Brazilian Green Propolis Extract Synergizes with Protoporphyrin IX-mediated Photodynamic Therapy via Enhancement of Intracellular Accumulation of Protoporphyrin IX and Attenuation of NF-κB and COX-2
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis of Extracts of BGP by HPLC-UV
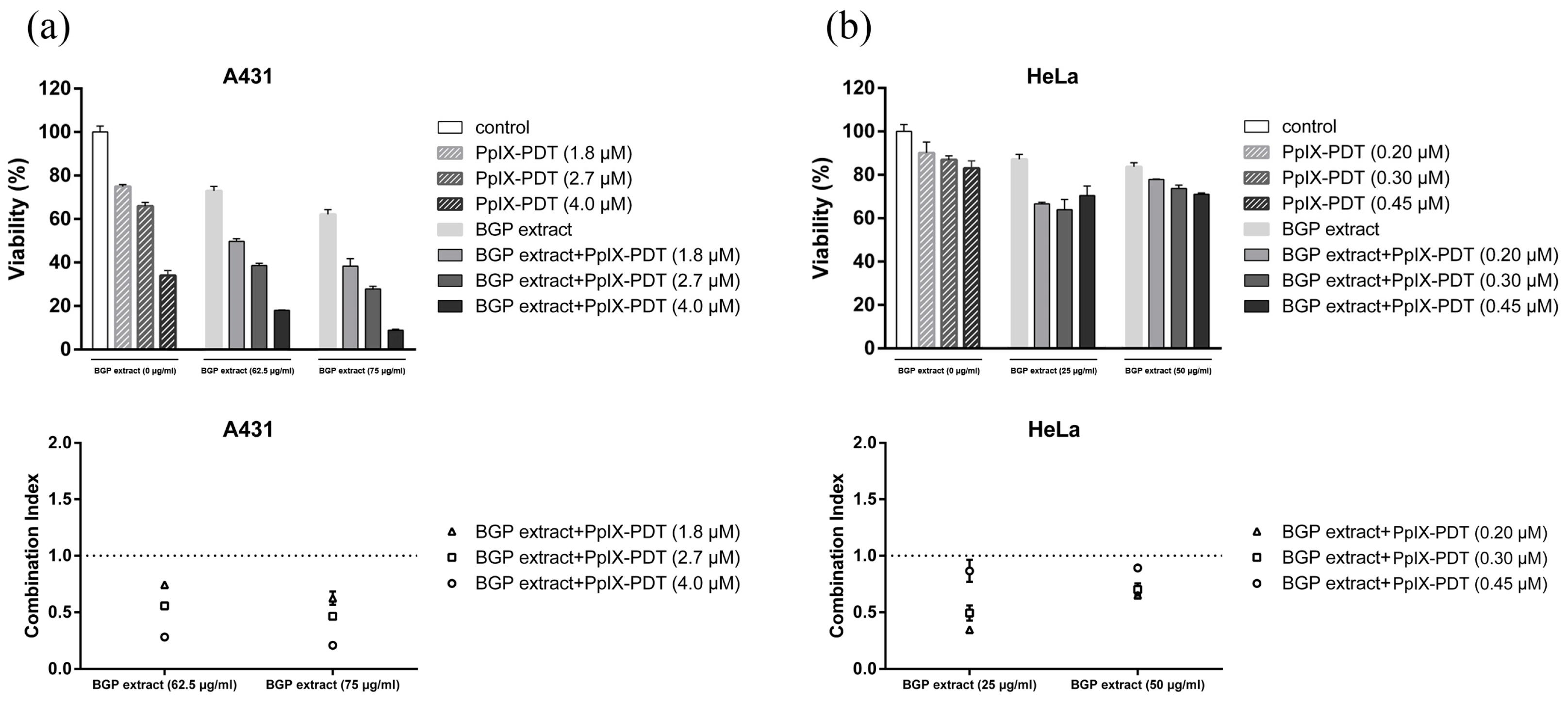
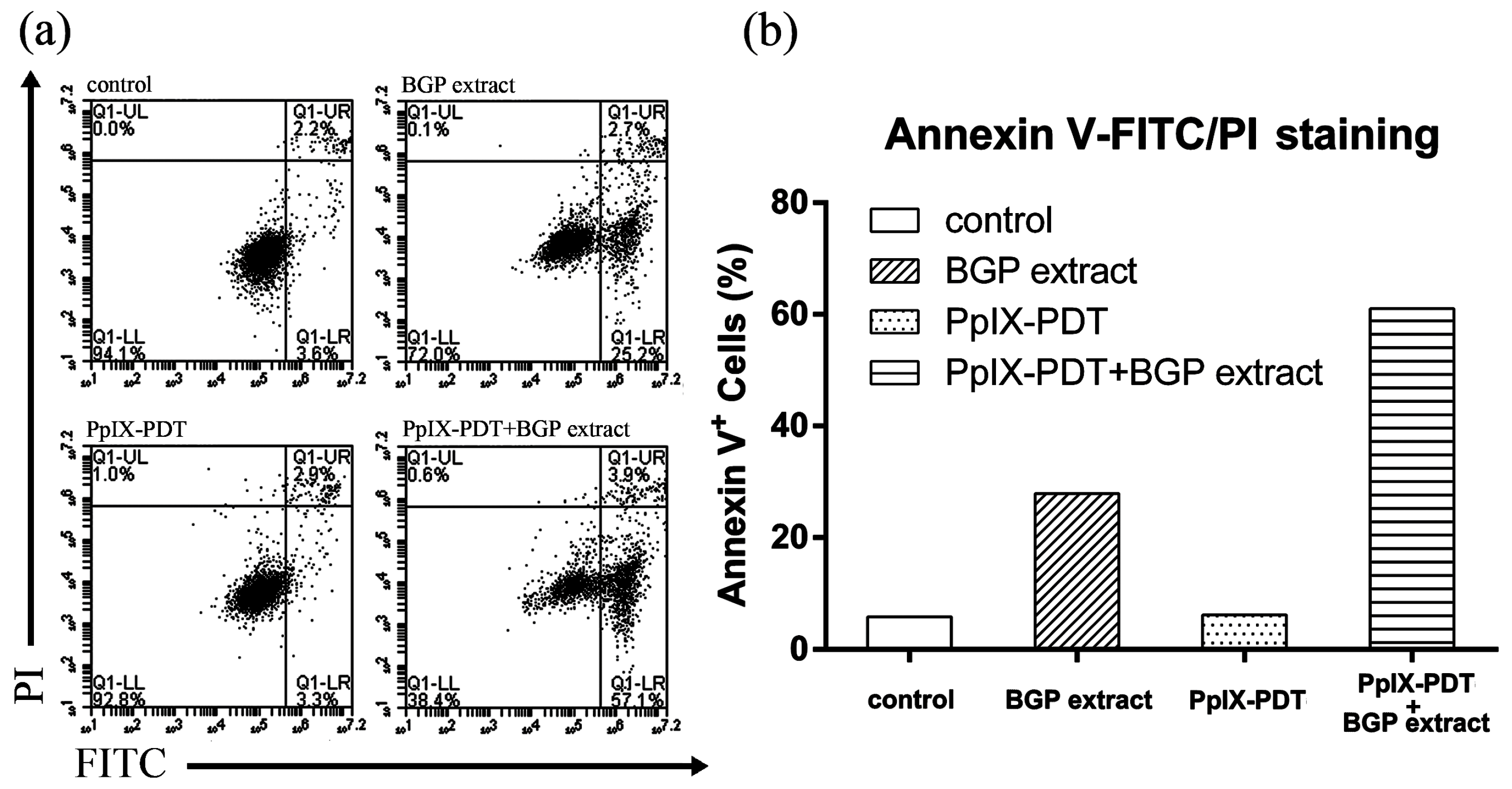
2.2. BGP Extract Increased PpIX-mediated Photocytotoxicity through Induction of Apoptosis
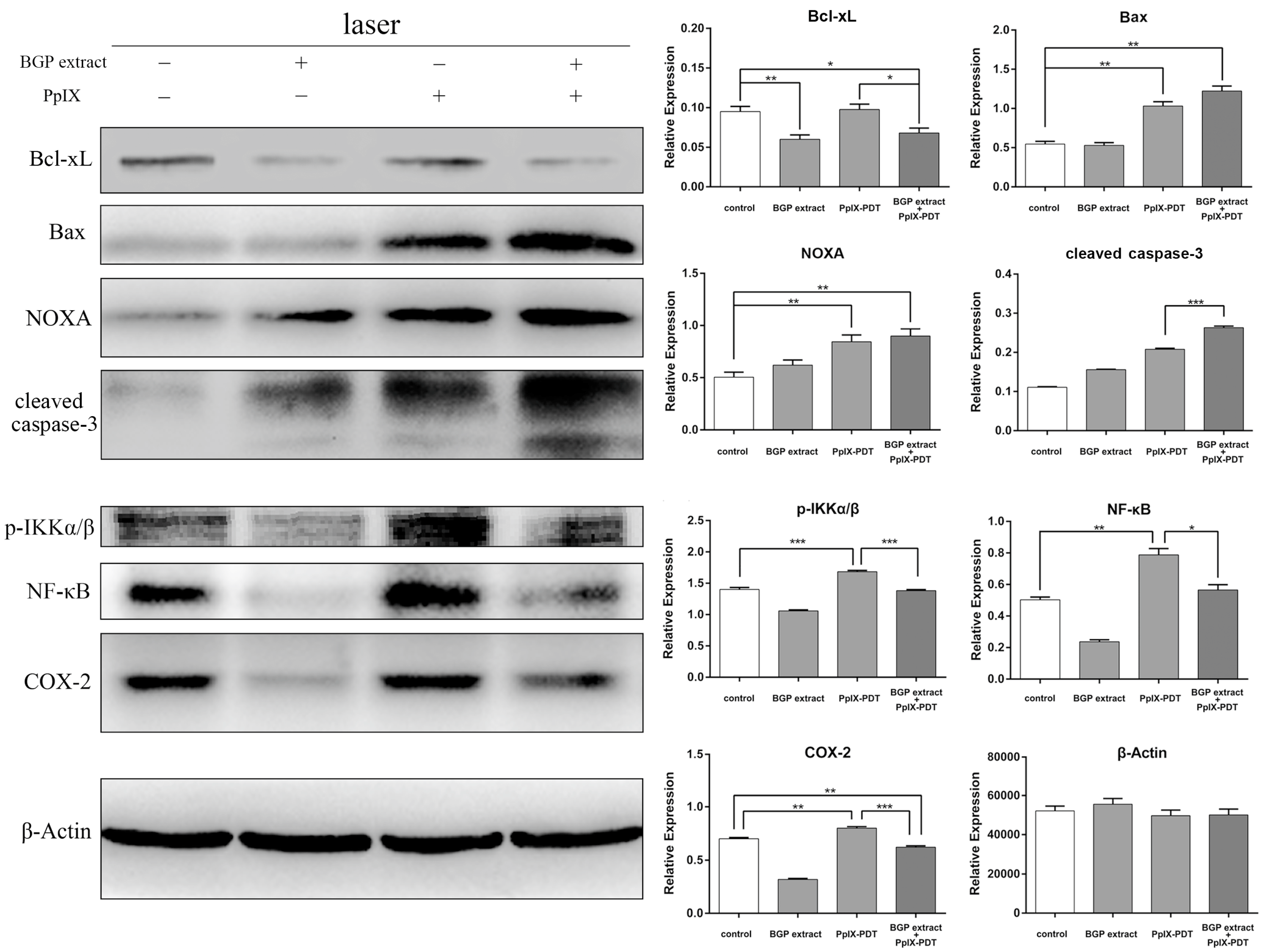
2.3. BGP Extract Attenuated PDT-Mediated Elevation of NF-κB and COX-2
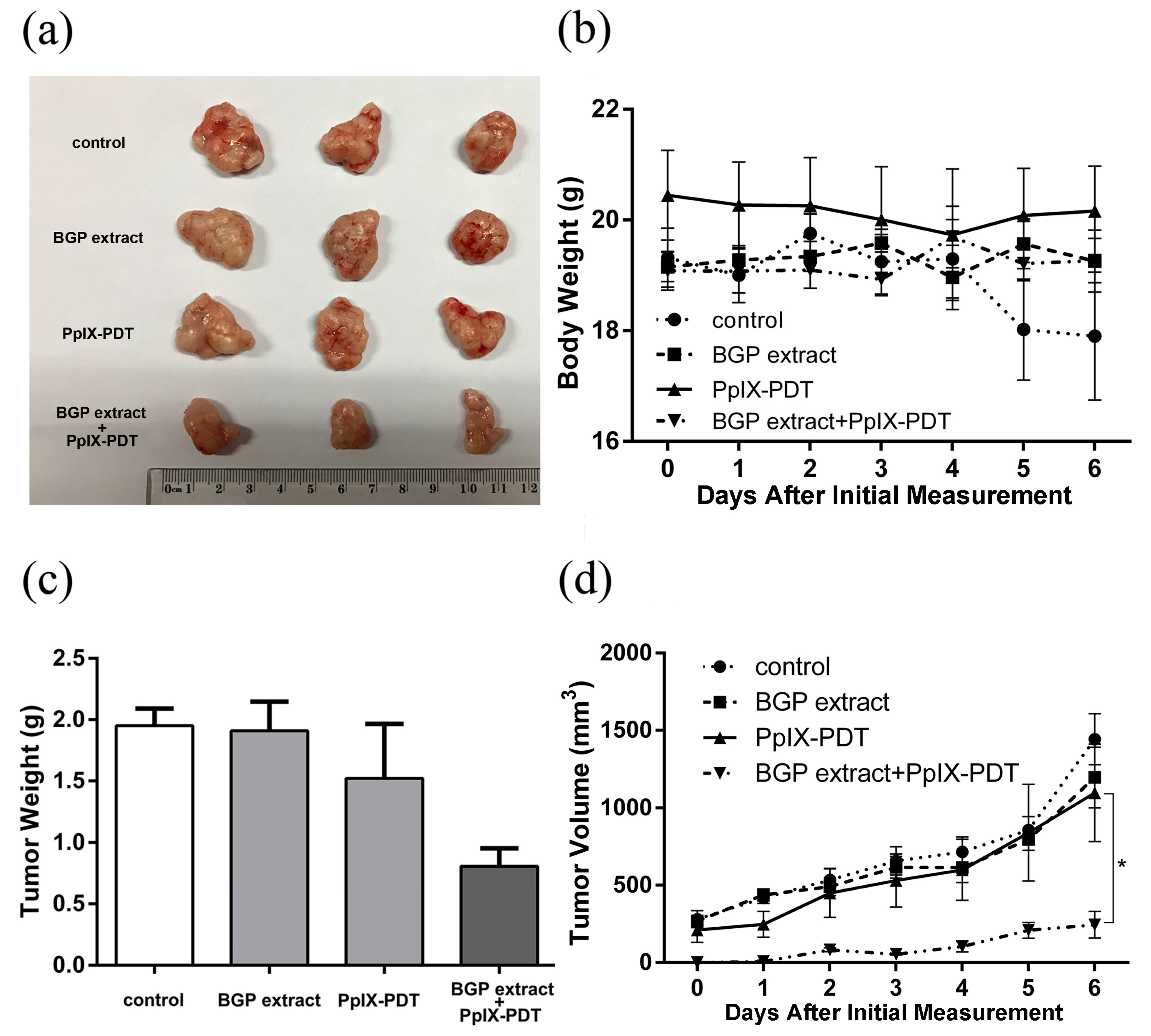
2.4. BGP Extract Enhanced the Anti-tumorigenicity of PpIX-PDT in a Xenograft Model
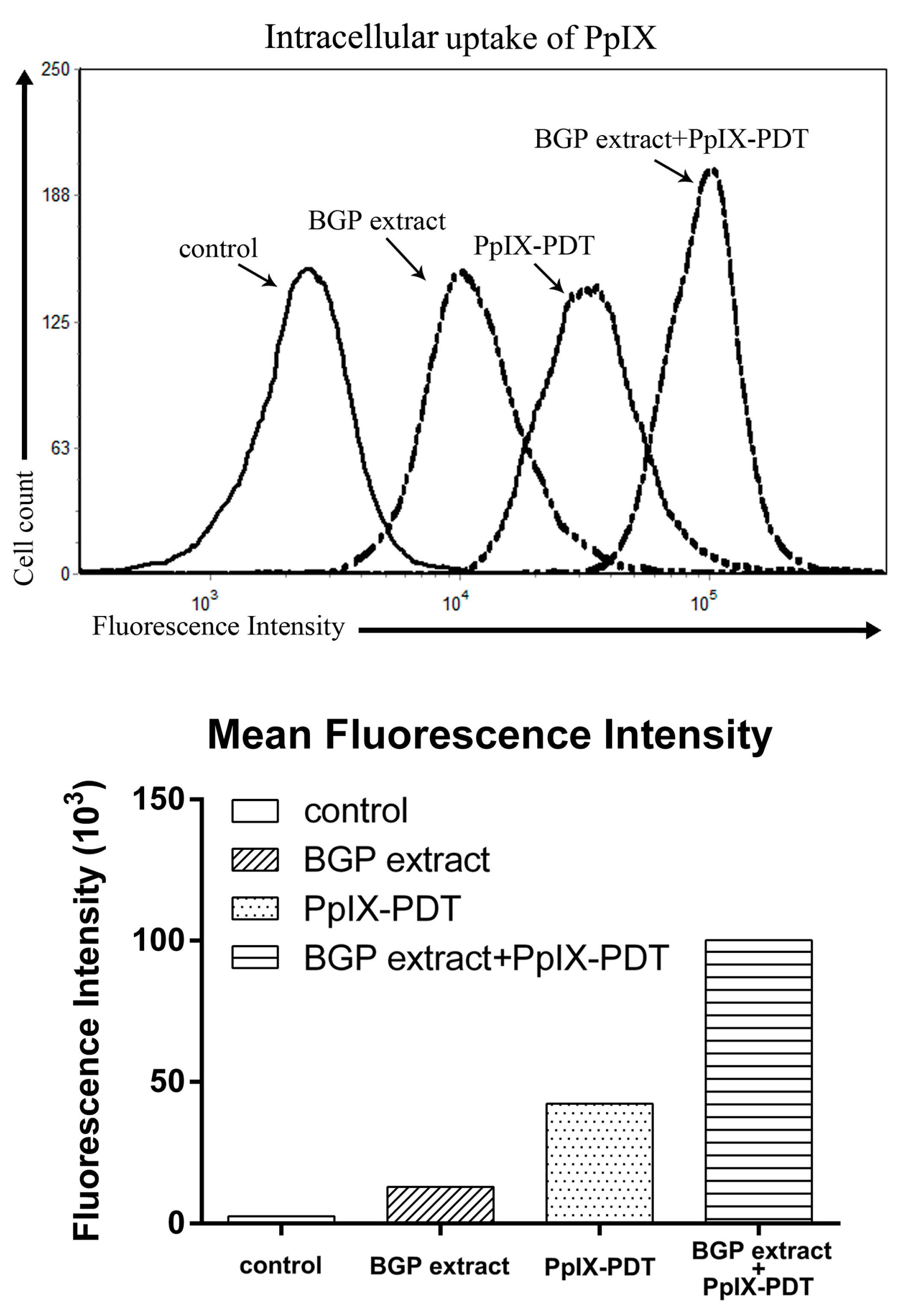
2.5. BGP Extract Increased Intracellular Accumulation of PpIX in PDT
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of BGP Extract
4.3. HPLC Analysis of BGP Extract
4.4. ESI-IT-TOF-MS Analysis of BGP Extract
4.5. Cell lines and Culture Conditions
4.6. MTT Assay
4.7. Evaluating the Interaction between BGP Extract and PpIX-PDT
4.8. TUNEL Assay
4.9. Annexin V-FITC/PI Staining Assay
4.10. Western Blotting Analysis
4.11. Animal Experiments
4.12. Intracellular Accumulation of PpIX
4.13. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Salatino, A.; Fernandes-Silva, C.C.; Righi, A.A.; Salatino, M.L. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011, 28, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Bankova, V.; Marcucci, M.C.; Simova, S.; Nikolova, N.; Kujumgiev, A.; Popov, S. Antibacterial diterpenic acids from Brazilian propolis. Z. Naturforsch. C. 1996, 51, 277–280. [Google Scholar] [PubMed]
