Constrained Geometry Organotitanium Catalysts Supported on Nanosized Silica for Ethylene (co)Polymerization
Abstract
:1. Introduction
2. Results
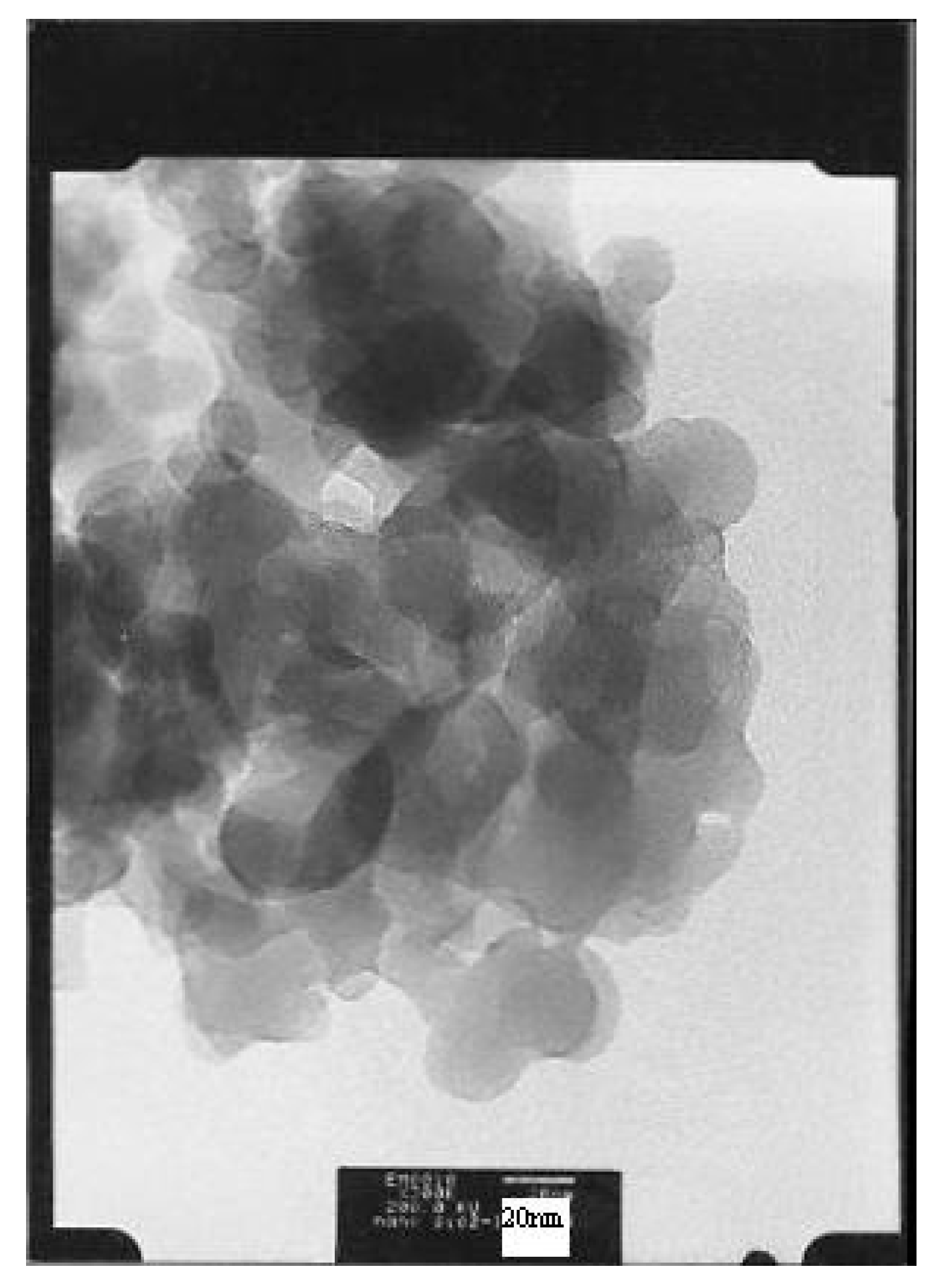
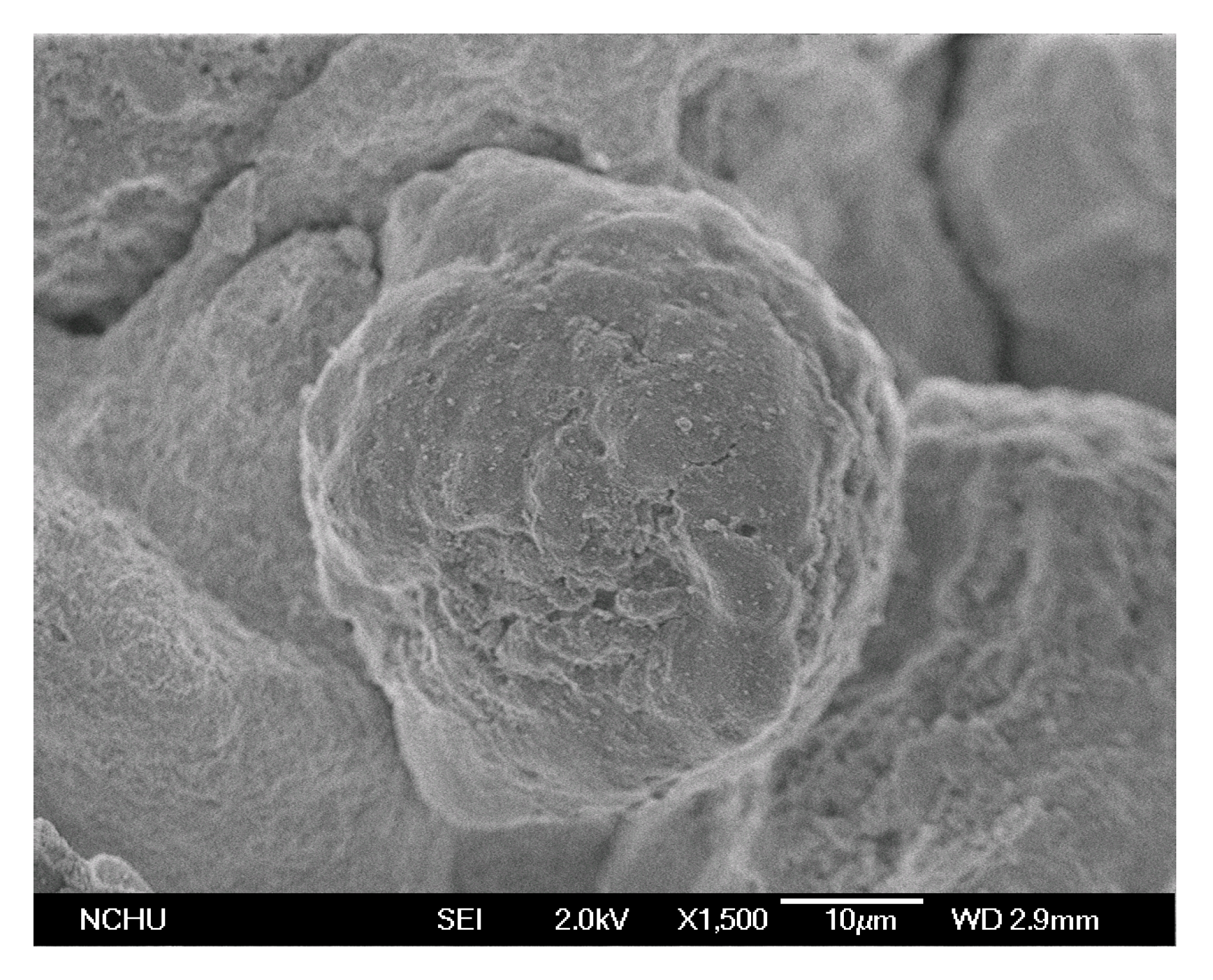
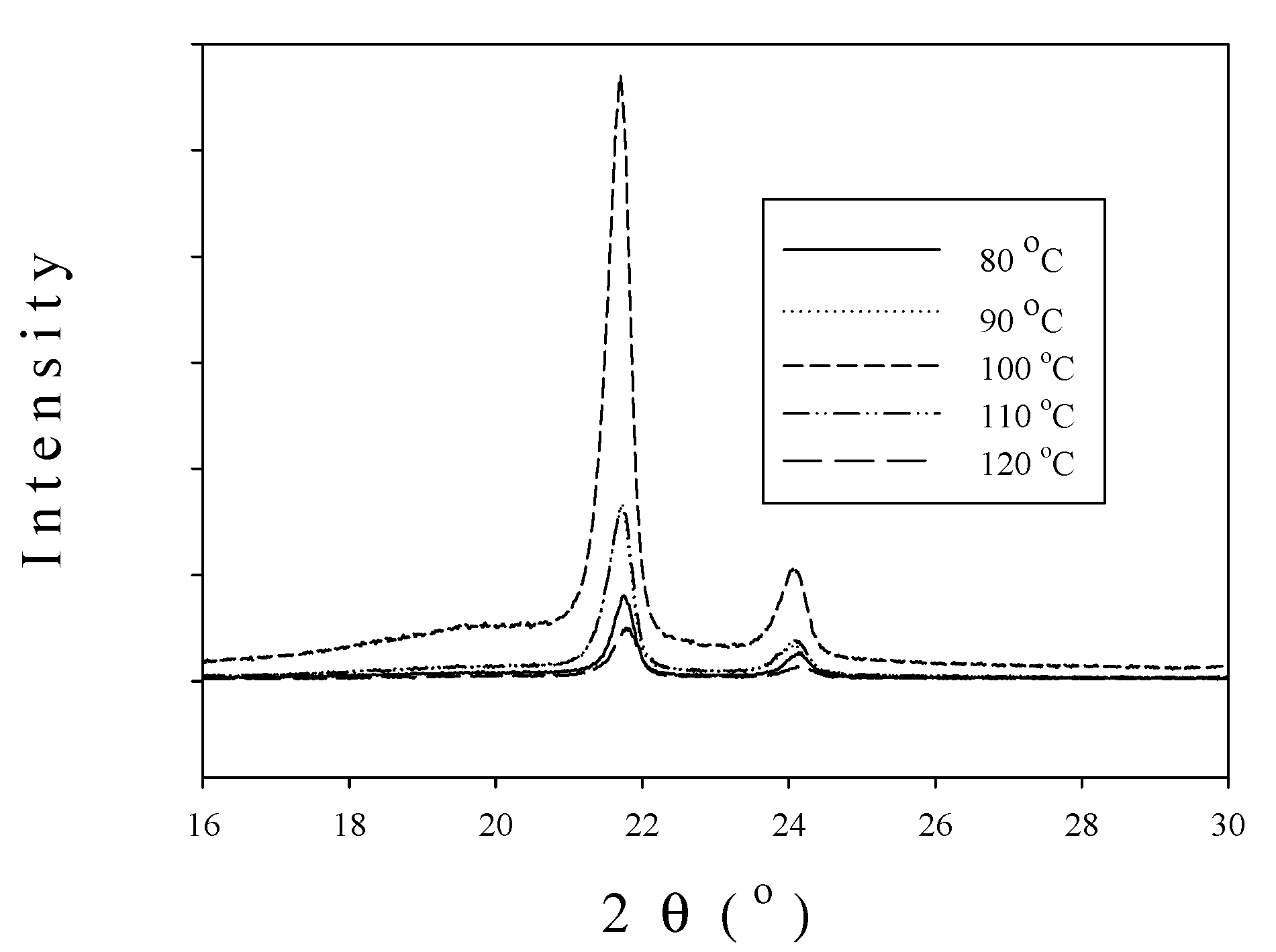
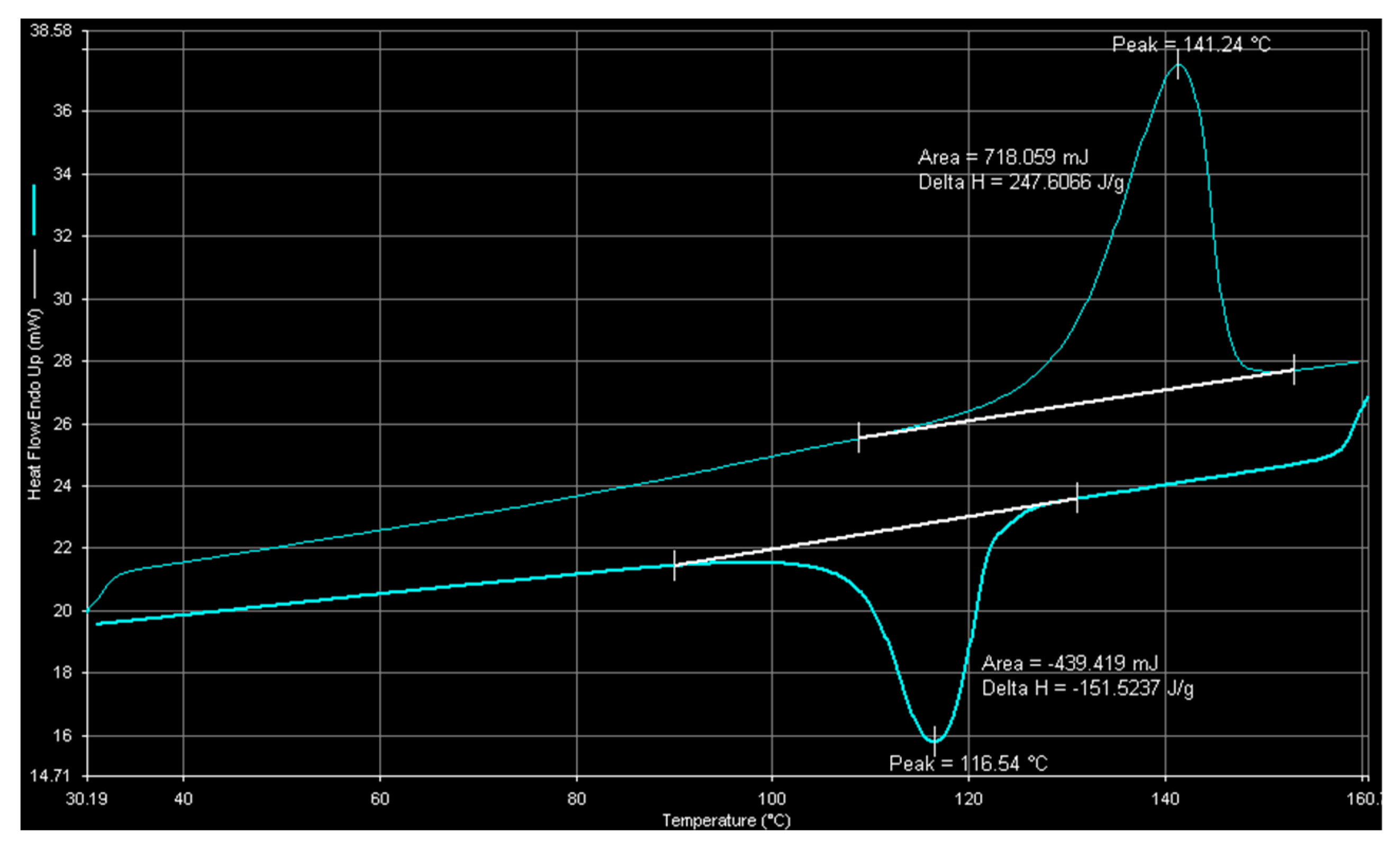
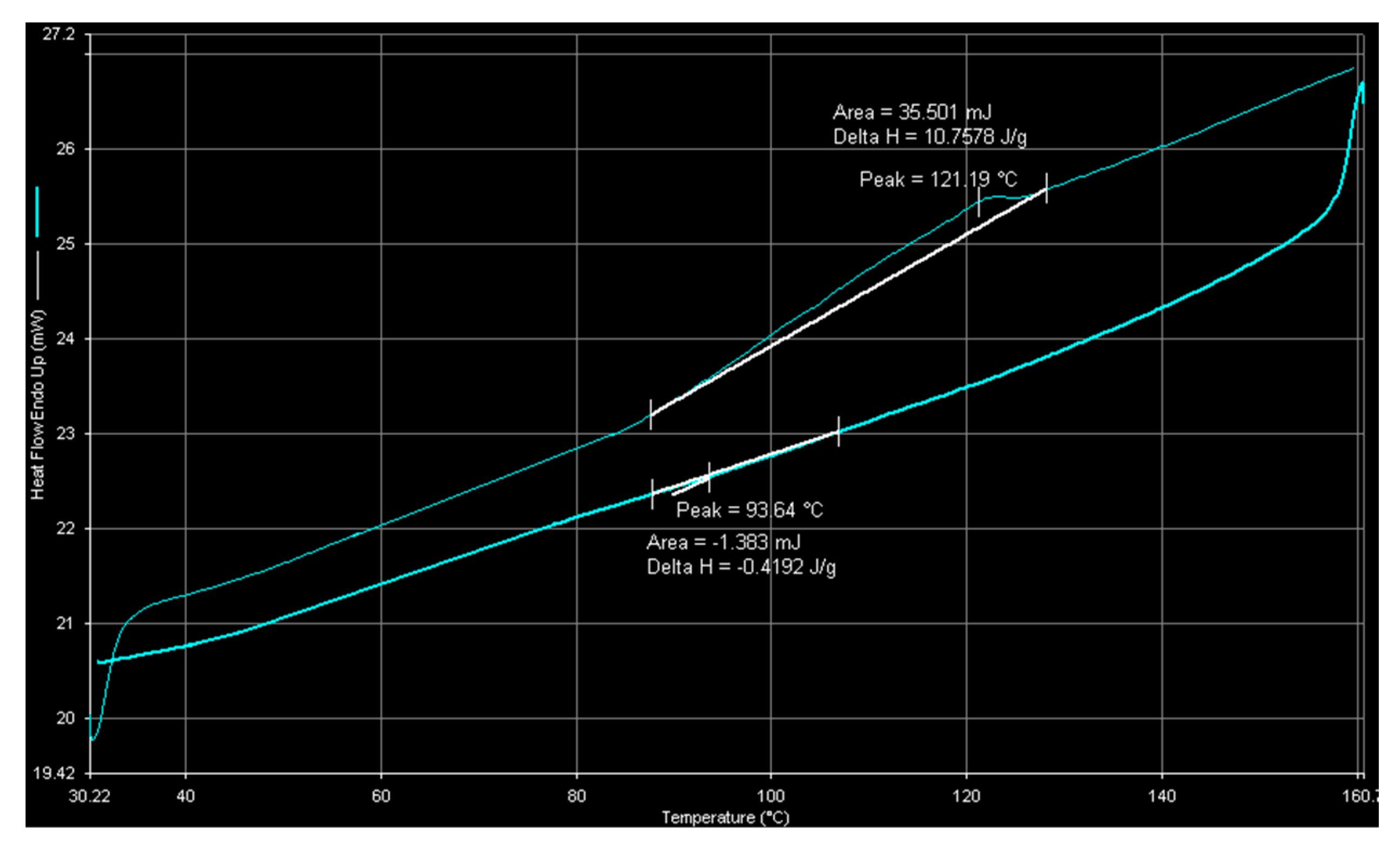
2.1. Catalyst Characterization
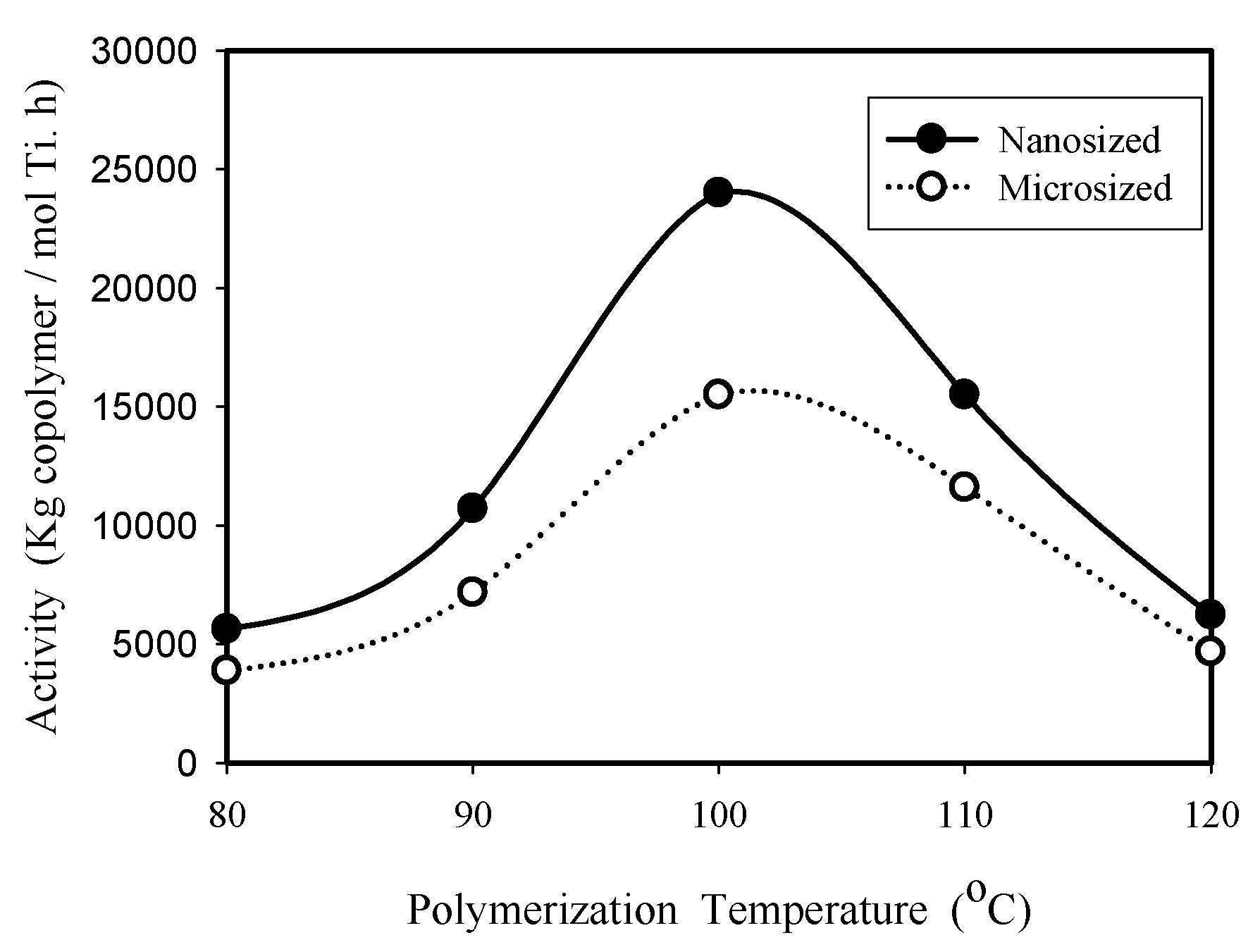
2.2. Ethylene Homopolymerization and Copolymerization
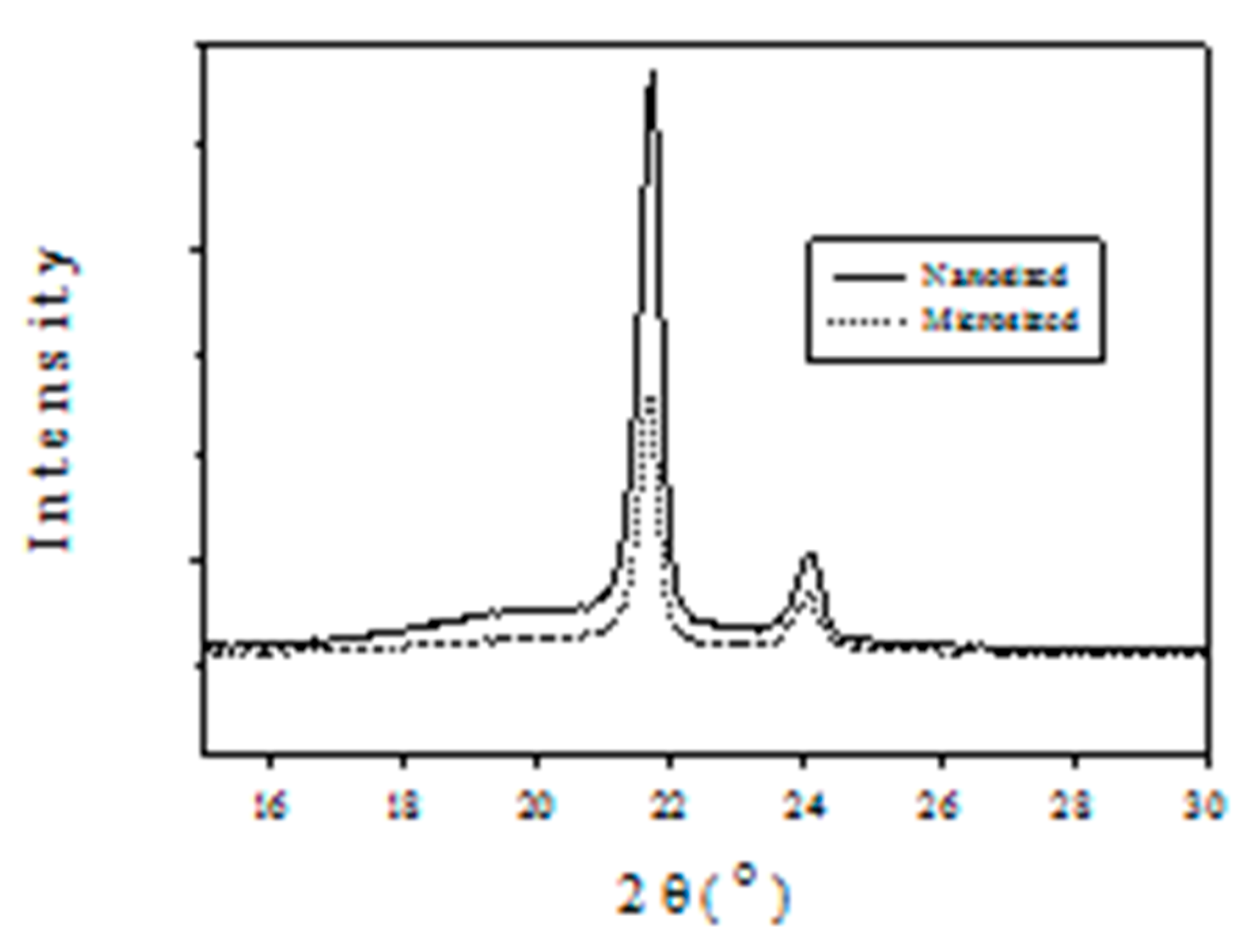
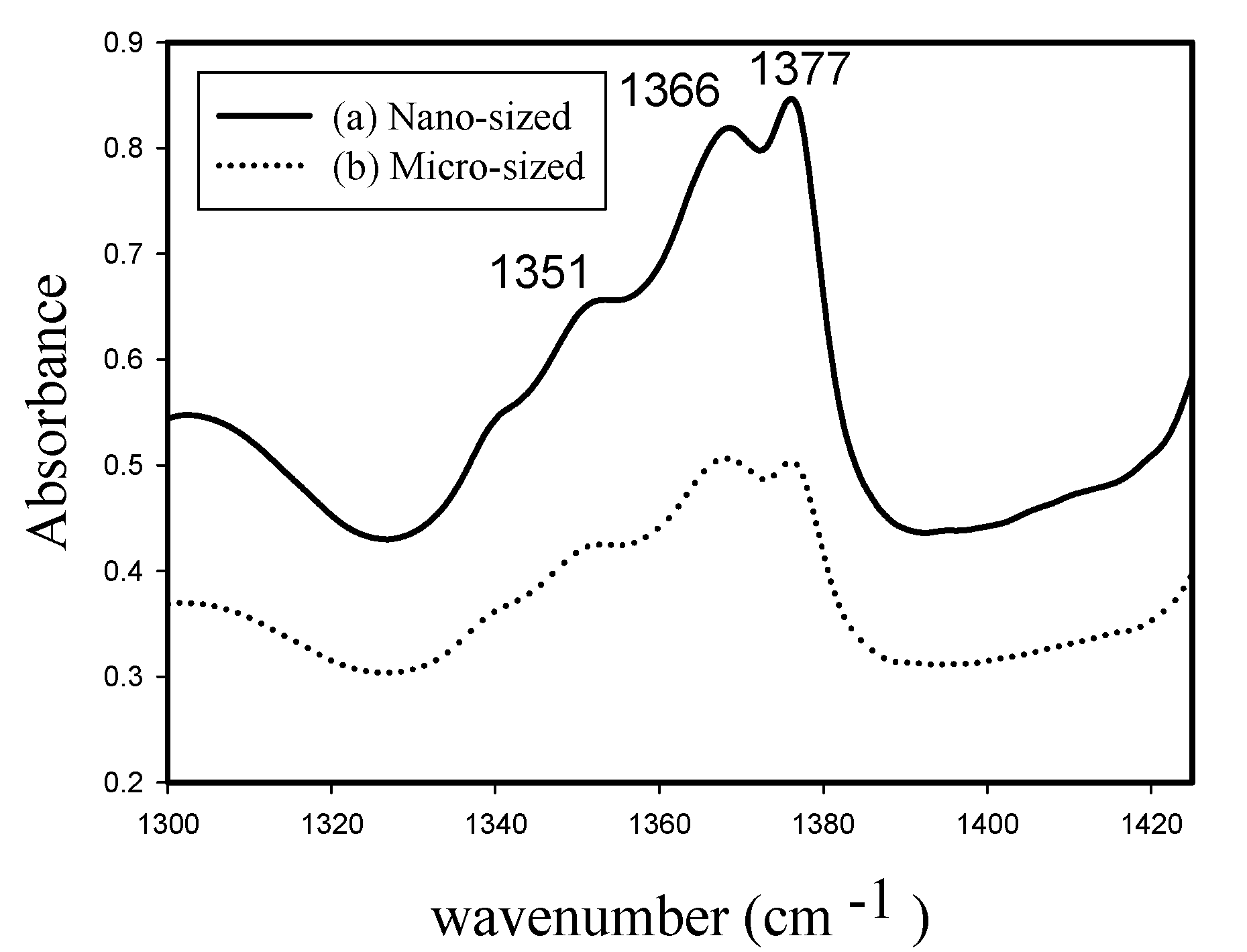
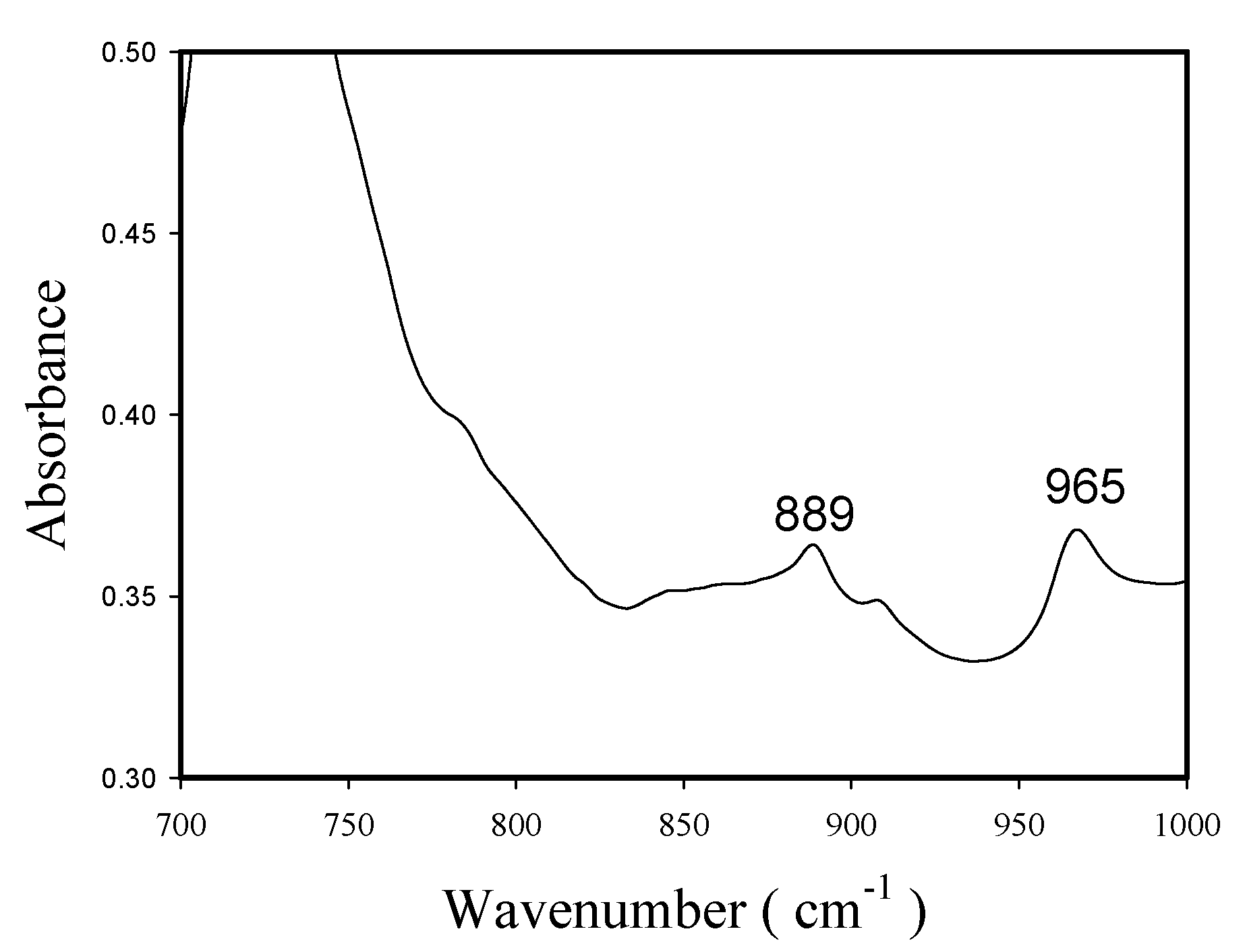
2.3. Polymer Characterization
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klosin, J.; Fontaine, P.P.; Figueroa, R. Development of group IV molecular catalysts for high temperature ethylene–α-olefin copolymerization reactions. Acc. Chem. Res. 2015, 48, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Doak, K.W.; James, D.E.; Beach, D.L.; Kissin, Y.V. Ethylene Polymers. In Encyclopedia of Polymer Science and Engineering; Mark, H.F., Bikales, N.M., Overberger, C.G., Menges, G., Eds.; Wiley: New York, NY, USA, 1986; Volume 6, pp. 383–490. [Google Scholar]
- Li, K.T.; Weng, W.T. Ethylene polymerization over Cr/MCM-41 and Cr/MCM-48 catalysts prepared by chemical vapor deposition method. J. Taiwan Inst. Chem. Eng. 2009, 40, 48–54. [Google Scholar] [CrossRef]
- Chum, P.S.; Kruper, W.J.; Guest, M.J. Material properties derived from INSITE metallocene catalysts. Adv. Mater. 2000, 12, 1759–1767. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-cyclopentaddienyl-amino catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, H.; Breitling, F.M. Constrained geometry complexes-Synthesis and applications. Coord. Chem. Rev. 2006, 250, 2691–2720. [Google Scholar] [CrossRef]
- Severn, J.R.; Chadwick, J.C.; Duchateau, R.; Friederichs, N. “Bound but not gagged”–Immobilizing single-site α-olefin polymerization catalysts. Chem. Rev. 2005, 105, 4073–4147. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.M.; Lourenco, J.P.; Cramail, H.; Ribeiro, M.R. Nanostructured silica materials in olefin polymerization: From catalytic behavior to polymer characteristics. Prog. Polym. Sci. 2012, 37, 1764–1804. [Google Scholar] [CrossRef]
- Kim, J.D.; Soares, H.B.P. Coploymerization of ethylene and α-olefin with combined metallocene catalysts. II. Mathematical modeling of polymerization with single metallocene catalysts. J. Polym. Sci. A Polym. Chem. 2000, 38, 1417–1426. [Google Scholar] [CrossRef]
- Ray, S.S.; Sarin, R.; Tuli, D.K.; Rai, M.M.; Ghosh, S.; Bhatnagar, A.K.; Sivaram, S.; Mohandas, T.P.; Gholap, D.H.; Yanjarappa, M.J.G. Process for