Rapid Quantification and Quantitation of Alkaloids in Xinjiang Fritillaria by Ultra Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. Characterization of Chemical Constituents
2.2. Quantitative Analysis
2.2.1. Method Validation
2.2.2. Quantitative Investigations
2.2.3. Producing Area Selection
2.2.4. Index Components Selection
2.2.5. Sample Pretreatment
2.2.6. Result Analysis of the Content Determination
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Method
4.2.1. Characterization of Chemical Constituents
4.2.2. Quantitative Analysis
4.3. Preparation of Sample Solutions
4.3.1. Characterization of Chemical Constituents
4.3.2. Quantitative Analysis
4.4. Quantitative Method Validation
4.5. Standard Addition Recovery Experiments
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- China Pharmacopoeia Committee. Part 1. Pharmacopoeia of the People’s Republic of China; China Chemical Industry Press: Beijing, China, 2015; pp. 141–142. [Google Scholar]
- Wang, D.; Wang, S.; Chen, X.; Xu, X.; Zhu, J.; Nie, L.; Long, X. Antitussive, expectorant and anti-inflammatory activities of four alkaloids isolated from Bulbus of Fritillaria wabuensis. J. Ethnopharmacol. 2012, 139, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, J.; Du, Q.; Li, H.; Wang, S. The total alkaloid fraction of bulbs of Fritillaria cirrhosa displays anti-inflammatory activity and attenuates acute lung injury. J. Ethnopharmacol. 2016, 193, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Sheng, P.; Yao, L.; Shi, H.; Zhang, X. ISSR Analysis on genetic diversity of 8 Species of plants in Fritillaria L. from Xinjiang. Chin. Wild Plant Resour. 2015, 4, 1–6. [Google Scholar]
- Xu, X.Q.; Xu, X.W. Review on the research of Fritillaria. Qiu Yi Wen Yao. 2013, 11, 319–320. (In Chinese) [Google Scholar]
- Hao, D.C.; Gu, X.J.; Xiao, P.G. Phytochemical and biological research of Fritillaria medicinal resources. Chin. J. Nat. Med. 2013, 11, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.S.; Ma, Y.; Wang, Z.Y.; Liu, X.S.; Liu, Y. Analysis on chemical constituents in Epimedii Herba by UPLC/Q-TOF-MS. Drugs Clin. 2014, 29, 349–352. [Google Scholar]
- Zhou, N.; Guo, D.Q.; Shen, L.; Chen, Q.Y.; Qin, Y. Comparative contents of four alkaloids in bulbs of Fritillaria taipaiensis and Fritillaria unibracteata. Food Sci. 2014, 35, 133–136. [Google Scholar]
- Peng, R.; Tan, J.; Ma, P. Study on HPLC fingerprint of alkaloids in Fritillaria taipaiensis. Mod. Tradit. Chin. Med. Materia Medica-World Sci. Technol. 2015, 17, 152–155. [Google Scholar]