- Machado, J.L.; Assuncao, A.K.; da Silva, M.C.; Dos Reis, A.S.; Costa, G.C.; Arruda Dde, S.; Rocha, B.A.; Vaz, M.M.; Paes, A.M.; Guerra, R.N.; et al. Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity. Evid. Based Complement. Alternat. Med. 2012, 2012, 157652. [Google Scholar] [CrossRef] [PubMed]
- Paulino, N.; Teixeira, C.; Martins, R.; Scremin, A.; Dirsch, V.M.; Vollmar, A.M.; Abreu, S.R.; de Castro, S.L.; Marcucci, M.C. Evaluation of the analgesic and anti-inflammatory effects of a Brazilian green propolis. Planta Med. 2006, 72, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Kucharska, A.Z.; Sokol-Letowska, A.; Mertas, A.; Czuba, Z.P.; Krol, W. Chemical composition and anti-Inflammatory effect of ethanolic extract of Brazilian green propolis on activated J774A.1 macrophages. Evid. Based Complement. Alternat. Med. 2013, 2013, 976415. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhu, A.; Takayama, F.; Okada, R.; Liu, Y.; Harada, Y.; Wu, S.; Nakanishi, H. Brazilian green propolis suppresses the hypoxia-induced neuroinflammatory responses by inhibiting NF-κB activation in microglia. Oxid. Med. Cell. Longev. 2013, 2013, 906726. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Sumitou, Y.; Sakamoto, T.; Araki, Y.; Hara, H. Antihypertensive effects of flavonoids isolated from Brazilian green propolis in spontaneously hypertensive rats. Biol. Pharm. Bull. 2009, 32, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Mishima, S.; Yoshida, C.; Akino, S.; Sakamoto, T. Antihypertensive