the Preparation of Supported Aluminum Chloride Catalyst Containing Organoaluminium Compound. U.S. Patent 6096678, 1 August 2000. [Google Scholar]
- Li, K.T.; Kao, Y.T. Nanosized silica-supported metallocene/MAO catalyst for propylene polymerization. J. Appl. Polym. Sci. 2006, 101, 2573–2580. [Google Scholar] [CrossRef]
- Li, K.T.; Dai, C.L.; Kuo, C.W. Ethylene polymerization over a nano-sized silica supported Cp2ZrCl2/MAO catalyst. Catal. Commun. 2007, 8, 1209–1213. [Google Scholar] [CrossRef]
- Li, K.T.; Ko, F.S. Dimethylsilybis(1-indenyl)zirconium dichloride/methylaluminoxane catalyst supported on nanosized silica for propylene polymerization. J. Appl. Polym. Sci. 2008, 107, 1387–1394. [Google Scholar] [CrossRef]
- Li, K.T.; Dai, C.L.; Li, C.Y. Synthesis of linear low density polyethylene with a nano-sized silica supported Cp2ZrCl2/MAO catalyst. Polym. Bull. 2010, 64, 749–759. [Google Scholar] [CrossRef]
- Li, K.T.; Li, C.Y. Nano-sized silica supported Me2Si(Ind)2ZrCl2/MAO catalyst for ethylene polymerization. J. Appl. Polym. Sci. 2012, 123, 1169–1175. [Google Scholar] [CrossRef]
- Canich, J.A.M.; Licciardi, G.F. Mono-Cp Heteroatom Containing Group IVB Transition Metal Complexes with MAO: Supported Polymerization. U.S. Patent 5057475, 15 October 1991. [Google Scholar]
- Odian, G. Principles of Polymerization, 2nd ed.; Wiley: New York, NY, USA, 1981; pp. 613–614. [Google Scholar]
- Meier, G.B.; Weickert, G.; van Swaaij, W.P.M. Comparison of gas and liquid phase polymerization with heterogeneous metallocene catalyst. J. Appl. Polym. Sci. 2001, 81, 1193–1206. [Google Scholar] [CrossRef]
- Atiqullah, M.; Hammawa, H.; Hamid, H. Modeling in the solubility of ethylene and propylene in a typical polymerization diluent: Some selected situation. Eur. Polym. J. 