- Wang, Y.; Zhang, Q.L.; Chen, X.Y.; Tang, Z.H.; Wu, Z. Quantitative determination of peiminine in Bulbus Fritillaria available on market by HPLC. Chin. Tradit. Herb. Drugs 2001, 32, 24–25. [Google Scholar]
- Chen, M.; Liu, M.Y.; Hu, C. Determination of Peiminine in Wenglitong tablets by TLC. Chin. Pharm. 2008, 12, 936–937. [Google Scholar]
- Zhou, J.; Li, P.; Li, H.; Jiang, Y.; Ren, M.; Liu, Y. Development and validation of a liquid chromatography/electrospray ionization time-of-flight mass spectrometry method for relative and absolute quantification of steroidal alkaloids in Fritillaria species. J. Chromatogr. A 2008, 1177, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, W.; Zeng, X.; Chen, B. Fingerprint analysis of Fritillaria thunbergii using rapid resolution liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Chin. J. Chin. Mater. Med. 2013, 38, 2832–2837. [Google Scholar]
- Klockmann, S.; Reiner, E.; Bachmann, R.; Hackl, T.; Fischer, M. Food fingerprinting: metabolomic approaches for geographical origin discrimination of hazelnuts (Corylus avellana) by UPLC-QTOF-MS. J. Agric. Food Chem. 2016, 64, 9253–9262. [Google Scholar] [CrossRef] [PubMed]
- In, G.; Seo, H.K.; Park, H.W.; Jang, K.H. A Metabolomic approach for the discrimination of red ginseng root parts and targeted validation. Molecules 2017, 22, 471. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, L.; Dai, X.; Huang, L.; Yang, M.; Chen, S. Identification and quantitative analysis of nucleosides and nucleobases in aqueous extracts of Fritillaria Cirrhosa D. Don. Using HPLC–DAD and HPLC-ESI-MS. Anal. Lett. 2011, 44, 2491–2502. [Google Scholar] [CrossRef]
- Liu, M.; Xu, W.; Xu, C.; Chen, D.; Wang, J. Two new steroidal alkaloids from bulbs of Fritillaria pallidiflora. Chin. Tradit. Herb. Drugs 2016, 47, 876–880. [Google Scholar]
- Xu, W.; Liu, M.; Chen, D.; Wang, J. Chemical constituents from bulbs of Fritillaria pallidiflora Schrenk. Biochem. Syst. Ecol. 2014, 57, 198–202. [Google Scholar] [CrossRef]
- Pan, B.; Su, X.; Hu, B.; Yang, N.; Chen, Q.; Wu, W. Fusarium redolens 6WBY3, an endophytic fungus isolated from Fritillaria unibracteata var. wabuensis, produces peimisine and imperialine-3β-d-glucoside. Fitoterapia 2015, 103, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Quality Study on Fritillariae Cirrhosae Bulbus. Ph.D. Thesis, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China, 2013. [Google Scholar]
