Synthesis of Europium-Doped Fluorapatite Nanorods and Their Biomedical Applications in Drug Delivery
Abstract
:1. Introduction
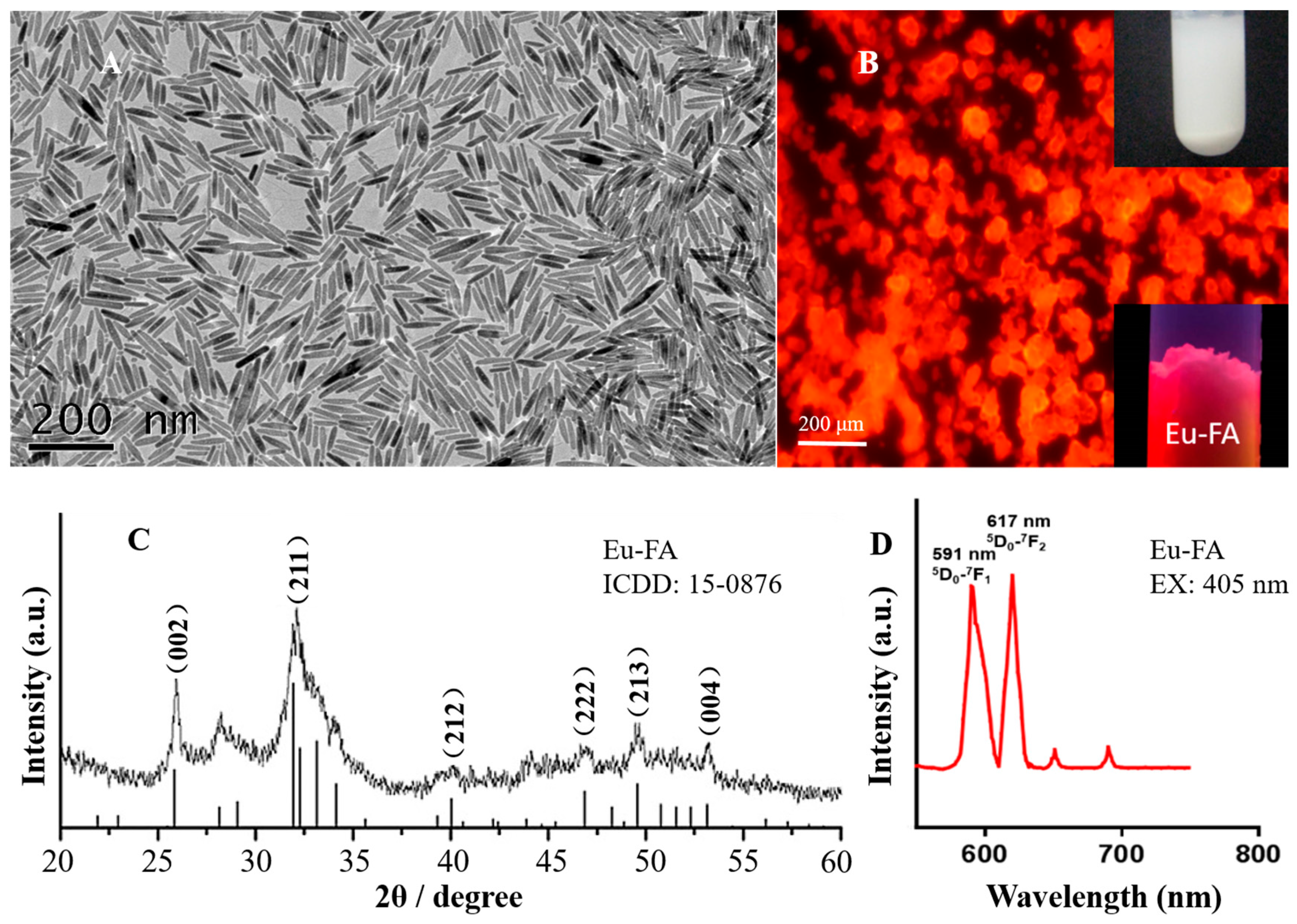
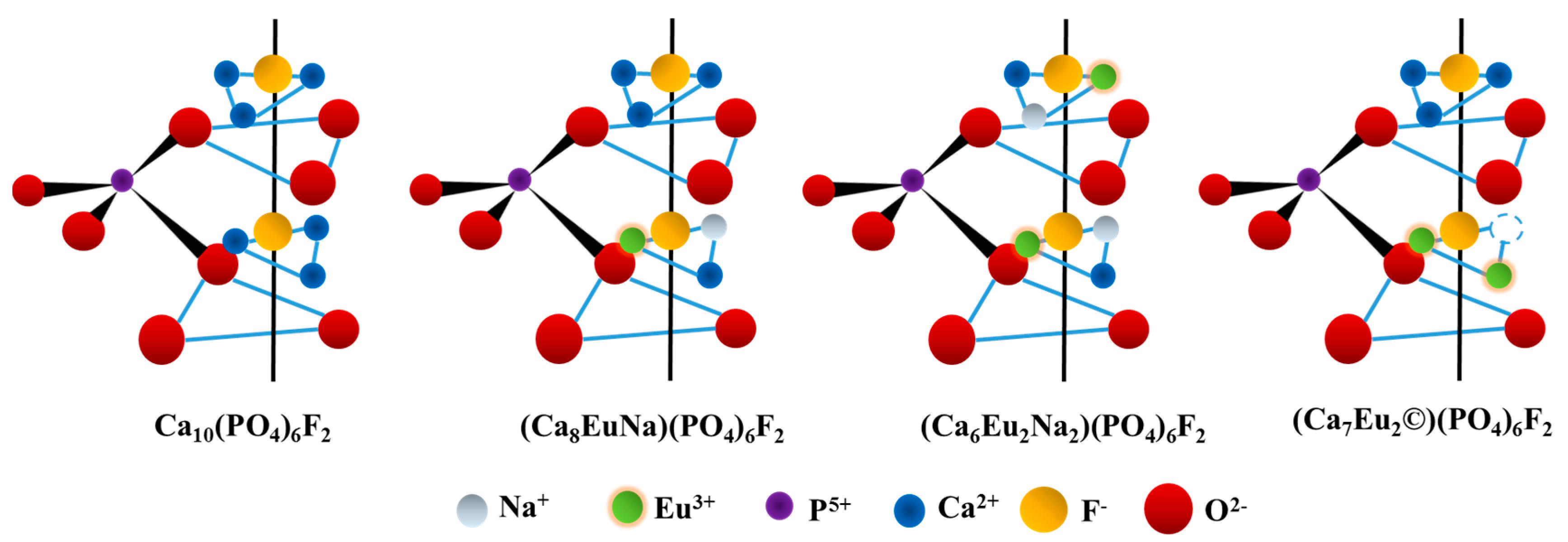
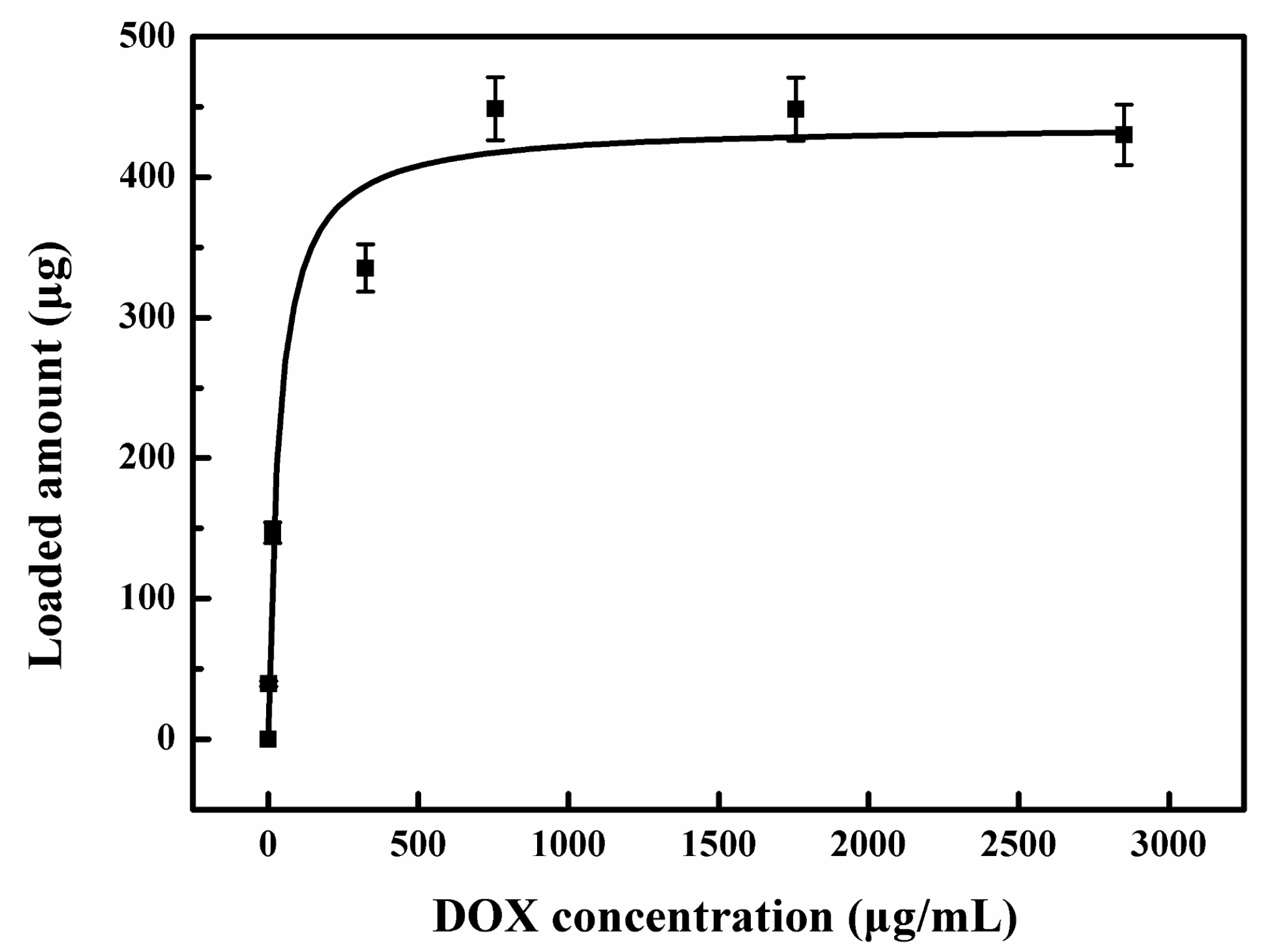
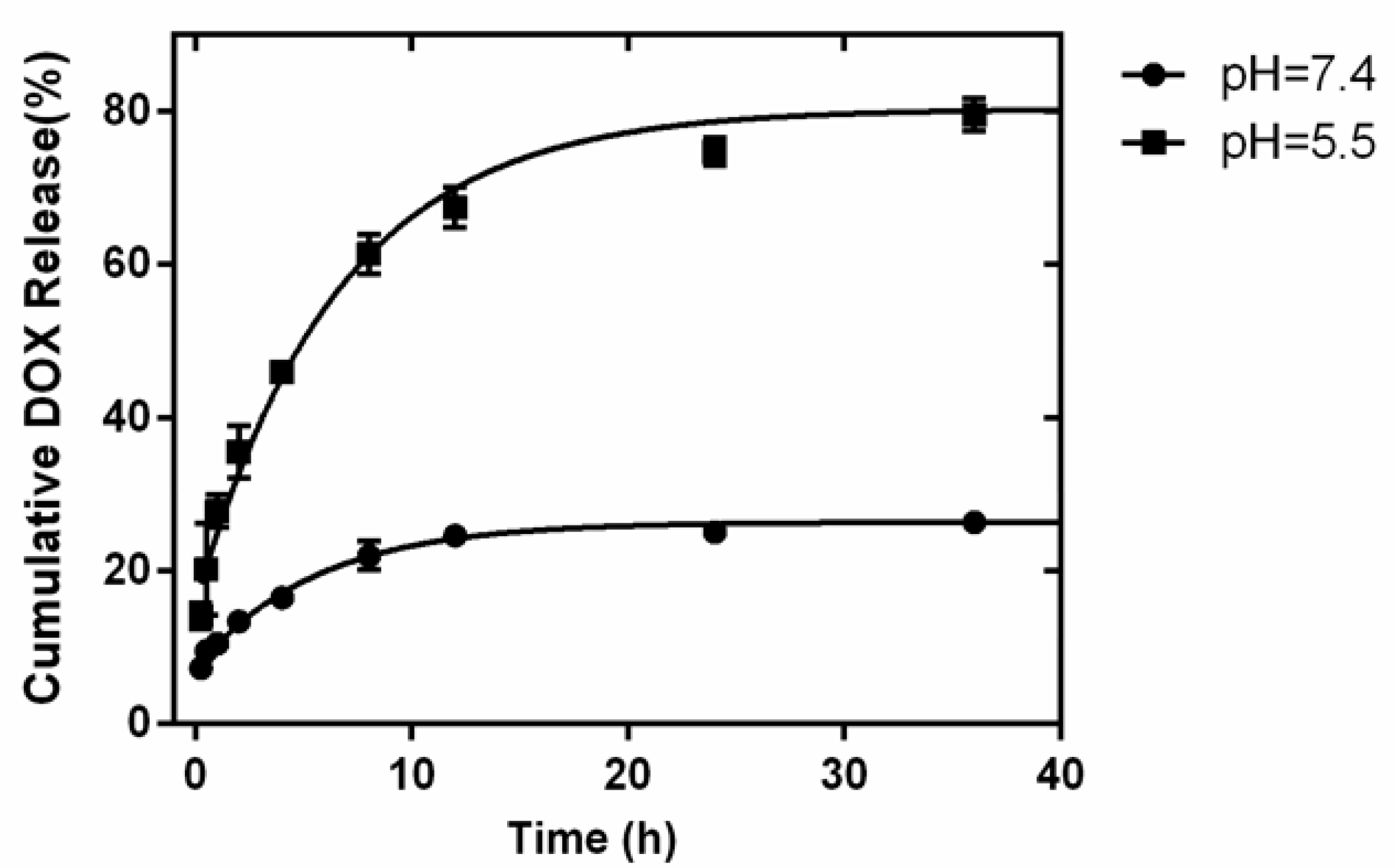
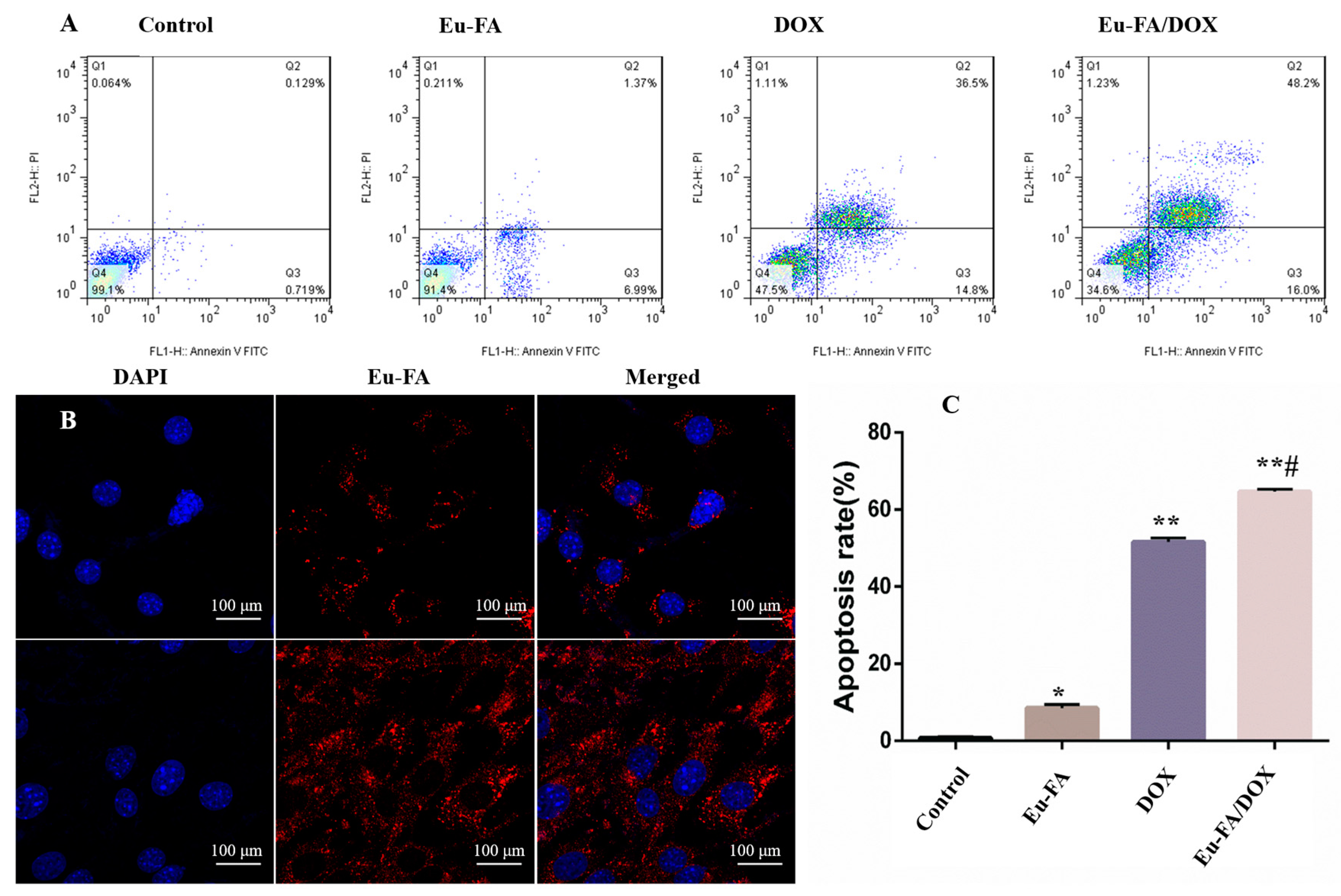
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Eu-FHA Nanorods
3.2. Characterization of Eu-FHA Nanorods
3.3. Preparation of the Drug Loading and Release System
3.4. Cell Culture and Fluorescence Imaging
3.5. Cell Apoptosis Detection
3.6. Statistics Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, Y.J.; Tseng, Y.H.; Chan, J.C.C. Morphology control of fluorapatite crystallites by citrate ions. Cryst. Growth Des. 2010, 10, 4240–4242. [Google Scholar] [CrossRef]
- Jantová, S.; Theiszová, M.; Letasiová, S.; Birosová, L.; Palou, T.M. In vitro effects of fluor-hydroxyapatite, fluorapatite and hydroxyapatite on colony formation, DNA damage and mutagenicity. Mutat. Res. 2008, 652, 139–144. [Google Scholar]
