Characterization of Compounds with Tumor–Cell Proliferation Inhibition Activity from Mushroom (Phellinus baumii) Mycelia Produced by Solid-State Fermentation
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Inhibitory Effects of Extracts on HepG2 Tumor Cells
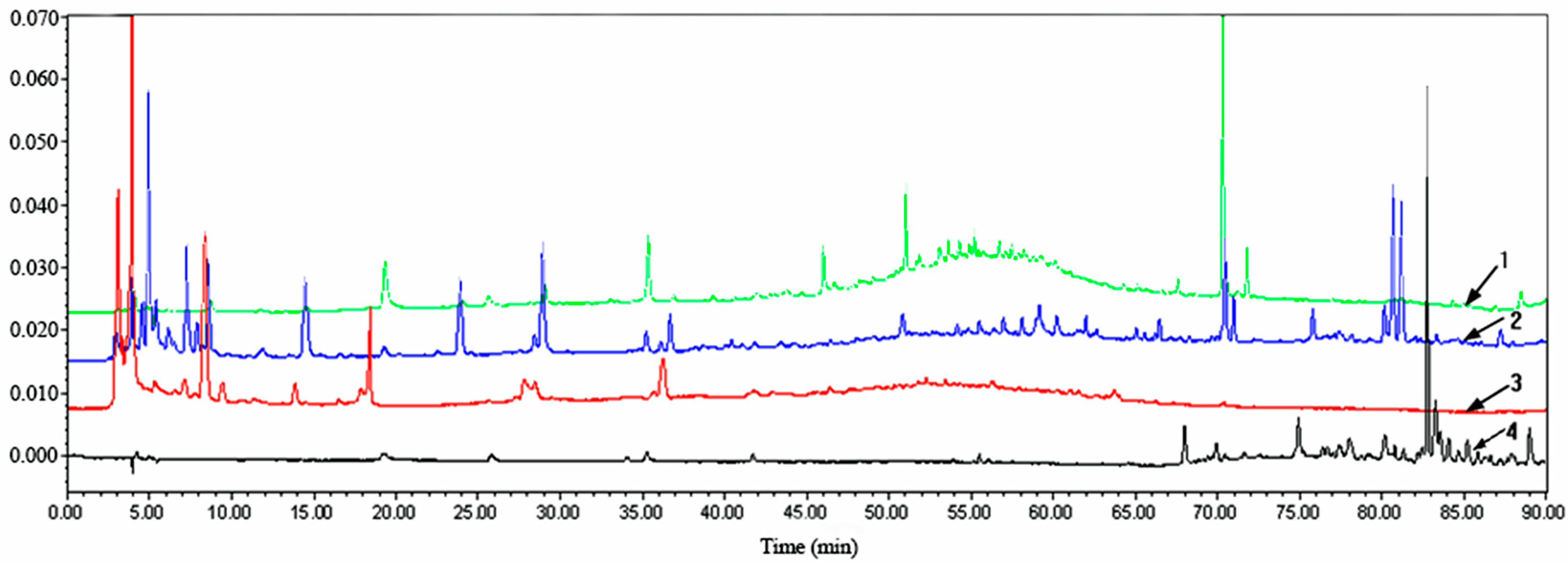
2.2. HPLC Analysis of Extracts from P. baumii Mycelia Fermented on Rice
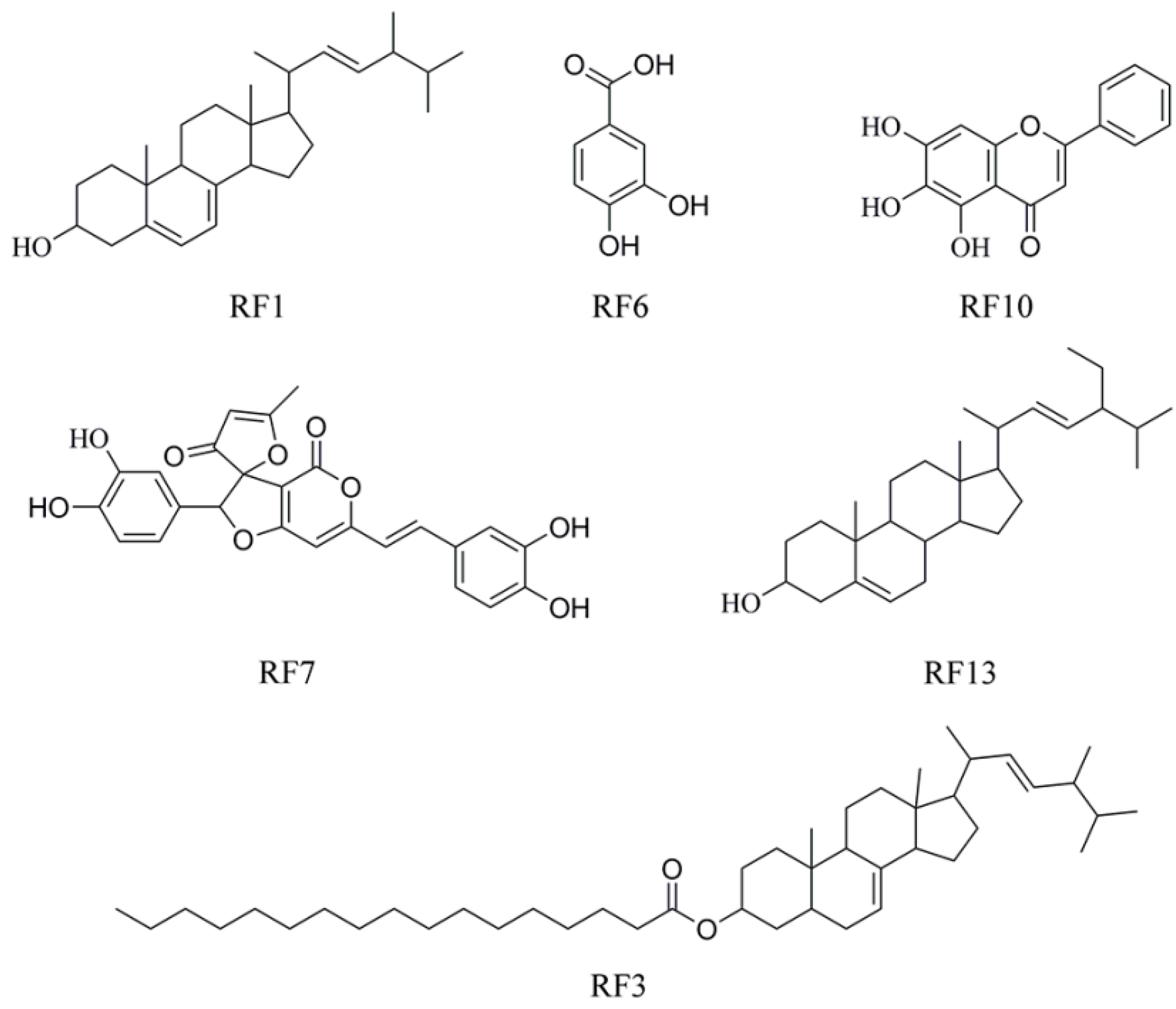
2.3. Compound Identification and Structure Elucidation
2.4. Evaluation of the Cytotoxicity of Compounds
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Culture Media and Conditions
3.3. The Preparation of the Extracts of P. baumii
3.4. HPLC Analyses
3.5. The Isolation of Compounds
3.6. Structure Analyses
3.7. Cell Culture and Cell Antiproliferation Assay
3.7.1. Cell Lines and Culture
3.7.2. Inhibitory Effects of Compounds from P. baumii on Tumor Cell Proliferation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 20 June 2011).