- Li, Y.; Zhang, L.; Wu, H.; Wu, X.; Ju, L.; Zhang, Y. Metabolomic study to discriminate the di erent Bulbus Fritillariae species using rapid resolution liquid chromatography-quadrupole time-of- ight mass spectrometry coupled with multivariate statistical analysis. Anal. Methods 2014, 6, 2247–2259. [Google Scholar] [CrossRef]
- Yan, L.; Abulimiti, Y.; Jun, L.; Aziz, M.; Haji, A.A. New isosteroidal alkaloids with tracheal relaxant effect from the bulbs of Fritillaria pallidiflora Schrenk. Bioorg. Med. Chem. Lett. 2016, 26, 1983–1987. [Google Scholar]
- Jiang, Y.; Dai, J.; Xiao, W.; Zhao, L. Isolation and purification of peimisine from Fritillaria taipaiensis bulbs by High-speed Counter-current Chromatography. Chem. Ind. For. Prod. 2015, 35, 86–90. [Google Scholar]
- Liang, J.; Cao, X.; Jian, W.; Ren, H.; Wu, S. Analysis of volatile components of flowers of Fritillaria thunbergii by GC-TOF-MS. Zhongguo Zhong Yao Za Zhi 2011, 36, 2689–2692. (In Chinese) [Google Scholar] [PubMed]
- Zhang, L.; Sun, J.; Wen, Q.; Ma, Y.; Feng, L. LC-MSn Analysis of the components in bulbus Fritilariae Ussuriensis and the urine of rats after oral administered bulbus Fritillariae Ussuriensis. Chin. J. Pharmacovigil. 2014, 11, 203–205. [Google Scholar]
- Shang, Y.; Li, S.; Xiao, L. Review on extraction technology of alkaloids from plants. Mod. Chem. Ind. 2002, 22, 51–54. [Google Scholar]
- Lu, A.; Wu, H. Research progress of nucleosides in traditional Chinese Medicine. Chin. J. Inf. Tradit. Chin. Med. 2006, 13, 94. [Google Scholar]
- China Pharmacopoeia Committee. Part 3. Pharmacopoeia of the People’s Republic of China; China Chemical Industry Press: Beijing, China, 2015; pp. 213–214. [Google Scholar]
- Zhou, J.L.; Xin, G.Z.; Shi, Z.Q.; Ren, M.T.; Qi, L.W.; Li, H.J.; Li, P. Characterization and identification of steroidal alkaloids in Fritillaria species using liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7109–7122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
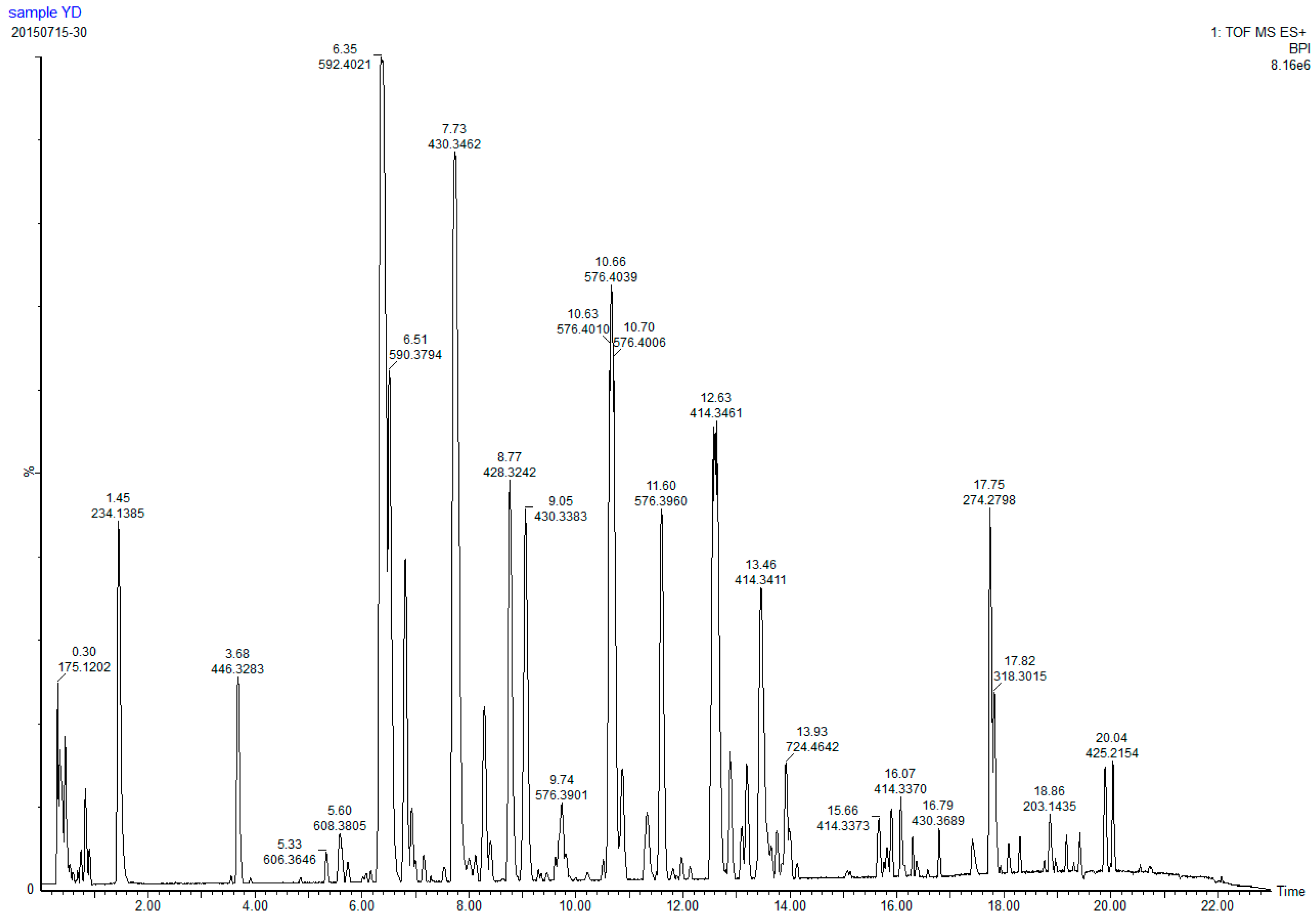
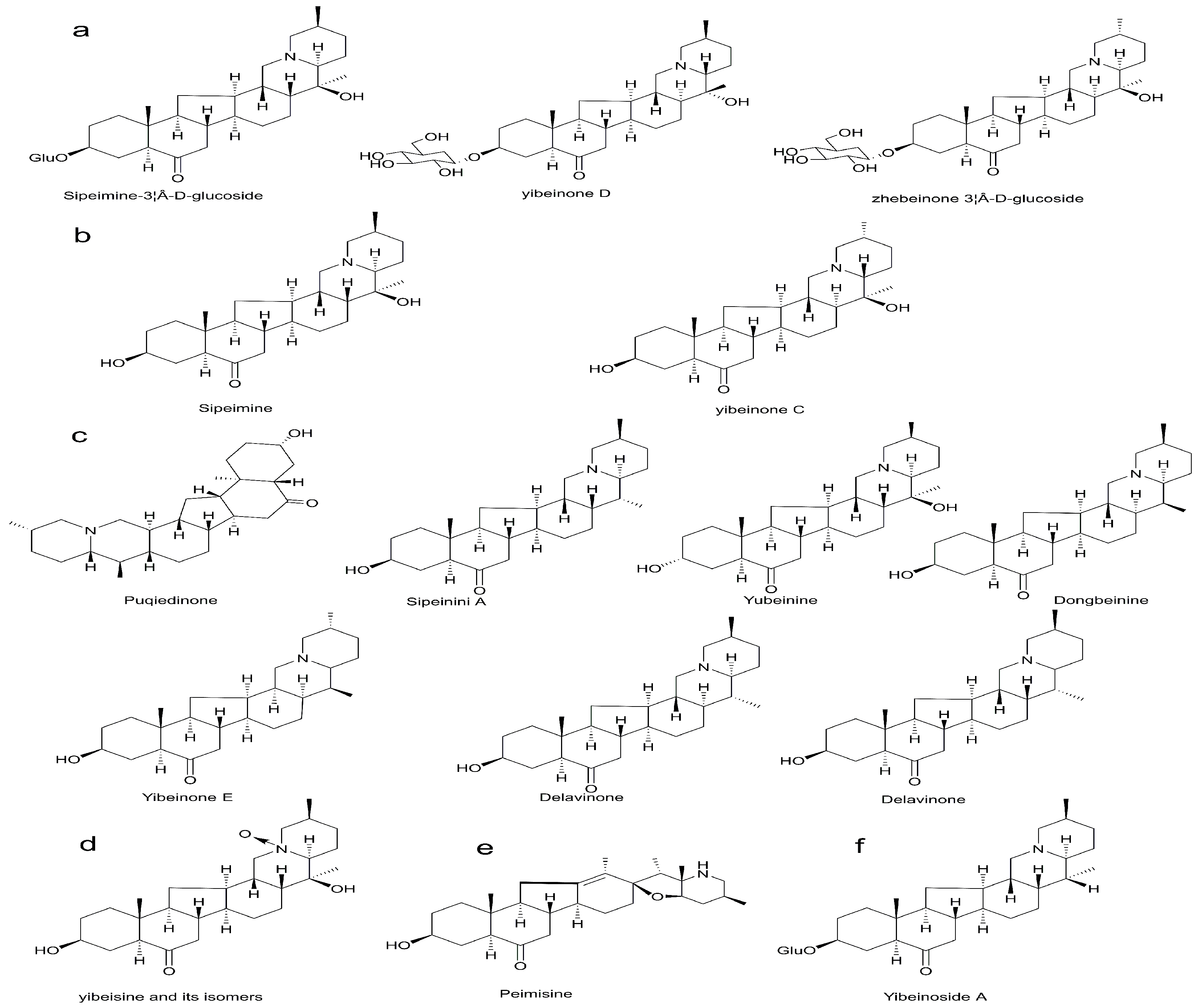
| Peak | tR/min | Formula | Experiment Value m/z | Theroretical Value m/z | Error | Fragments | Compound | Relative Mass Fraction % | References | Structural Formula | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mDa | ppm | ||||||||||
| 1 | 0.3 | C6H14N4O2 | 175.1202 | 175.1195 | 0.7 | 4 | 0.61 | ||||
