Photoactive Hybrid Catalysts Based on Natural and Synthetic Polymers: A Comparative Overview
Abstract
:1. Introduction
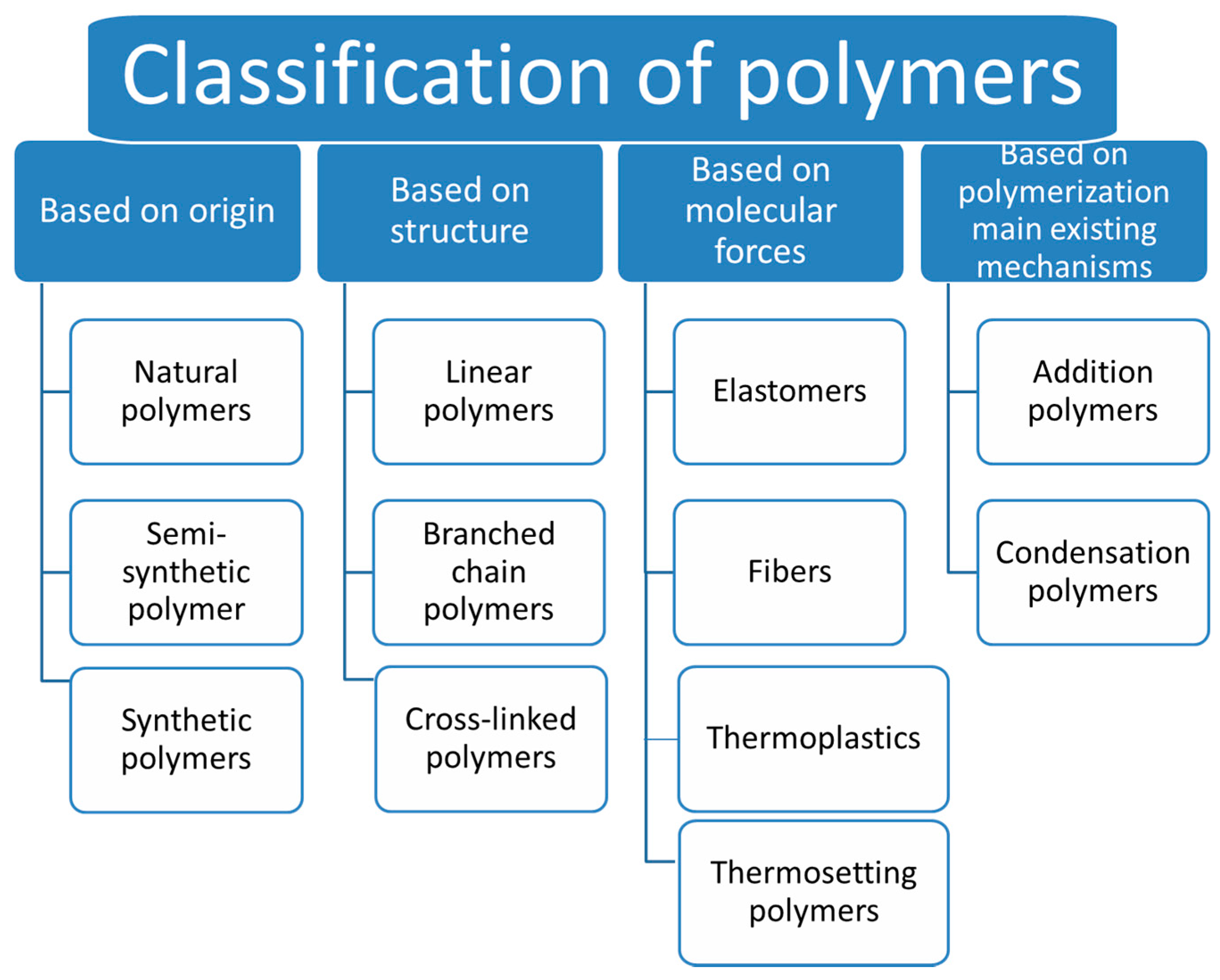
2. Synthetic and Natural Polymers
2.1. Photocatalytic Hybrid Materials Based on Synthetic Polymers
2.2. Photocatalytic Hybrid Materials Based on Natural Polymers
3. Summary and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pichat, P.; Oills, D. Photocatalytic Treatment of Water: Irradiance Influences. In Photocatalysis and Water Purification: From Fundamentals to Recent Applications, 1st ed.; Pichat, P., Ed.; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2013; pp. 311–333. [Google Scholar]
- George, C.; Beeldens, A.; Barmpas, F.; Doussin, J.F.; Manganelli, G.; Herrmann, H.; Kleffmann, J.; Mellouki, A. Impact of photocatalytic remediation of pollutants on urban air quality. Front. Environ. Sci. Eng. 2016, 10. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, W.; Yu, J.C.; Liu, H.; Wei, L. Design and fabrication of heterojunction photocatalysts for energy conversion and pollutant degradation. Chin. J. Catal. 2014, 35, 1609–1618. [Google Scholar] [CrossRef]
- Peng, Y.; Shang, L.; Bian, T.; Zhao, Y.; Zhou, C.; Yu, H.; Tunga, C.; Zhang, T. Flower-like CdSe ultrathin nanosheet assemblies for enhanced visible-light-driven photocatalytic H2 production. Chem. Commun. 2015, 51, 4677–4680. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, N.; Shanmugam, S.; Xu, J.; Boyer, C. Photocatalysis in organic and polymer synthesis. Chem. Soc. Rev. 2016, 45, 6165–6212. [Google Scholar] [CrossRef] [PubMed]
- Penga, Y.; Shanga, L.; Cao, Y.; Wang, Q.; Zhao, Y.; Zhou, C.; Bian, T.; Wu, L.Z.; Tung, C.H.; Zhang, T. Effects of surfactants on visible-light-driven photocatalytic hydrogen evolution activities of AgInZn7S9 nanorods. Appl. Surf. Sci. 2015, 358, 485–490. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, G.; Bian, T.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Smith, L.J.; O’Hare, D.; Zhang, T. Defect-Rich Ultrathin ZnAl-Layered Double Hydroxide Nanosheets for Efficient Photoreduction of CO2 to CO with Water. Adv. Mater. 2015, 27, 7823–7831. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T. Alkali-Assisted Synthesis of Nitrogen Deficient Graphitic Carbon Nitride with Tunable Band Structures for Efficient Visible-Light-Driven Hydrogen Evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, J.C.; Varma, R.S.; Lisowski, P. Sustainable hybrid photocatalysts: Titania immobilized on carbon materials derived from renewable and biodegradable resources. Green Chem. 2016, 18, 5736–5750. