | - | 1.5267 | 1.8400 | 2.3667 | 2.7067 |
| A17 | 228.351 | 3130.2 | 6-hydroxy-2-[2-(4′-methoxyphenyl)ethyl]chromone | C18H16O4 | - | - | - | 0.7833 | 0.9000 | - | 0.6900 |
| A18 | 233.746 | 3206.2 | 6,8-dihydroxy-2-[2-(3′-methoxy-4'-hydroxyl phenylethyl)]chromone | C18H16O5 | - | - | 0.03 | - | 1.0700 | - | - |
| A19 | 262.132 | 3606.1 | 6,8-dihydroxy-2-[2-(3′-methoxy-4′-hydroxyl phenylethyl)]chromone | C18H16O5 | - | - | - | 0.3867 | - | - | 0.4100 |
| Relative percentage content of total chromonetration (n = 3) | - | - | 6.767 ± 2.30 | 22.84 ± 6.60 | 42.29 ± 3.52 | 35.84 ± 10.27 | 44.87 ± 19.44 | ||||
| No. | RT b | RI c | Chemical Name | Formula | Relative Percentage Content/ % (Sample Number of Each Compound Can Be Retrieved) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Month | 2 Months | 4 Months | 6 Months | 8 Months | 10 Months | 12 Months | |||||
| A20 | 30.411 | 1575.4 | Isoaromadendrene epoxide | C15H24O | - | - | 0.8900 | 0.5267 | 0.2433 | 0.2733 | 0.2233 |
| A21 | 33.98 | 1620.6 | Aromadendrene oxide-(1) | C15H24O | - | - | 0.5067 | 0.1200 | - | 0.0300 | 0.0467 |
| A22 | 34.736 | 1628.4 | Agarospirol | C15H26O | - | - | 1.0733 | 0.0633 | - | 0.0333 | 0.3200 |
| A23 | 36.234 | 1644 | Guaiol | C15H26O | - | - | 2.9500 | 0.6400 | 0.0933 | 0.2100 | 0.2567 |
| A24 | 41.098 | 1694.6 | Santalol | C15H24O | - | - | 0.1533 | 0.0767 | - | 0.1500 | 0.0667 |
| A25 | 42.373 | 1705.4 | Aromadendrene oxide-(2) | C15H24O | - | - | 0.1167 | 0.0967 | 0.0767 | - | - |
| A26 | 43.736 | 1715.3 | 2-(4a,8-Dimethyl-1,2,3,4,4a,5,6,7-octahydro-naphthalen-2-yl)-prop-2-en-1-ol | C15H24O | - | - | 0.2500 | 0.4567 | 0.0433 | 0.2200 | 0.1233 |
| A27 | 46.384 | 1734.4 | Longipinocarvone | C15H22O | - | - | 0.3733 | 0.4733 | 0.0733 | 0.0200 | - |
| A28 | 46.719 | 1737 | Germacrone | C15H22O | - | - | 0.2767 | 0.4067 | 0.2267 | 0.1533 | 0.1000 |
| A29 | 47.54 | 1742.8 | Viridiflorol | C15H26O | - | - | 0.9233 | 0.4233 | 0.0800 | 0.1933 | - |
| A30 | 49.743 | 1758.7 | γ-Gurjunenepoxide-(2) | C15H24O | - | - | 0.7100 | 0.2233 | 0.0467 | - | - |
| A31 | 58.158 | 1817.5 | Baimuxinal | C15H24O2 | - | - | 4.5400 | 2.5200 | 0.3733 | 1.7333 | 1.5033 |
| A32 | 67.806 | 1865.4 | Longifolenaldehyde | C15H24O | - | - | 0.8233 | 0.3467 | 0.1133 | 1.1700 | 0.2367 |
| A33 | 81.632 | 1927.4 | Eudesma-5,11(13)-dien-8,12-olide | C15H20O2 | - | - | 1.6833 | - | 1.0867 | 1.5867 | 4.0500 |
| A34 | 81.939 | 1928.6 | Velleral | C15H20O2 | - | - | - | - | 5.9500 | - | 3.8500 |
| A35 | 89.478 | 1957 | Vellerdiol | C15H24O2 | - | - | 3.4367 | - | 0.3567 | 0.1700 | 0.3100 |
| A36 | 100.903 | 2000.1 | 6-(1-Hydroxymethylvinyl)-4,8a-dimethyl-3,5,6,7,8,8a-hexahydro-1H-naphthalen-2-one | C15H22O2 | - | - | 2.8433 | 2.5167 | - | - | - |
| Relative percentage content of total sesquiterpenetration (n = 3) | - | - | 21.55 ± 3.63 | 8.890 ± 2.46 | 8.763 ± 1.56 | 5.943 ± 1.73 | 11.09 ± 9.45 | ||||