| 2 | 0.35 | C6H14N4O2 | 175.1198 | 175.1195 | 0.3 | 1.7 | 1.47 | ||||
| 3 | 0.45 | C9H17NO8 | 268.1047 | 268.1046 | 0.1 | 0.4 | 136.0632, 119.036 | Adenosine | 0.97 | [16,17,18] | |
| 4 | 0.54 | C12H21NO6 | 276.1445 | 276.1447 | −0.2 | −0.7 | 0.24 | ||||
| 5 | 0.6 | C10H23NO6 | 254.1617 | 254.1617 | 0 | 0 | 0.10 | ||||
| 6 | 0.68 | C9H15NO4 | 202.1081 | 202.1079 | 0.2 | 1 | 0.09 | ||||
| 7 | 0.75 | C6H9NO3 | 144.0663 | 144.0661 | 0.2 | 1.4 | 0.20 | ||||
| 8 | 0.83 | C8H9N | 120.0818 | 120.0813 | 0.5 | 4.2 | 0.60 | ||||
| 9 | 0.9 | C15H35NO10 | 390.2355 | 390.2355 | 0.1 | 0.3 | 0.22 | ||||
| 10 | 1.45 | C10H19NO5 | 234.1385 | 234.1341 | 4.4 | 3.2 | 3.17 | ||||
| 11 | 3.56 | C33H51NO9 | 606.3644 | 606.3642 | 1.2 | 2 | 0.10 | ||||
| 12 | 3.68 | C27H43NO4 | 446.3283 | 446.3284 | 0.4 | 0.9 | yibeisine | 1.65 | [6,19] | Figure 2D | |
| 13 | 3.92 | C33H51NO10 | 622.3589 | 622.3605 | −1.6 | −2.6 | 0.09 | ||||
| 14 | 4.85 | C33H51NO9 | 606.3632 | 606.3642 | −1 | −1.6 | 0.08 | ||||
| 15 | 4.93 | C33H51NO9 | 606.364 | 606.3642 | −0.2 | −0.3 | 0.05 | ||||
| 16 | 5.33 | C33H51NO9 | 606.3646 | 606.3642 | 0.4 | 0.7 | 0.25 | ||||
| 17 | 5.6 | C33H53NO9 | 608.3805 | 608.3799 | 0.5 | 0.8 | 0.51 | ||||
| 18 | 5.73 | C34H55NO8 | 606.4001 | 606.4006 | −0.5 | −0.8 | 0.22 | ||||
| 19 | 6.02 | C39H63NO13 | 754.4374 | 754.4378 | −1.7 | −2.3 | 0.07 | ||||
| 20 | 6.08 | C39H63NO13 | 754.438 | 754.4378 | 0.2 | 0.3 | 0.14 | ||||
| 21 | 6.17 | C33H53NO8 | 592.385 | 592.385 | 0 | 0 | 138.1285, 574.3744, 412.3216, 394.3110, | Sipeimine-3β-d-glucoside | 0.11 | [20,21] | Figure 2A |
| 22 | 6.35 | C33H53NO8 | 592.4021 | 592.4015 | 0.6 | 1 | 138.1285, 574.3744, 412.3216, 394.3110 | Sipeimine-3β-d-glucoside its isomers | 11.95 | [20,21] | Figure 2A |
| 23 | 6.51 | C33H51NO8 | 590.3693 | 590.3588 | 0.6 | 1 | 428.3167, 412.3211, 114.0921, 142.0782 | Peimisine-3-O-β-d-glucopyranoside its isomers | 4.68 | [22] | |
| 24 | 6.81 | C33H53NO8 | 592.3846 | 592.3849 | −0.3 | −0.5 | 138.1285, 574.3744, 412.3216, 394.3110, | Sipeimine-3β-d-glucoside its isomers | 2.53 | [20,21] | Figure 2A |
| 25 | 6.93 | C33H55NO8 | 594.4009 | 594.4006 | 0.2 | 0.3 | 576.3896, 414.3364, 138.1285 | Peiminoside | 0.62 | [23] | |
| 26 | 6.99 | C33H51NO8 | 590.3691 | 590.3693 | −0.2 | −0.3 | 428.3167, 412.3211, 114.0921, 142.0782 | Peimisine-3-O-β-d-glucopyranoside its isomers | 0.22 | [22] | |
| 27 | 7.15 | C27H41NO4 | 444.3121 | 444.3114 | 0.5 | 1.1 | 428.3167, 412.3211, 114.0921, 142.0782 | Yibeissine isomers | 0.27 | [24] | |