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Kuna, E.; Lisowski, P. Synthesis of Photoactive Materials by Sonication: Application in Photocatalysis and Solar Cells. Top. Curr. Chem. 2016, 374. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, J.C.; Xu, Y.J. Heterogeneous Photocatalysis: From Fundamentals to Green Applications. In Heterogeneous Photocatalysis, 1st ed.; He, L.N., Rogers, R.D., Su, D., Tundo, P., Zhang, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Arora, A.K.; Jaswal, V.S.; Singh, K.; Singh, R. Applications of Metal/Mixed Metal Oxides as Photocatalyst: (A Review). Orient. J. Chem. 2016, 32, 2035–2042. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, X.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; O’Hare, D.; Zhang, T. Layered Double Hydroxide Nanostructured Photocatalysts for Renewable Energy Production. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, T.; Li, X.; Xu, J.; Zeng, H. Progress of Carbon Quantum Dots in Photocatalysis Applications. Part. Part. Syst. Charact. 2016, 33, 447–588. [Google Scholar] [CrossRef]
- Wang, W.; Tadéa, M.O.; Shao, Z. Research progress of perovskite materials in photocatalysis- and photovoltaics-related energy conversion and environmental treatment. Chem. Soc. Rev. 2015, 44, 5371–5408. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Schneider, J.; Bahnemann, D.; Ye, J.; Puma, G.L.; Dionysiou, D.D. Photocatalysis, Vol. 1: Fundamentals and Perspectives; Vol. 2: Applications, 1st ed.; RSC Publishing: Cambridge, UK, 2016; pp. 1–936. [Google Scholar]
- Pichat, P. Photocatalysis: Fundamentals, Materials and Potential, 1st ed.; MDPI: Basel, Switzerland, 2016; pp. 3–650. [Google Scholar]
- Nosaka, Y.; Nosaka, A. Introduction to Photocatalysis: From Basic Science to Applications, 1st ed.; RSC Publishing: Cambridge, UK, 2016; pp. 1–272. [Google Scholar]
- Portela, R.; Hernández-Alonso, M.D. Design of Advanced Photocatalytic Materials for Energy and Environmental Applications, 1st ed.; Coronado, J., Fresno, F., Hernández-Alonso, M.D., Portela, R., Eds.; Springer: London, UK, 2013; pp. 35–36. [Google Scholar]
- Chaturvedi, S.; Dave, P.N. Environmental Application of Photocatalysis. Mater. Sci. Forum 2012, 734, 273–294. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Feng, C. Application of Photocatalytic Technology in Environmental Safety. Procedia Eng. 2012, 45, 993–997. [Google Scholar] [CrossRef]