effects of Brazilian propolis: Identification of caffeoylquinic acids as constituents involved in the hypotension in spontaneously hypertensive rats. Biol. Pharm. Bull. 2005, 28, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Koya-Miyata, S.; Arai, N.; Mizote, A.; Taniguchi, Y.; Ushio, S.; Iwaki, K.; Fukuda, S. Propolis prevents diet-induced hyperlipidemia and mitigates weight gain in diet-induced obesity in mice. Biol. Pharm. Bull. 2009, 32, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Shimazawa, M.; Mishima, S.; Hara, H. Neuroprotective effects of Brazilian green propolis and its main constituents against oxygen-glucose deprivation stress, with a gene-expression analysis. Phytother. Res. PTR 2009, 23, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Bufalo, M.C.; Candeias, J.M.; Sforcin, J.M. In vitro cytotoxic effect of Brazilian green propolis on human laryngeal epidermoid carcinoma (HEp-2) cells. Evid. Based Complement. Alternat. Med. 2009, 6, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Ishiai, S.; Tahara, W.; Yamamoto, E.; Yamamoto, R.; Nagai, K. Histone deacetylase inhibitory effect of Brazilian propolis and its association with the antitumor effect in Neuro2a cells. Food Sci. Nutr. 2014, 2, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Messerli, S.M.; Ahn, M.R.; Kunimasa, K.; Yanagihara, M.; Tatefuji, T.; Hashimoto, K.; Mautner, V.; Uto, Y.; Hori, H.; Kumazawa, S.; et al. Artepillin C (ARC) in Brazilian green propolis selectively blocks oncogenic PAK1 signaling and suppresses the growth of NF tumors in