| 28 | 7.29 | C27H41NO4 | 444.3139 | 444.3127 | 2.5 | 5.6 | 428.3167, 412.3211, 114.0921, 142.0782 | Yibeissine isomers | 0.13 | [24] | |
| 29 | 7.54 | C27H43NO4 | 446.3274 | 446.327 | 0.4 | 0.9 | Yibeinine its isomers | 0.21 | [6,19] | Figure 2D | |
| 30 | 7.73 | C27H43NO3 | 430.3462 | 430.3342 | 2.1 | 4.9 | 412.3279, 138.1301 | Sipeimine | 9.46 | [23] | Figure 2B |
| 31 | 8.00 | C27H43NO3 | 430.3316 | 430.3321 | −0.6 | −1.4 | 412.3279, 138.1301 | Sipeimine its isomers | 0.07 | [23] | Figure 2B |
| 32 | 8.12 | C27H43NO3 | 430.3313 | 430.3321 | −0.8 | −1.9 | 412.3279, 138.1301 | Sipeimine its isomers | 0.13 | [23] | Figure 2B |
| 33 | 8.29 | C27H43NO3 | 430.3323 | 430.3321 | 0.2 | 0.5 | 412.3279, 138.1301 | Sipeimine its isomers | 1.55 | [23] | Figure 2B |
| 34 | 8.4 | C27H45NO3 | 432.3474 | 432.3478 | −0.4 | −0.9 | 414.3366, 398.3054, 138.1284 | Verticine its isomers | 0.42 | [23] | |
| 35 | 8.77 | C27H41NO3 | 428.3165 | 428.3165 | 0 | 0 | 412.3211, 114.0921, 142.0782 | Peimisine | 4.13 | [20,23,25] | Figure 2E |
| 36 | 9.05 | C27H43NO3 | 430.3383 | 430.3321 | 0.1 | 0.2 | 412.3279, 138.1301 | Sipeimine | 3.73 | [23] | Figure 2B |
| 37 | 9.3 | C23H33NO | 340.2599 | 340.2581 | 0.8 | 2.4 | 0.17 | ||||
| 38 | 9.35 | C27H41NO3 | 428.3165 | 428.3165 | 0 | 0 | 412.3211, 114.0921, 142.0782 | Peimisine its isomers | 0.15 | [20,23,25] | Figure 2E |
| 39 | 9.45 | C27H43NO4 | 446.3271 | 446.3271 | 0 | 0 | Yibeinine its isomers | 0.21 | [6,19] | Figure 2D | |
| 40 | 9.62 | C34H55NO8 | 606.4005 | 606.4006 | −0.1 | −0.2 | 0.28 | ||||
| 41 | 9.74 | C33H53NO7 | 576.3902 | 576.3901 | 0.1 | 0.2 | 414.3369, 396.3263 | Yibeinoside A its isomers | 1.01 | [25] | Figure 2F |
| 42 | 9.81 | C33H55NO7 | 578.4049 | 578.4057 | −0.7 | −1.2 | 416.3516, 398.3409 | hupeheninoside | 0.45 | [25] | |
| 43 | 10.00 | C33H53NO7 | 576.39 | 576.39 | 0 | 0 | 414.3369, 396.3263 | Yibeinoside A its isomers | 0.08 | [25] | Figure 2F |
| 44 | 10.21 | C33H53NO8 | 592.3848 | 592.3849 | −0.2 | −0.3 | 138.1285, 574.3744, 412.3216, 394.3110 | Sipeimine-3β-d-glucoside its isomers | 0.30 | [20,21] | Figure 2A |
| 45 | 10.51 | C27H41NO | 396.8019 | 396.8019 | 0 | 0 | 0.25 | ||||
| 46 | 10.66 | C33H53NO7 | 576.391 | 576.39 | 1 | 1.7 | 414.3369, 396.3263 | Yibeinoside A | 6.78 | [20] | Figure 2F |
| 47 | 10.87 | C33H53NO7 | 576.3898 | 576.39 | −0.2 | −0.3 | 414.3369, 396.3263 | Yibeinoside A its isomers | 1.05 | [20] | Figure 2F |