- Nakataa, K.; Fujishimaa, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Sanchez, C.; Ribot, F.; Lebeau, B. Molecular design of hybrid organic-inorganic nanocomposites synthesized via sol-gel chemistry. J. Mater. Chem. 1999, 9, 35–44. [Google Scholar] [CrossRef]
- Singh, S.; Mahalingam, H.; Singh, P.K. Polymer-supported titanium dioxide photocatalysts for environmental remediation: A review. Appl. Catal. A 2013, 462–463, 178–195. [Google Scholar] [CrossRef]
- Vilatela, J.J.; Eder, D. Nanocarbon composites and hybrids in sustainability: A review. ChemSusChem 2012, 12, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.S.; Chaudhary, R.; Singh, C. Fundamentals and applications of the photocatalytic treatment for the removal of industrial organic pollutants and effects of operational parameters: A review. J. Renew. Sustain. Energy Rev. 2010, 2, 042701. [Google Scholar] [CrossRef]
- Peng, Y.; Shang, L.; Cao, Y.; Waterhouse, G.I.N.; Zhou, C.; Bian, T.; Wu, L.Z.; Tunga, C.H.; Zhang, T. Copper(I) cysteine complexes: Efficient earth-abundant oxidation co-catalysts for visible light-driven photocatalytic H2 production. Chem. Commun. 2015, 51, 12556–12599. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Hassan, M.; Gomes, V.G. Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl. Catal. A Gen. 2015, 489, 1–16. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Ghosh, T.; Oh, W.C. Carbon Based Titania Photocatalysts. Asian J. Chem. 2012, 24, 5419–5423. [Google Scholar]
- Muggeridge, A.; Cockin, A.; Webb, K.; Frampton, H.; Collins, I.; Moulds, T.; Salino, P. Recovery rates, enhanced oil recovery and technological limits. Philos. Trans. R. Soc. A 2013, 372, 20120320. [Google Scholar] [CrossRef] [PubMed]
- How Much Oil Is Used to Make Plastic? Available online: http://www.eia.gov/tools/faqs/faq.cfm?id=34&t=6 (accessed on 25 April 2016).
- Gervet, B. The Use of Crude Oil in Plastic Making Contributes to Global Warming; Department of Civil and Environmental Engineering Luleå University of Technology: Luleå, Sweden, May 2007. [Google Scholar]
- Compendium of Polymer Terminology and Nomenclature. Available online: https://www.iupac.org/cms/wp-content/uploads/2016/01/Compendium-of-Polymer-Terminology-and-Nomenclature-IUPAC-Recommendations-2008.pdf (accessed on 19 January 2009).