mice. Phytother. Res. PTR 2009, 23, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Frion-Herrera, Y.; Diaz-Garcia, A.; Ruiz-Fuentes, J.; Rodriguez-Sanchez, H.; Sforcin, J.M. Brazilian green propolis induced apoptosis in human lung cancer A549 cells through mitochondrial-mediated pathway. J. Pharm. Pharmacol. 2015, 67, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Zydowicz, G.; Janoszka, B.; Dobosz, C.; Kowalczyk-Ziomek, G.; Krol, W. Ethanolic extract of Brazilian green propolis sensitizes prostate cancer cells to TRAIL-induced apoptosis. Inter. J. Oncol. 2011, 38, 941–953. [Google Scholar]
- Szliszka, E.; Zydowicz, G.; Mizgala, E.; Krol, W. Artepillin C (3,5-diprenyl-4-hydroxycinnamic acid) sensitizes LNCaP prostate cancer cells to TRAIL-induced apoptosis. Int. J. Oncol. 2012, 41, 818–828. [Google Scholar] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Moor, A.C. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B Biol. 2000, 57, 1–13. [Google Scholar] [CrossRef]
- Gomer, C.J. Induction of prosurvival molecules during treatment: Rethinking therapy options for photodynamic therapy. J. Natl. Compr. Cancer Netw. JNCCN 2012, 10 (Suppl. 2), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Milla Sanabria, L.; Rodriguez, M.E.; Cogno, I.S.; Rumie Vittar, N.B.; Pansa, M.F.; Lamberti, M.J.; Rivarola, V.A. Direct and indirect photodynamic therapy effects on the cellular and molecular components of the tumor microenvironment. Biochim. Biophys. Acta 2013, 1835, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, S.O.; Evans, S.S.; Baumann, H.; Owczarczak, B.; Maier, P.; Vaughan, L.; Wang, W.C.; Unger, E.; Henderson, B.W. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br. J. Cancer 2003, 88, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhong, Y.; Zhou, Y.; Sun, H.; Zheng, X.; Zhao, C.; Yan, Y.; Lin, Y.; Liao, L.; Wang, X. Autocrine TNF-alpha-mediated NF-κB activation is a determinant for evasion of CD40-induced cytotoxicity in cancer cells. Biochem. Biophys. Res. Commun. 2013, 436, 467–472. [Google Scholar] [CrossRef] [PubMed]
- de Groot, D.J.; de Vries, E.G.; Groen, H.J.; de Jong, S. Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: From lab to clinic. Crit. Rev. Oncol. Hematol. 2007, 61, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Zhou, J.H.; Du, X.X.; Jia de, X.; Wu, C.L.; Huang, P.; Han, Y.; Sui, H.; Wei, X.L.; Liu, L.; et al. Dihydroartemisinin accentuates the anti-tumor effects of photodynamic therapy via inactivation of NF-κB in Eca109 and Ec9706 esophageal cancer cells. Cell. Physiol. Biochem. 2014, 33, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wei, Y.; Chen, Q.; Xing, D. Cyclooxygenase 2-mediated apoptotic and inflammatory responses in photodynamic therapy treated breast adenocarcinoma cells and xenografts. J. Photochem. Photobiol. B Biol. 2014, 134, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Lisicic, D.; Benkovic, V.; Ethikic, D.; Blazevic, A.S.; Mihaljevic, J.; Orsolic, N.; Knezevic, A.H. Addition of propolis to irinotecan therapy prolongs survival in ehrlich ascites tumor-bearing mice. Cancer Biother. Radiopharm. 