| 48 | 11.33 | C33H53NO7 | 576.39 | 576.39 | 0 | 0 | 414.3369, 396.3263 | Yibeinoside A its isomers | 0.87 | [20] | Figure 2F |
| 49 | 11.6 | C33H53NO7 | 576.396 | 576.4076 | −3.6 | −6.2 | 414.3369, 396.3263 | Yibeinoside A its isomers | 3.56 | [20] | Figure 2F |
| 50 | 11.81 | C33H53NO7 | 576.396 | 576.396 | 0 | 0 | 414.3369, 396.3263 | Yibeinoside A its isomers | 0.12 | [20] | Figure 2F |
| 51 | 11.97 | C28H45NO2 | 428.3163 | 428.3165 | −0.2 | −0.5 | puqietinedinone its isomers | 0.30 | [26] | ||
| 52 | 12.14 | C33H53NO8 | 592.3859 | 592.3849 | 1 | 1.7 | 138.1285, 574.3744, 412.3216, 394.3110, | Sipeimine-3β-d-glucoside its isomers | 0.21 | [20,21] | Figure 2A |
| 53 | 12.63 | C27H43NO2 | 414.3461 | 414.6438 | 0.9 | 1.6 | 396.3253, 105.0697 | puqiedinoneits isomers | 8.84 | [20,23] | Figure 2C |
| 54 | 12.89 | C33H53NO7 | 576.3909 | 576.3901 | 0.9 | 1.6 | 414.3369, 396.3263 | Yibeinoside A | 1.11 | [20] | Figure 2F |
| 55 | 13.1 | C40H67NO12 | 754.4739 | 754.4742 | −0.3 | −0.2 | 0.55 | ||||
| 56 | 13.19 | C33H53NO7 | 576.3902 | 576.3901 | 0.2 | 0.3 | 414.3369, 396.3263 | Yibeinoside A its isomers | 0.93 | [20] | Figure 2F |
| 57 | 13.46 | C27H43NO2 | 414.3411 | 414.3472 | 3.9 | 9.4 | 396.3253, 105.0697 | puqiedinone its isomers | 4.03 | [20,23] | Figure 2C |
| 58 | 13.64 | C34H57NO8 | 608.4161 | 608.4162 | −0.1 | −0.2 | pingbeininoside | 0.39 | [24] | ||
| 59 | 13.76 | C33H53NO8 | 592.385 | 592.385 | 0 | 0 | 138.1285, 574.3744, 412.3216, 394.3110 | Sipeimine-3β-d-glucoside its isomers | 0.51 | [20,21] | Figure 2A |
| 60 | 13.93 | C39H65NO11 | 724.4642 | 724.4636 | 0.6 | 0.8 | 1.11 | ||||
| 61 | 14 | C27H43NO3 | 430.3323 | 430.3321 | 0.2 | 0.5 | 412.3279, 138.1301 | Sipeimine its isomers | 0.64 | [23] | Figure 2B |
| 62 | 14.13 | C39H65NO12 | 740.4592 | 740.4585 | 0.7 | 0.9 | 0.24 | ||||
| 63 | 15.07 | C27H45NO2 | 416.3525 | 416.3529 | −0.5 | −1.2 | 398.3418 | Songbeinine | 0.27 | [23] | |
| 64 | 15.13 | C33H55NO7 | 578.406 | 578.4059 | 0.1 | 0.2 | 416.3516, 398.3409 | hupeheninoside | 0.19 | [20] | |
| 65 | 15.66 | C27H43NO2 | 414.3373 | 414.3373 | 0 | 0 | 396.3253, 105.0697 | puqiedinone its isomers | 0.58 | [20,23] | Figure 2C |
| 66 | 15.75 | C28H47NO2 | 430.3636 | 430.3685 | −4.9 | −11.4 | puqietinone | 0.23 | [27] | ||
| 67 | 15.82 | C28H45NO2 | 428.3527 | 428.3529 | −0.2 | −0.5 | puqietinedinone its isomers | 0.39 | [27] | ||
| 68 | 15.9 | C33H55NO6 | 562.4106 | 562.4108 | −0.2 | −0.4 | 0.49 | ||||