- Olatunji, O. Classification of Natural Polymers; Springer: Basel, Switzerland, 2015; pp. 1–17. [Google Scholar]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers—A Review on Recent Trends and Emerging Perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J. Biodegradable and Biobased Polymers. In Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications, 1st ed.; Ebnesajjad, S., Andrew, W., Eds.; Elsevier: Oxford, UK, 2013; pp. 109–124. [Google Scholar]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers—Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Yu, Z.; Mielczarski, E.; Mielczarski, J.; Laub, C.; Buffat, P.; Klehm, U.; Albers, P.; Lee, K.; Kulike, A.; Kiwi-Minerska, L.; et al. Preparation, stabilization and characterization of TiO2 on thin polyethylene films (LDPE). Photocatalytic applications. Water Res. 2007, 41, 862–874. [Google Scholar]
- Ma, S.; Meng, J.; Li, J.; Zhang, Y.; Ni, L. Synthesis of catalytic polypropylene membranes enabling visible-light-driven photocatalytic degradation of dyes in water. J. Membr. Sci. 2014, 453, 221–229. [Google Scholar] [CrossRef]
- Zan, L.; Tian, L.; Liu, Z.; Peng, Z. A new polystyrene–TiO2 nanocomposite film and its photocatalytic degradation. Appl. Catal. A Gen. 2004, 264, 237–242. [Google Scholar]
- Taylor, D.M.; Lewis, T.J. Electrical conduction in polyethylene terephthalate and polyethylene films. J. Phys. D Appl. Phys. 1971, 4, 1346–1354. [Google Scholar] [CrossRef]
- Wang, D.; Shi, L.; Luo, Q.; Li, X.; An, J. An efficient visible light photocatalyst prepared from TiO2 and polyvinyl chloride. J. Mater. Sci. 2012, 47, 2136–2145. [Google Scholar] [CrossRef]
- Araújo, V.D.; Tranquilin, R.L.; Motta, F.V.; Paskocimas, C.A.; Bernardi, M.I.B.; Cavalcante, L.S.; Andres, J.; Longo, E.; Bomio, M.R.D. Effect of polyvinyl alcohol on the shape, photoluminescence and photocatalytic properties of PbMoO4 microcrystals. Mater. Sci. Semicond. Process. 2014, 26, 425–430. [Google Scholar] [CrossRef]
- Fateh, R.; Dillert, R.; Bahnemann, D. Preparation and characterization of transparent hydrophilic photocatalytic TiO2/SiO2 thin films on polycarbonate. Langmuir 2015, 29, 3730–3739. [Google Scholar] [CrossRef] [PubMed]
- Tennakone, K.; Tilakaratne, C.T.K.; Kottegoda, I.R.M. Photocatalytic degradation of organic contaminants in water with TiO2 supported on polyethene films. J. Photochem. Photobiol. A Chem. 1995, 87, 177–179. [Google Scholar] [CrossRef]
- Ariffin, S.N.; Lima, H.N.; Jumeri, F.A.; Zobir, M.; Abdullah, A.H.; Ahmad, M.; Ibrahim, N.A.; Huang, N.M.; Teo, P.S.; Muthoosamy, K.; et al. Modification of polypropylene filter with metal oxide and reduced graphene oxide for water treatment. Ceram. Int. 2014, 40, 6927–6936. [Google Scholar] [CrossRef]