2014, 29, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic extract of propolis (EEP) enhances the apoptosis- inducing potential of TRAIL in cancer cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Bronikowska, J.; Mertas, A.; Paradysz, A.; Krol, W. Ethanolic Extract of Propolis Augments TRAIL-Induced Apoptotic Death in Prostate Cancer Cells. Evid. Based Complement. Altern. Med. 2011, 2011, 535172. [Google Scholar] [CrossRef] [PubMed]
- Granville, D.J.; Carthy, C.M.; Jiang, H.; Levy, J.G.; McManus, B.M.; Matroule, J.Y.; Piette, J.; Hunt, D.W. Nuclear factor-kappaB activation by the photochemotherapeutic agent verteporfin. Blood 2000, 95, 256–262. [Google Scholar] [PubMed]
- Kulikova, L.; Mikes, J.; Hyzdalova, M.; Palumbo, G.; Fedorocko, P. NF-kappaB is not directly responsible for photoresistance induced by fractionated light delivery in HT-29 colon adenocarcinoma cells. Photochem. Photobiol. 2010, 86, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M. Complement upregulation in photodynamic therapy-treated tumors: Role of Toll-like receptor pathway and NFκB. Cancer Lett. 2009, 281, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Matroule, J.Y.; Volanti, C.; Piette, J. NF-kappaB in photodynamic therapy: Discrepancies of a master regulator. Photochem. Photobiol. 2006, 82, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; den Bergh, H.; Griffioen, A.W.; Nowak-Sliwinska, P. Angiogenesis inhibition for the improvement of photodynamic therapy: The revival of a promising idea. Biochim. Biophys. Acta 2012, 1826, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Milas, L.; Mason, K.A.; Crane, C.H.; Liao, Z.; Masferrer, J. Improvement of radiotherapy or chemoradiotherapy by targeting COX-2 enzyme. Oncology 2003, 17, 15–24. [Google Scholar] [PubMed]