| 69 | 16.07 | C27H43NO2 | 414.337 | 414.3372 | −0.3 | −0.7 | 396.3253, 105.0697 | puqiedinone its isomers | 0.63 | [20,23] | Figure 2C |
| 70 | 16.30 | C27H43NO4 | 446.3635 | 446.3634 | 0 | 0 | Yibeinine its isomers | 0.31 | [6,19] | Figure 2D | |
| 71 | 16.37 | C14H31NO2 | 246.2432 | 246.2433 | −0.1 | −0.4 | 0.44 | ||||
| 72 | 16.58 | C16H35NO3 | 290.2693 | 290.2695 | −0.2 | −0.7 | 0.24 | ||||
| 73 | 16.79 | C28H47NO2 | 430.3689 | 430.3685 | 0.4 | 0.9 | puqietinone | 0.35 | [27] | ||
| 74 | 17.41 | C13H10O | 183.081 | 183.081 | 0 | 0 | 1.00 | ||||
| 75 | 17.75 | C16H35NO2 | 274.2798 | 274.2759 | 1.3 | 4.7 | 3.04 | ||||
| 76 | 17.82 | C18H39NO3 | 318.3015 | 318.3008 | 0.7 | 2.2 | 1.50 | ||||
| 77 | 17.94 | C16H33NO2 | 272.2589 | 272.259 | −0.2 | −0.7 | 0.18 | ||||
| 78 | 18.09 | C16H35N | 242.2849 | 242.2848 | 0.1 | 0.4 | Thymidine | 0.36 | [18] | ||
| 79 | 18.29 | C21H30O2 | 359.2323 | 359.2324 | −0.1 | −0.3 | 0.32 | ||||
| 80 | 18.76 | C18H39NO2 | 302.3057 | 302.3059 | −0.2 | −0.7 | 0.20 | ||||
| 81 | 18.86 | C14H18O | 203.1437 | 203.1437 | 0.1 | 0.5 | 0.68 | ||||
| 82 | 18.96 | C18H30O2 | 279.2321 | 279.2324 | −0.3 | −1.1 | 0.25 | ||||
| 83 | 19.17 | C17H24O3 | 277.1801 | 277.1804 | −0.3 | −1.1 | 0.37 | ||||
| 84 | 19.3 | C21H37N | 304.3001 | 304.3004 | −0.3 | −1 | 0.20 | ||||
| 85 | 19.42 | C18H20O4 | 301.1417 | 301.144 | −2.3 | −7.6 | 0.43 | ||||
| 86 | 19.89 | C15H26O | 223.2063 | 223.2062 | 0.3 | 1.3 | Patchouli alcohol | 0.92 | [26] | ||
| 87 | 20.04 | C22H32O8 | 425.2154 | 425.2175 | −1.6 | −3.8 | 0.80 | ||||
| 88 | 20.55 | C16H33NO | 256.264 | 256.264 | 0 | 0 | 0.18 | ||||
| 89 | 20.73 | C18H35NO | 282.2795 | 282.2797 | −0.6 | −2.1 | 0.26 | ||||
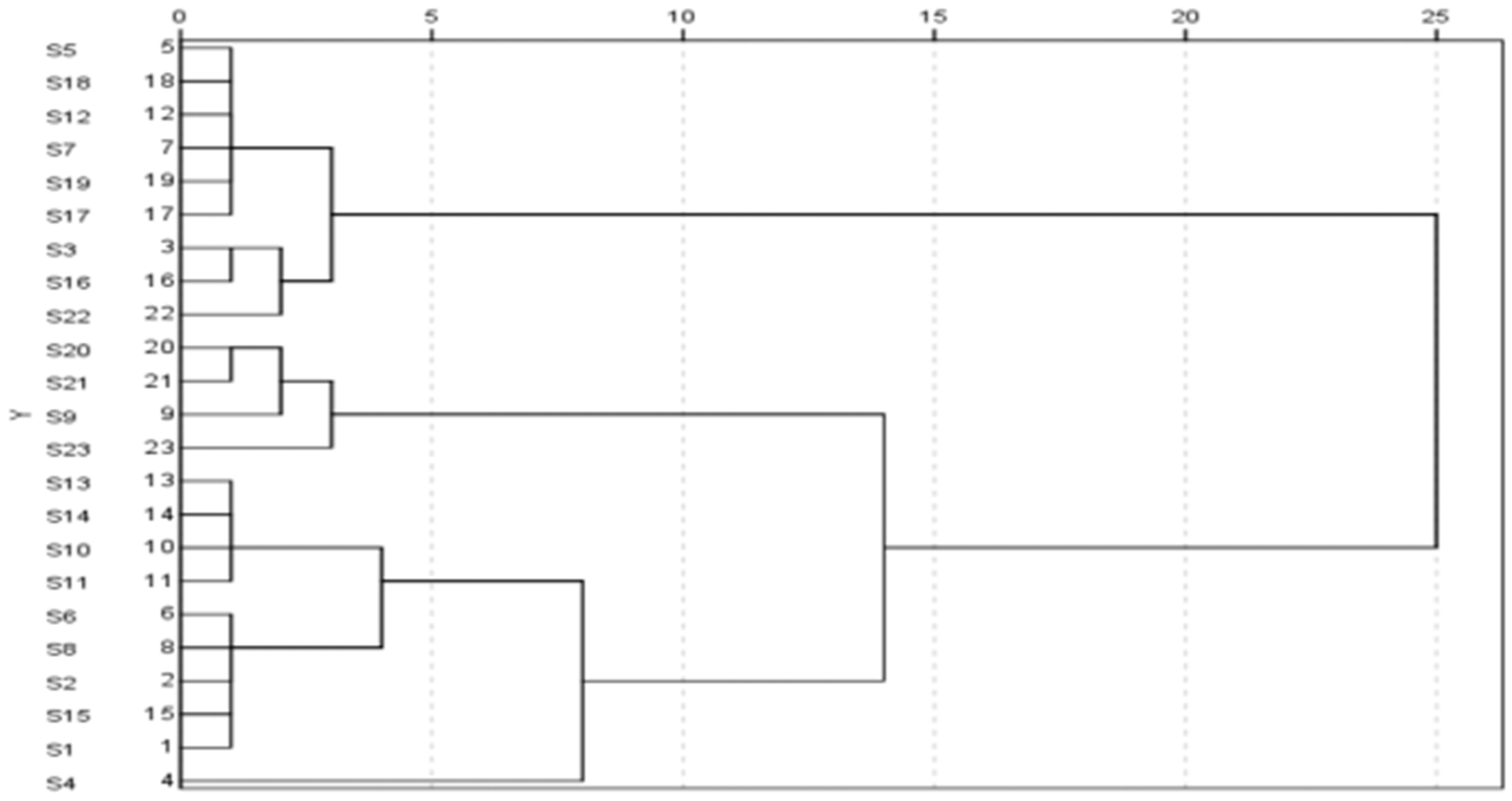
| Number | Sipeimine-3β-d-glucoside | Sipeimine | Peimisine | Yibeinoside A |
|---|---|---|---|---|
| S1 | 0.0576 | 0.0573 | 0.0155 | 0.0349 |
| S2 | 0.0565 | 0.0395 | 0.0087 | 0.0337 |
| S3 | 0.0084 | 0.0275 | 0.0338 | 0.0236 |
| S4 | 0.0665 | 0.0141 | 0.012 | 0.1398 |
| S5 | 0.0044 | 0.0105 | 0.0096 | 0.0034 |
| S6 | 0.0375 | 0.0453 | 0.0096 | 0.0202 |
| S7 | 0.0099 | 0.0216 | 0.0079 | 0.0038 |
| S8 | 0.0338 | 0.0493 | 0.0099 | 0.018 |
| S9 | 0.0427 | 0.1218 | 0.0343 | 0.0087 |
| S10 | 0.0749 | 0.0319 | 0.0083 | 0.0357 |
| S11 | 0.1033 | 0.0337 | 0.0073 | 0.0499 |
| S12 | 0.0018 | 0.0113 | 0.0033 | 0.0038 |
| S13 | 0.0815 | 0.0337 | 0.0073 | 0.0558 |
| S14 | 0.0763 | 0.0413 | 0.0126 | 0.0494 |
| S15 | 0.0545 | 0.0471 | 0.0093 | 0.0366 |
| S16 | 0.0039 | 0.0358 | 0.0437 | 0.0132 |