- Li, M.; Li, G.; Fan, Y.; Jiang, J.; Ding, Q.; Dai, X.; Mai, K. Effect of nano-ZnO-supported 13X zeolite on photo-oxidation degradation and antimicrobial properties of polypropylene random copolymer. Polym. Bull. 2014, 71, 2981–2997. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Kuna, E.; Jakubiak, S.; Michalski, J.; Kurzydłowski, K. Polypropylene nonwoven filter with nanosized ZnO rods: Promising hybrid photocatalyst for water purification. Appl. Catal. B Environ. 2015, 170–171, 273–282. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Preparation and Characterization of Composite PES/Nanoparticle Membranes. Available online: https://web.wpi.edu/Pubs/E-project/Available/E-project-042313–223351/unrestricted/Sheppard_MQP_Report.pdf (accessed on 25 April 2013).
- Gong, B.; Peng, Q.; Na, J.S.; Parsons, G.N. Highly active photocatalytic ZnO nanocrystalline rods supported on polymer fiber mats: Synthesis using atomic layer deposition and hydrothermal crystal growth. Appl. Catal. A Gen. 2011, 407, 211–216. [Google Scholar] [CrossRef]
- Hong, J.; He, Y. Polyvinylidene fluoride ultrafiltration membrane blended with nano-ZnO particle for photo-catalysis self-cleaning. Desalination 2014, 332, 67–75. [Google Scholar] [CrossRef]
- Moafi, H.F.; Shojaie, A.F.; Zanjanchi, M.A. Semiconductor-assisted self-cleaning polymeric fibers based on zinc oxide nanoparticles. J. Appl. Polym. Sci. 2011, 121, 3641–3650. [Google Scholar] [CrossRef]
- Sun, L.; Shi, Y.; Li, B.; Li, X.; Wang, Y. Preparation and characterization of polypyrrole/TiO2 nanocomposites by reverse microemulsion polymerization and its photocatalytic activity for the degradation of methyl orange under natural light. Polym. Compos. 2013, 34, 1076–1080. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Cho, M.H. Polythiophene nanocomposites for photodegradation applications: Past, present and future. J. Saudi Chem. Soc. 2015, 19, 494–504. [Google Scholar] [CrossRef]
- Aizawa, M.; Watanabe, S.; Shinohara, H.; Shirakawa, H. Photodoping of polyacetylene films. J. Chem. Soc. Chem. Commun. 1985, 2, 62–63. [Google Scholar] [CrossRef]
- Yang, M.; Dan, Y. Preparation of poly(methyl methacrylate)/titanium oxide composite particles via in-situ emulsion polymerization. J. Appl. Polym. Sci. 2006, 101, 4056–4063. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, T.; Xu, J.; Zeng, H. Polythiophene/Bi2MoO6: A novel conjugated polymer/nanocrystal hybrid composite for photocatalysis. J. Mater. Sci. 2016, 51, 3846–3853. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kouame, N.A.; Remita, S.; Ramos, L.; Goubard, F.; Aubert, P.H.; Dazzi, A.; Deniset-Besseau, A.; Remita, H. Visible-light active conducting polymer nanostructures with superior photocatalytic activity. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Polypyrrole/TiO2 composites for the application of photocatalysis. Sens. Actuators B Chem. 2017, 241, 1161–1169. [Google Scholar] [CrossRef]