- Liu, H.; Chen, F.; Xi, P.; Chen, B.; Huang, L.; Cheng, J.; Shao, C.; Wang, J.; Bai, D.; Zeng, Z. Biocompatible fluorescent hydroxyapatite: Synthesis and live cell imaging applications. J. Phys. Chem. 2011, 115, 18538–18544. [Google Scholar] [CrossRef]
- Chen, F.; Huang, P.; Zhu, Y.J.; Wu, J.; Zhang, C.L.; Cui, D.X. The photoluminescence, drug delivery and imaging properties of multifunctional eu3+/gd3+ dual-doped hydroxyapatite nanorods. Biomaterials 2011, 32, 9031–9039. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X. Multicolor tuning of lanthanide-doped nanoparticles by single wavelength excitation. Acc. Chem. Res. 2014, 47, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tu, D.; Zhu, H.; Chen, X. Cheminform abstract: Lanthanide-doped luminescent nanoprobes: Controlled synthesis, optical spectroscopy, and bioapplications. Chem. Soc. Rev. 2013, 42, 6924. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Jiang, F.L.; Wu, M.Y.; Chen, L.; Yan, C.F.; Hong, M.C. Structures and photoluminescent properties of the lanthanide coordination complexes with hydroxyquinoline carboxylate ligands. Cryst. Growth Des. 2010, 10, 2306–2313. [Google Scholar] [CrossRef]