1998, 34, 1511–1520. [Google Scholar] [CrossRef]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2006; pp. 833–834. [Google Scholar]
- Wang, W.J.; Kolodka, E.; Zhu, S.; Hamielec, A.E. Continuous solution copolymerization of ethylene and octene-1 with constrained geometry metallocene catalyst. J. Polym. Sci. A Polym. Chem. 1999, 37, 2949–2957. [Google Scholar] [CrossRef]
- Krimm, S.; Tobolsky, A.V. Quantitative X-ray studies of order in amorphous and crystalline polymers. Quantative X-ray determination of crystallinity in polyethylene. J. Polym. Sci. 1951, 7, 57–76. [Google Scholar] [CrossRef]
- Young, R.J.; Lovell, P.A. Introduction to Polymers, 2nd ed.; Chapman & Hall: London, UK, 1991; p. 266. [Google Scholar]
- Mahdavi, H.; Nook, M.E. Characterization and microstructure study of low-density polyethylene by Fourier transform infrared spectroscopy and temperature rising elution fractionation. J. Appl. Polym. Sci. 2008, 109, 3492–3501. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2001, 21, 557–563. [Google Scholar] [CrossRef]
- Kupper, L.; Gulmine, J.V.; Janissek, P.R.; Heise, H.M. Attenuated total reflection infrared spectroscopy for micro-domain analysis of polyethylene samples after accelerated aging within weathering chambers. Vib. Spectrosc. 2004, 34, 63–72. [Google Scholar] [CrossRef]
- ASTM D 2238–92: Standard Test Method for Absorbance of Polyethylene due to Methyl Groups at 1378 cm−1; American Society for Testing and Materials: West Conshohocken, PA, USA, 1992.
- Caro, E.; Comas, E. Polyethylene comonomer characterization by using FTIR and a multivariate classification technique. Talanta 2017, 163, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Uozumi, T.; Nakamura, S.; Toneri, T.; Teranishi, T.; Sano, T.; Arai, T. Structure of polyethylene and copolymers of ethylene with 1-octene and oligoethylene produced with the Cp2ZrCl2 and [(C5Me4)SiMe2N(t-Bu)] Ti Cl2 catalysts. Macromol. Chem. Phys. 1996, 197, 4237–4251. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.-T.; Wu, L.-H. Constrained Geometry Organotitanium Catalysts Supported on Nanosized Silica for Ethylene (co)Polymerization. Molecules 2017, 22, 751. https://doi.org/10.3390/molecules22050751
Li K-T, Wu L-H. Constrained Geometry Organotitanium Catalysts Supported on Nanosized Silica for Ethylene (co)Polymerization. Molecules. 2017; 22(5):751. https://doi.org/10.3390/molecules22050751
Chicago/Turabian StyleLi, Kuo-Tseng, and Ling-Huey Wu. 2017. "Constrained Geometry Organotitanium Catalysts Supported on Nanosized Silica for Ethylene (co)Polymerization" Molecules 22, no. 5: 751. https://doi.org/10.3390/molecules22050751