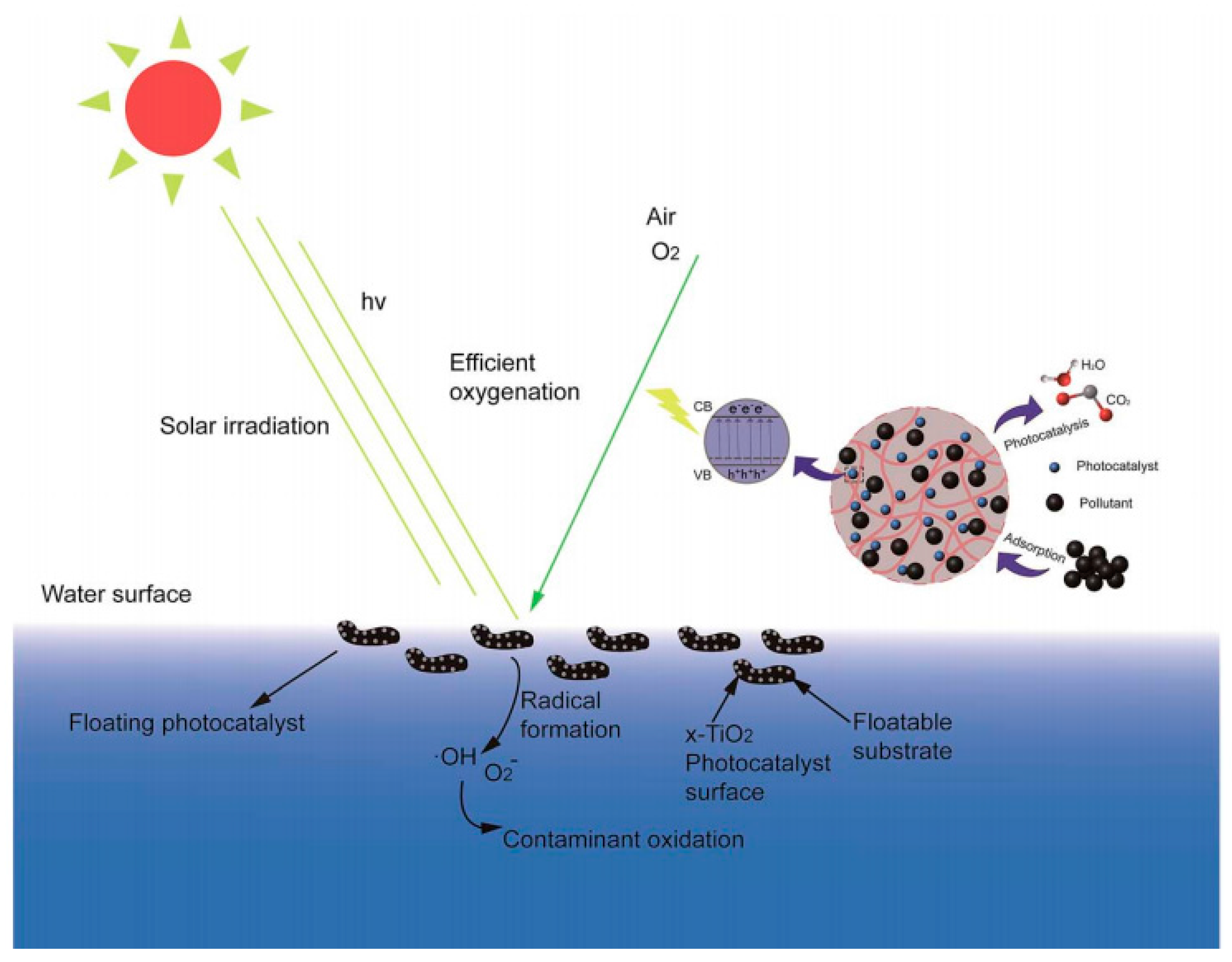
- Magalhães, F.; Mourab, F.C.C.; Lago, R.M. TiO2/LDPE composites: A new floating photocatalyst for solar degradation of organic contaminants. Desalination 2011, 276, 266–271. [Google Scholar] [CrossRef]
- Naskar, S.; Pillay, A.S.; Chanda, M. Photocatalytic degradation of organic dyes in aqueous solution with TiO2 nanoparticles immobilized on foamed polyethylene sheet. J. Photochem. Photobiol. A Chem. 1998, 3, 257–264. [Google Scholar] [CrossRef]
- Jin, L.; Wu, H.; Morbidelli, M. Synthesis of Water-Based Dispersions of Polymer/TiO2 Hybrid Nanospheres. Nanomaterials 2015, 5, 1454–1468. [Google Scholar] [CrossRef] [PubMed]
- Nabid, M.R.; Golbabaee, M.; Moghaddam, A.B.; Dinarvand, R.; Sedghi, R. Polyaniline/TiO2 Nanocomposite: Enzymatic Synthesis and Electrochemical Properties. Int. J. Electrochem. Sci. 2008, 3, 1117–1126. [Google Scholar]
- Wang, X.; Wang, X.; Wang, W.; Zhao, J. Enhanced visible light photocatalytic activity of a floating photocatalyst based on B-N-codoped TiO2 grafted on expanded perlite. RSC Adv. 2015, 5, 41385–41392. [Google Scholar] [CrossRef]
- Kan, W.Q.; Liu, B.; Yang, J.; Liu, Y.Y.; Ma, J.F. A Series of Highly Connected Metal–Organic Frameworks Based on Triangular Ligands and d10 Metals: Syntheses, Structures, Photoluminescence, and Photocatalysis. Cryst. Growth Des. 2012, 12, 2288–2298. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; deKrafft, K.E.; Lin, W. Light-Harvesting Cross-Linked Polymers for Efficient Heterogeneous Photocatalysis. ACS Appl. Mater. Interfaces 2012, 4, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Ranocchiari, M.; Bokhoven, J.A. Metal organic frameworks for photo-catalytic water splitting. Energy Environ. Sci. 2015, 8, 1923–1937. [Google Scholar] [CrossRef]
- Li, C.; Chen, R.; Zhang, X.; Shu, S.; Xiong, J.; Zheng, Y.; Dong, W. Electrospinning of CeO2–ZnO composite nanofibers and their photocatalytic property. Mater. Lett. 2011, 65, 1327–1330. [Google Scholar] [CrossRef]
- Baruah, S.; Thanachayanont, C.; Dutta, J. Growth of ZnO nanowires on nonwoven polyethylene fibers. Sci. Technol. Adv. Mater. 2008, 9, 025009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, C.; Li, X.; Zhang, L.; Xue, H.; Wang, C.; Liu, Y. Electrospun Nanofibers of ZnO−SnO2 Heterojunction with High Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 7920–7925. [Google Scholar] [CrossRef]