- Ou, Y.C.; Gao, X.; Zhou, Y.; Chen, Y.C.; Wang, L.F.; Wu, J.Z.; Tong, M.L. Magnetic properties and photoluminescence of lanthanide coordination polymers constructed with conformation-flexible cyclohexane-tetracarboxylate ligands. Cryst. Growth Des. 2015, 16, 946–952. [Google Scholar] [CrossRef]
- Kariem, M.; Yawer, M.; Sharma, S.; Sheikh, H.N. Syntheses, crystal structure, luminescence, porosity and magnetic properties of three-dimensional lanthanide coordination polymers with 2-aminoterepthalic acid. ChemistrySelect 2016, 1, 4489–4501. [Google Scholar] [CrossRef]
- Zeng, H.; Li, X.; Xie, F.; Teng, L.; Chen, H. Dextran-coated fluorapatite nanorods doped with lanthanides in labelling and directing osteogenic differentiation of bone marrow mesenchymal stem cells. J. Mater. Chem. 2014, 2, 3609–3617. [Google Scholar] [CrossRef]
- Li, X.; Zeng, H.; Teng, L.; Chen, H. Comparative investigation on the crystal structure and cell behavior of rare-earth doped fluorescent apatite nanocrystals. Mater. Lett. 2014, 125, 78–81. [Google Scholar] [CrossRef]