- Piette, J. Signalling pathway activation by photodynamic therapy: NF-κB at the crossroad between oncology and immunology. Photochem. Photobiol. Sci. 2015, 14, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Kaidi, A.; Qualtrough, D.; Williams, A.C.; Paraskeva, C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006, 66, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.; Wong, S.; Ferrario, A.; Gomer, C.J. Cyclooxygenase-2 expression induced by photofrin photodynamic therapy involves the p38 MAPK pathway. Photochem. Photobiol. 2008, 84, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Tohnai, N. Cyclooxygenase isozymes and their gene structures and expression. Prostag. Other Lipid Mediat. 2002, 69, 95–114. [Google Scholar] [CrossRef]
- Saenz, C.; Ethirajan, M.; Iacobucci, G.; Pandey, A.; Missert, J.R.; Dobhal, M.P.; Pandey, R.K. Indium as a central metal enhances the photosensitizing efficacy of benzoporphyrin derivatives. J. Porphyr. Phthalocyanines 2011, 15, 1310. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Wilson, C.; Hasan, T.; Maytin, E.V. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res. 2011, 71, 6040–6050. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, F.S.; Bentley, M.V. Photodynamic therapy of skin cancers: Sensitizers, clinical studies and future directives. Pharm. Res. 2000, 17, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Wang, Y.