| S17 | 0.0083 | 0.0091 | 0.0113 | 0.0203 |
| S18 | 0.0013 | 0.0066 | 0.0094 | 0.0019 |
| S19 | 0.007 | 0.0152 | 0.0124 | 0.0051 |
| S20 | 0.0737 | 0.1086 | 0.0071 | 0.008 |
| S21 | 0.0882 | 0.0913 | 0.0058 | 0.0076 |
| S22 | 0.0088 | 0.0755 | 0.0206 | 0.0075 |
| S23 | 0.1357 | 0.1019 | 0.0067 | 0.0116 |
| Sampel No. | Provenance | Origin | |
|---|---|---|---|
| S1 | (Wild) | F. pallidiflora | Gongliu County, Yili |
| S2 | (Cultivate) | F. pallidiflora | Gongliu County, Yili |
| S3 | (Wild) | F. walujewii | Xinyuan County, Yili |
| S4 | (Wild) | F. pallidiflora | Huocheng County, Yili |
| S5 | (Wild) | F. tortifolia | Toli County, Tacheng |
| S6 | (Cultivate) | F. yuminensis | Yumin County, Tacheng |
| S7 | (Wild) | F. yuminensis | Yumin County, Tacheng |
| S8 | (Cultivate) | F. tortifolia | Toli County, Tacheng |
| S9 | (Cultivate) | F. walujeweii | Altai, Xinjiang |
| S10 | (Wild) | F. pallidiflora | Tekes County, Yili |
| S11 | (Wild) | F. pallidiflora | Tekes County, Yili |
| S12 | (Cultivate) | Fritillaria | Kazakhstan |
| S13 | (Cultivate) | F. pallidiflora | Yili, Xinjiang |
| S14 | (Cultivate) | F. pallidiflora | Yili, Xinjiang |
| S15 | (Wild) | F. pallidiflora | Yili, Xinjiang |
| S16 | (Wild) | F. walujeweii | Altai, Xinjiang |
| S17 | (Wild) | F. verticillata | Jeminay County,Altai |
| S18 | (Wild) | F. tortifolia | Toli County, Tacheng |
| S19 | (Wild) | F. yuminensis | Yumin County, Tacheng |
| S20 | (Wild) | Fritillaria | Kazakhstan |
| S21 | (Wild) | Fritillaria | Kazakhstan |
| S22 | (Wild) | F. yuminensis | Yumin County, Tacheng |
| S23 | (Wild) | Fritillaria | Kazakhstan |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammat, A.; Yili, A.; Aisa, H.A. Rapid Quantification and Quantitation of Alkaloids in Xinjiang Fritillaria by Ultra Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry. Molecules 2017, 22, 719. https://doi.org/10.3390/molecules22050719
Mohammat A, Yili A, Aisa HA. Rapid Quantification and Quantitation of Alkaloids in Xinjiang Fritillaria by Ultra Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry. Molecules. 2017; 22(5):719. https://doi.org/10.3390/molecules22050719
Chicago/Turabian StyleMohammat, Aziz, Abulimiti Yili, and Haji Akber Aisa. 2017. "Rapid Quantification and Quantitation of Alkaloids in Xinjiang Fritillaria by Ultra Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry" Molecules 22, no. 5: 719. https://doi.org/10.3390/molecules22050719