- Rulis, P.; Ouyang, L.; Ching, W.Y. Electronic structure and bonding in calcium apatite crystals: Hydroxyapatite, fluorapatite, chlorapatite, and bromapatite. Phys. Rev. B 2004, 70, 2806–2810. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Nezafati, N.; Saber-Samandari, S. The effective role of hydroxyapatite based composites in anticancer drug delivery systems. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 41. [Google Scholar] [CrossRef] [PubMed]
- Di, W.; Wang, X.; Chen, B.; Lu, S.; Zhao, X. Effect of oh- on the luminescent efficiency and lifetime of tb3+-doped yttrium orthophosphate synthesized by solution precipitation. J. Phys. Chem. B 2005, 109, 13154. [Google Scholar] [CrossRef] [PubMed]
- Sanson, C.; Schatz, C.; Meins, J.F.L.; Soum, A.; Thévenot, J.; Garanger, E.; Lecommandoux, S. A simple method to achieve high doxorubicin loading in biodegradable polymersomes. J. Control. Release 2010, 147, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Singh, R.K.; Kim, T.H.; Patel, K.D.; Kim, J.J.; Chrzanowski, W.; Kim, H.W. Luminescent mesoporous nanoreservoirs for the effective loading and intracellular delivery of therapeutic drugs. Acta Biomater. 