- Tian, Y.T.; Zhao, Y.T.; Zeng, H.L.; Zhang, Y.L.; Zheng, B.D. Structural characterization of a novel neutral polysaccharide from Lentinus giganteus and its antitumor activity through inducing apoptosis. Carbohydr. Polym. 2016, 154, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Zeng, P.; Li, X.; Shi, L.G. Antitumor and immunomodulation activities of polysaccharide from Phellinus baumii. Int. J. Biol. Macromol. 2016, 91, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Staffa, J.A.; Chang, J.; Geen, L. Cerivastain and reports of fatal rhabdomyolysis. N. Engl. J. Med. 2002, 346, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Song, T.Y.; Lin, H.C.; Yang, N.C.; Hu, M.L. Antiproliferative and antimetastatic effects of the ethanolic extract of Phellinus igniarius (Linnearus: Fries) Quelet. J. Ethnopharmacol. 2008, 115, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Chen, Y.; Nie, S.P.; Xie, M.Y.; He, M.; Zhang, S.S.; Zhu, K.X. Ganoderma atrum polysaccharide induces anti-tumor activity via the mitochondrial apoptotic pathway related to activation of host immune response. J. Cell. Biochem. Suppl. 2011, 112, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Chieh, S.Y.; Tso, T.K.; Chien, T.Y.; Lin, H.T.; Tsai, Y.C. Orally administered mycelial culture of Phellinus linteus exhibits antitumor effects in hepatoma cell-bearing mice. J. Ethnopharmacol. 2011, 133, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, J.; Liu, Y.; Feng, N.; Chen, H.; Tang, Q.; Ye, L.; Zhang, J. Antioxidant and cytotoxic activities of ethanolic extracts and isolated fractions of species of the genus Phellinus Quel. (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2011, 13, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.C.; Zhou, L.W.; Cui, B.K.; Chen, Y.Q.; Decock, C. Current advances in Phellinus sensu lato: Medicinal species, functions, metabolites and mechanisms. Appl. Microbiol. Biotechnol. 2010, 87, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, Q.J.; Zhu, M.J.; Wang, H.X.; Zhang, G.Q. An extracellular laccase with antiproliferative activity from the sanghuang mushroom Inonotus baumii. J. Mol. Catal. B Enzym. 2014, 99, 20–25. [Google Scholar] [CrossRef]
- Lung, M.Y.; Tsai, J.C.; Huang, P.C. Antioxidant properties of edible basidiomycete Phellinus igniarius in submerged cultures. J. Food Sci. 2010, 75, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pan, J.Z.; Li, X.; Zhou, Y.; Meng, Q.L.; Wang, Q. Endo-polysaccharide of Phellinus igniarius exhibited anti-tumor effect through enhancement of cell mediated immunity. Int. Immunopharmacol. 2011, 11, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.H.; Syu, J.L.; Lee, Y.L.; Mau, J.L. Nonvolatile taste components of solid-state fermented adlay and rice by Phellinus linteus. LWT–Food Sci. Technol. 2009, 42, 1738–1743. [Google Scholar] [CrossRef]
- Shao, Q.; Yang, Y.; Li, T.T.; Feng, J.; Liu, Y.F.; Yan, M.Q.; Zhu, L.N.; Tang, C.H. Biological activities of ethanol extracts of Phellinus baumii (Higher Basidiomycetes) obtained by different fermentation methods. Int. J. Med. Mushrooms 2015, 17, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Krzyczkowski, W.; Malinowska, E.; Suchocki, P.; Kleps, J.; Olejnik, M.; Herold, F. Isolation and quantitative determination of ergosterolperoxidein various edible mushroom species. Food Chem. 2009, 113, 351–355. [Google Scholar] [CrossRef]
- Ziegenbein, F.C.; Hanssen, H.P.; König, W.A. Secondary metabolites from Ganoderma lucidum and Spongiporus leucomallellus. Phytochemistry 2006, 67, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, D.A.; Vieira-Coelho, M.A.; Benes, J.; Alves, P.C.; Borges, N.; Freitas, A.P.; Soares-Da-Silva, P. Synthesis of 1-(3,4-dihydroxy-5-nitrophenyl)-2-phenyl-ethanone and derivatives as potent and long-acting peripheral inhibitors of catechol-O-methyltransferase. J. Med. Chem. 2002, 45, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.S.; Lee, I.K.; Choi, H.J.; Yun, B.S. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg. Med. Chem. Lett. 2015, 25, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Kim, Y.S.; Seok, S.J.; Yun, B.S. Inoscavin E, a free radical scavenger from the fruiting bodies of Inonotus xeranticus. J. Antibiot. 2007, 60, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Keshari, A.K.; Kumar, G.; Kushwaha, P.S.; Bhardwaj, M.; Kumar, P.; Rawat, A.; Kumar, D.; Prakash, A.; Ghosh, B.; Saha, S. Isolated flavonoids from Ficus racemosa stem bark possess antidiabetic, hypolipidemic and protective effects in albino Wistar rats. J. Ethnopharmacol. 2016, 181, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Matsubara, Y.; Ghosh, P.; Thakur, S.; Shimizu, N. The 24α- and 24β-epimers of 24-ethylcholesta-5,22-dien-3β-ol in two Clerodendrum species. Phytochemistry 1988, 27, 1169–1172. [Google Scholar] [CrossRef]