- Gao, M.; Peh, C.K.G.; Onga, W.L.; Ho, G.W. Green chemistry synthesis of a nanocomposite graphene hydrogel with three-dimensional nano-mesopores for photocatalytic H2 production. RSC Adv. 2013, 3, 13169–13177. [Google Scholar] [CrossRef]
- Petzold, J.C.; Herrington, T.M. The measurement of concentration and stability of weak acids and a study of the ionisation of polysaccharides using buffer capacity curves. Macromol. Chem. Phys. 2003, 192, 1741–1748. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, X.; Yang, Y. Facile Synthesis of Monodisperse Porous ZnO Spheres by a Soluble Starch-Assisted Method and Their Photocatalytic Activity. J. Phys. Chem. C 2011, 115, 7145–7152. [Google Scholar] [CrossRef]
- Hamdi, A.; Boufi, S.; Bouattour, S. Phthalocyanine/chitosan-TiO2 photocatalysts: Characterization and photocatalytic activity. Appl. Surf. Sci. 2015, 339, 128–136. [Google Scholar] [CrossRef]
- Benabid, F.Z.; Zouai, F. Natural polymers: Cellulose, chitin, chitosan, gelatin, starch, carrageenan, xylan and dextran. Alger. J. Nat. Prod. 2016, 4, 348–357. [Google Scholar]
- Fu, S.; Song, P.; Liu, X. Thermal and flame retardancy properties of thermoplastics/natural fiber biocomposites. In Advanced High Strength Natural Fibre Composites in Construction, 1st ed.; Gwen, J., Ed.; Wood Publishing: Duxford, UK, 2017; pp. 479–508. [Google Scholar] [CrossRef]
- Thakura, V.K.; Thakurb, M.K. Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carbohydr. Polym. 2014, 109, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fu, S.; Peng, L. Surface modification of cellulose fibers by layer-by-layer self-assembly of lignosulfonates and TiO2 nanoparticles: Effect on photocatalytic abilities and paper properties. Fibers Polym. 2013, 14, 1794–1802. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, S.; Cai, J.; Zhang, L. TiO2 Immobilized in Cellulose Matrix for Photocatalytic Degradation of Phenol under Weak UV Light Irradiation. J. Phys. Chem. C 2010, 114, 7806–7811. [Google Scholar] [CrossRef]
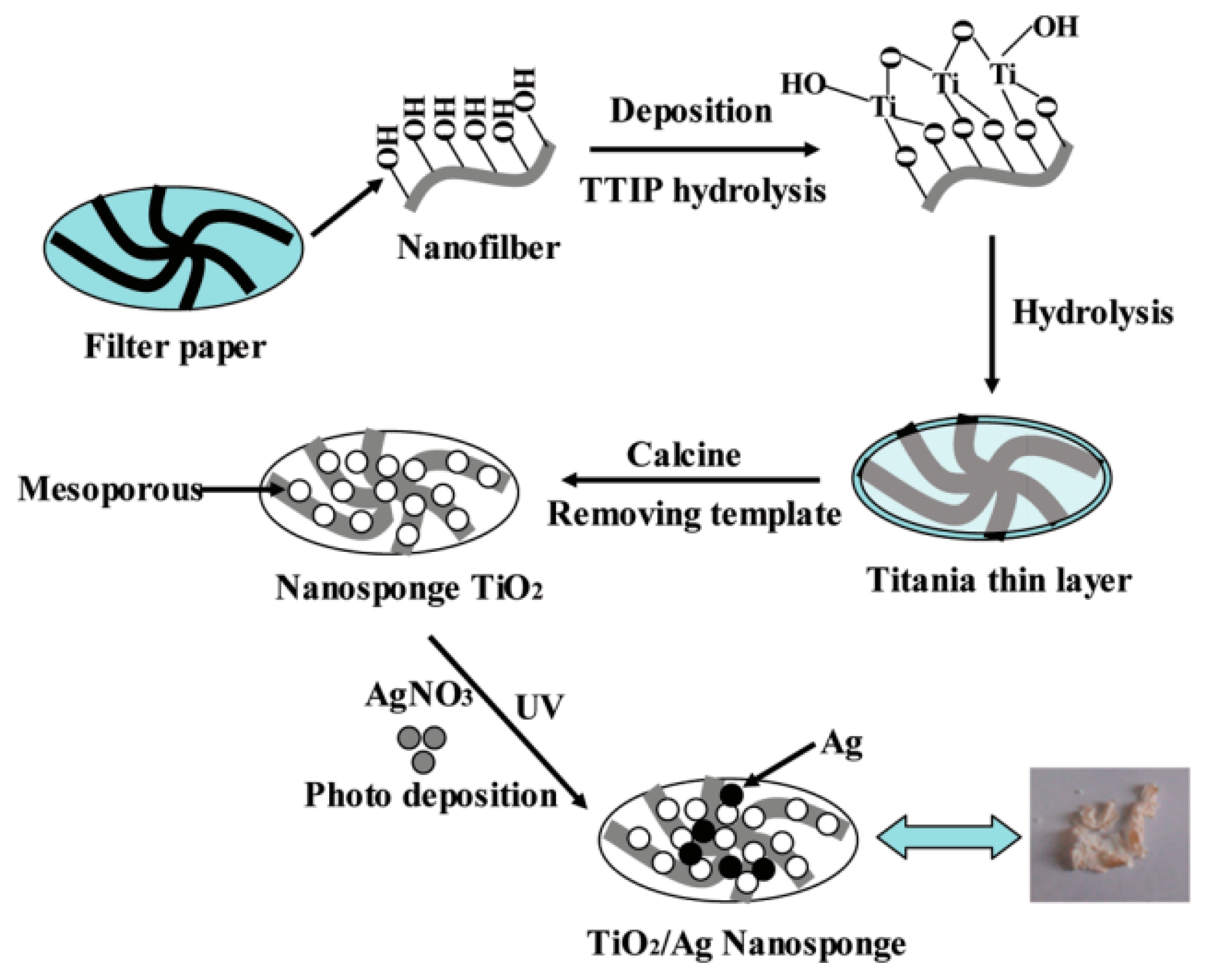
- Yu, D.H.; Yu, X.; Wang, C.; Liu, X.C.; Xing, Y. Synthesis of Natural Cellulose-Templated TiO2/Ag Nanosponge Composites and Photocatalytic Properties. ACS Appl. Mater. Interfaces 2012, 4, 2781–2788. [Google Scholar] [CrossRef] [PubMed]
- Ahmadizadegan, H. Surface modification of TiO2 nanoparticles with biodegradable nanocellolose and synthesis of novel polyimide/cellulose/TiO2 membrane. J. Colloid Interface Sci. 2017, 491, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Foruzanmehr, M.R.; Vuillaume, P.Y.; Robert, M.; Elkoun, S. The effect of grafting a nano-TiO2 thin film on physical and mechanical properties of cellulosic natural fibers. Mater. Des. 2015, 85, 671–678. [Google Scholar] [CrossRef]