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | A11 | A12 | A13 | A14 | A15 | A16 | A17 | A18 | A19 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHS1 | Pearson correlation | 0.372 | 0.922 ** | 0.873 * | 0.261 | 0.338 | 0.870 * | 0.467 | −0.229 | −0.074 | −0.261 | 0.426 | −0.338 | 0.436 | 0.165 | 0.201 | 0.536 | 0.358 | −0.275 | 0.749 |
| Bilateral significance | 0.467 | 0.009 | 0.023 | 0.617 | 0.512 | 0.024 | 0.35 | 0.663 | 0.889 | 0.618 | 0.4 | 0.512 | 0.387 | 0.755 | 0.703 | 0.273 | 0.485 | 0.598 | 0.087 | |
| CHS2 | Pearson correlation | 0.498 | −0.002 | 0.168 | 0.323 | 0.518 | −0.312 | 0.055 | −0.291 | −0.108 | 0.953 ** | 0.013 | −0.042 | 0.246 | −0.06 | −0.488 | 0.342 | −0.492 | −0.327 | −0.266 |
| Bilateral significance | 0.315 | 0.997 | 0.751 | 0.533 | 0.292 | 0.548 | 0.918 | 0.576 | 0.839 | 0.003 | 0.98 | 0.938 | 0.639 | 0.91 | 0.326 | 0.507 | 0.322 | 0.527 | 0.61 | |
| A20 | A21 | A22 | A23 | A24 | A25 | A26 | A27 | A28 | A29 | A30 | A31 | A32 | A33 | A34 | A35 | A36 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHS1 | Pearson correlation | −0.174 | −0.156 | 0.079 | −0.174 | −0.038 | −0.39 | −0.065 | −0.266 | −0.243 | −0.331 | −0.257 | −0.036 | −0.238 | 0.821 * | 0.347 | −0.165 | −0.229 |
| Bilateral significance | 0.742 | 0.767 | 0.882 | 0.742 | 0.943 | 0.445 | 0.903 | 0.611 | 0.642 | 0.521 | 0.624 | 0.946 | 0.65 | 0.045 | 0.5 | 0.755 | 0.662 | |
| CHS2 | Pearson correlation | −0.111 | −0.188 | −0.242 | −0.184 | 0.576 | −0.428 | 0.195 | −0.243 | −0.114 | −0.063 | −0.252 | 0.03 | 0.786 | −0.004 | −0.433 | −0.203 | −0.238 |
| Bilateral significance | 0.835 | 0.722 | 0.644 | 0.727 | 0.231 | 0.397 | 0.711 | 0.643 | 0.83 | 0.905 | 0.63 | 0.955 | 0.064 | 0.994 | 0.391 | 0.699 | 0.649 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhu, X.; Feng, M.; Zhong, Z.; Zhou, X.; Chen, X.; Ye, W.; Zhang, W.; Gao, X. Relationship between Expression of Chalcone Synthase Genes and Chromones in Artificial Agarwood induced by Formic Acid Stimulation Combined with Fusarium sp. A2 Inoculation. Molecules 2017, 22, 686. https://doi.org/10.3390/molecules22050686
Chen X, Zhu X, Feng M, Zhong Z, Zhou X, Chen X, Ye W, Zhang W, Gao X. Relationship between Expression of Chalcone Synthase Genes and Chromones in Artificial Agarwood induced by Formic Acid Stimulation Combined with Fusarium sp. A2 Inoculation. Molecules. 2017; 22(5):686. https://doi.org/10.3390/molecules22050686
Chicago/Turabian StyleChen, Xiaodong, Xiaoling Zhu, Meirou Feng, Zhaojian Zhong, Xin Zhou, Xiaoying Chen, Wei Ye, Weimin Zhang, and Xiaoxia Gao. 2017. "Relationship between Expression of Chalcone Synthase Genes and Chromones in Artificial Agarwood induced by Formic Acid Stimulation Combined with Fusarium sp. A2 Inoculation" Molecules 22, no. 5: 686. https://doi.org/10.3390/molecules22050686