- Lee, I.K.; Han, M.S.; Lee, M.S.; Kim, Y.S.; Yun, B.S. Styrylpyrones from the medicinal fungus Phellinusbaumii and their antioxidant properties. Bioorg. Med. Chem. Lett. 2010, 20, 5459–5461. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Lin, Z.M.; Wang, L.N.; Guo, D.X.; Wang, S.Q.; Liu, Y.Q.; Yuan, H.Q.; Lou, H.X. Phenolic compounds with NF-κB inhibitory effects from the fungus Phellinus baumii. Bioorg. Med. Chem. Lett. 2011, 21, 3261–3267. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Song, J.G.; Hwang, B.S.; Kim, D.W.; Lee, Y.J.; Woo, E.E.; Kim, J.Y.; Lee, I.K.; Yun, B.S. Lipoxygenase inhibitory activity of Korean indigenous mushroom extracts and isolation of an active compound from Phellinus baumii. Mycobiology 2014, 21, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Wu, N.; Yang, Y.; Zhang, J.S.; Tang, Q.J.; Shao, Q. Compounds from the fruiting bodies of Phellinus baumii and their inhibition to tumor cell proliferation. Mycosystema 2015, 34, 124–130. [Google Scholar]
- McNulty, J.; Nair, J.J.; Bollareddy, E.; Keskar, K.; Thorat, A.; Crankshaw, D.J.; Holloway, A.C.; Khan, G.; Wright, G.D.; Ejim, L. Isolation of flavonoids from the heartwood and resin of Prunus avium and some preliminary biological investigations. Phytochemistry 2009, 70, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.F.; Shi, W.W.; Yu, C.H.; Jin, X.Y.; Zhang, H.H.; Zhang, W.Y.; Wang, L.C.; Yu, B. Baicalein attenuates vinorelbine-induced vascular endothelial cell injury and chemotherapeutic phlebitis in rabbits. Toxicol. Appl. Pharmacol. 2017, 23, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shane, S.A.; Smith, J.N.; Chen, Y.C. Anticancer properties of baicalein: A review. Med. Chem. Res. 2016, 25, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.H.; Tsheng, Y.M.; Lee, Y.J. Cytotoxicity effects of di- and tri-hydroxybenzaldehydes as a chemopreventive potential agent on tumor cells. Toxicology 2001, 161, 179–187. [Google Scholar] [CrossRef]
- Lin, G.Q.; You, Q.D.; Cheng, J.F. Chiral Drugs: Chemistry and Biological Action; John Wiley & Sons, Inc., Publication: Hoboken, NJ, USA, 2011. [Google Scholar]
- Zhang, Z.; Tudia, T.; Liu, Y.F.; Zhou, S.; Feng, N.; Yang, Y.; Tang, C.H.; Tang, Q.J.; Zhang, J.S. Preparative isolation of cordycepin, N6-(2-hydroxyethyl)-adenosine and adenosine from Cordycepsmilitaris by macroporous resin and purification by recycling high-speed counter-current chromatography. J. Chromatogr. B 2016, 1033, 18–225. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| RF10 | ||||||
|---|---|---|---|---|---|---|
| IC50 (μg/mL) | HepG2 | K562 | L1210 | LNCaP | MCF-7 | SW620 |
| 60.22 ± 1.98 | 39.61 ± 0.36 | 23.62 ± 0.72 | 19.515 ± 0.59 | 37.73 ± 1.23 | 33.17 ± 1.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Shao, Q.; Wang, W.; Zhang, J.; Zhang, Z.; Liu, Y.; Yang, Y. Characterization of Compounds with Tumor–Cell Proliferation Inhibition Activity from Mushroom (Phellinus baumii) Mycelia Produced by Solid-State Fermentation. Molecules 2017, 22, 698. https://doi.org/10.3390/molecules22050698
Zhang H, Shao Q, Wang W, Zhang J, Zhang Z, Liu Y, Yang Y. Characterization of Compounds with Tumor–Cell Proliferation Inhibition Activity from Mushroom (Phellinus baumii) Mycelia Produced by Solid-State Fermentation. Molecules. 2017; 22(5):698. https://doi.org/10.3390/molecules22050698
Chicago/Turabian StyleZhang, Henan, Qian Shao, Wenhan Wang, Jingsong Zhang, Zhong Zhang, Yanfang Liu, and Yan Yang. 2017. "Characterization of Compounds with Tumor–Cell Proliferation Inhibition Activity from Mushroom (Phellinus baumii) Mycelia Produced by Solid-State Fermentation" Molecules 22, no. 5: 698. https://doi.org/10.3390/molecules22050698
APA StyleZhang, H., Shao, Q., Wang, W., Zhang, J., Zhang, Z., Liu, Y., & Yang, Y. (2017). Characterization of Compounds with Tumor–Cell Proliferation Inhibition Activity from Mushroom (Phellinus baumii) Mycelia Produced by Solid-State Fermentation. Molecules, 22(5), 698. https://doi.org/10.3390/molecules22050698