2014, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor ph targeting nanotechnology. J. Control. Release 2011, 132, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bian, S.; Huang, Y.; Liang, J.; Fan, Y.; Zhang, X. High drug loading ph-sensitive pullulan-dox conjugate nanoparticles for hepatic targeting. J. Biomed. Mater. Res. Part A 2014, 102, 150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Meng, H.; Wang, N.; Donovan, M.J.; Fu, T.; You, M.; Chen, Z.; Zhang, X.; Tan, W. A controlled-release nanocarrier with extracellular pH value driven tumor targeting and translocation for drug delivery. Angew. Chem. Int. Ed. 2013, 52, 7487–7491. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Ghosh, D.; Sinha, M.K.; Sen, P.S.; Balla, V.K.; Das, N.; Basu, D. Doxorubicin-intercalated nano-hydroxyapatite drug-delivery system for liver cancer: An animal model. Ceram. Int. 2013, 39, 9557–9566. [Google Scholar] [CrossRef]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin v/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011, 50, 40. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahimhashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Li, X.; Sun, M.; Wu, S.; Chen, H. Synthesis of Europium-Doped Fluorapatite Nanorods and Their Biomedical Applications in Drug Delivery. Molecules 2017, 22, 753. https://doi.org/10.3390/molecules22050753
Zeng H, Li X, Sun M, Wu S, Chen H. Synthesis of Europium-Doped Fluorapatite Nanorods and Their Biomedical Applications in Drug Delivery. Molecules. 2017; 22(5):753. https://doi.org/10.3390/molecules22050753
Chicago/Turabian StyleZeng, Haifeng, Xiyu Li, Muyang Sun, Sufan Wu, and Haifeng Chen. 2017. "Synthesis of Europium-Doped Fluorapatite Nanorods and Their Biomedical Applications in Drug Delivery" Molecules 22, no. 5: 753. https://doi.org/10.3390/molecules22050753
APA StyleZeng, H., Li, X., Sun, M., Wu, S., & Chen, H. (2017). Synthesis of Europium-Doped Fluorapatite Nanorods and Their Biomedical Applications in Drug Delivery. Molecules, 22(5), 753. https://doi.org/10.3390/molecules22050753