-X.; Yu, N.-Q.; Hu, D.; Wang, X.-Y.; Chen, X.-G.; Liao, Y.-W.; Yao, J.; Wang, H.; He, L.; et al. Brazilian Green Propolis Extract Synergizes with Protoporphyrin IX-mediated Photodynamic Therapy via Enhancement of Intracellular Accumulation of Protoporphyrin IX and Attenuation of NF-κB and COX-2. Molecules 2017, 22, 732. https://doi.org/10.3390/molecules22050732
Wang C-C, Wang Y-X, Yu N-Q, Hu D, Wang X-Y, Chen X-G, Liao Y-W, Yao J, Wang H, He L, et al. Brazilian Green Propolis Extract Synergizes with Protoporphyrin IX-mediated Photodynamic Therapy via Enhancement of Intracellular Accumulation of Protoporphyrin IX and Attenuation of NF-κB and COX-2. Molecules. 2017; 22(5):732. https://doi.org/10.3390/molecules22050732
Chicago/Turabian StyleWang, Cheng-Cheng, Yu-Xuan Wang, Nian-Qin Yu, Die Hu, Xiao-Yan Wang, Xing-Guang Chen, You-Wei Liao, Jing Yao, Hao Wang, Ling He, and et al. 2017. "Brazilian Green Propolis Extract Synergizes with Protoporphyrin IX-mediated Photodynamic Therapy via Enhancement of Intracellular Accumulation of Protoporphyrin IX and Attenuation of NF-κB and COX-2" Molecules 22, no. 5: 732. https://doi.org/10.3390/molecules22050732
APA StyleWang, C.-C., Wang, Y.-X., Yu, N.-Q., Hu, D., Wang, X.-Y., Chen, X.-G., Liao, Y.-W., Yao, J., Wang, H., He, L., & Wu, L. (2017). Brazilian Green Propolis Extract Synergizes with Protoporphyrin IX-mediated Photodynamic Therapy via Enhancement of Intracellular Accumulation of Protoporphyrin IX and Attenuation of NF-κB and COX-2. Molecules, 22(5), 732. https://doi.org/10.3390/molecules22050732