- Horvath, A.L. Solubility of structurally complicated materials: I. wood. J. Phys. Chem. 2006, 35, 77–92. [Google Scholar] [CrossRef]
- Ten, E.; Vermerris, W. Functionalized Polymers from Lignocellulosic Biomass: State of the Art. Polymers 2013, 5, 600–642. [Google Scholar] [CrossRef]
- Dong, Y.Y.; Li, S.M.; Ma, M.G.; Zhao, J.J.; Sun, R.C.; Wang, S.P. Environmentally-friendly sonochemistry synthesis of hybrids from lignocelluloses and silver. Carbohydr. Polym. 2014, 102, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Siwińska-Stefańska, K.; Kołodyńska, D. Preparation and characterization of novel TiO2/lignin and TiO2-SiO2/lignin hybrids and their use as functional biosorbents for Pb(II). Chem. Eng. J. 2017, 314, 169–181. [Google Scholar] [CrossRef]
- Kansala, S.K.; Singh, M.; Sud, D. Studies on TiO2/ZnO photocatalysed degradation of lignin. J. Hazard. Mater. 2008, 153, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.K. A note on the influence of extractives on the photo-discoloration and photo-degradation of wood. Polym. Degrad. Stab. 2005, 87, 375–379. [Google Scholar] [CrossRef]
- Chen, X.; Chew, S.L.; Kerton, F.M.; Yan, N. Direct conversion of chitin into a N-containing furan derivative. Green Chem. 2014, 16, 2204–2212. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, W.; Zhang, L. Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J. Hazard. Mater. 2012, 209–210, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Kristiina, O.; Aji, P.M. Electrospun Chitosan Nanofiber Random Mats Reinforced with Chitin and Cellulose Nanocrystals for Wound Dressing Application. 2016. Available online: http://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1011676&dswid=-5388 (accessed on 11 May 2017).
- Muzzarelli, R.A. Biomedical exploitation of chitin and chitosan via mechano-chemical disassembly, electrospinning, dissolution in imidazolium ionic liquids, and supercritical drying. Mar. Drugs 2010, 9, 1510–1533. [Google Scholar] [CrossRef] [PubMed]
- Kamal, T.; Anwar, Y.; Khan, S.B.; Tariq Saeed Chania, M.; Asiria, A.B. Dye adsorption and bactericidal properties of TiO2/chitosan coating layer. Carbohydr. Polym. 2016, 148, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Haldorai, Y.; Shim, J.J. Novel chitosan-TiO2 nanohybrid: Preparation, characterization, antibacterial, and photocatalytic properties. Polym. Compos. 2014, 35, 327–333. [Google Scholar] [CrossRef]
- Salehi, R.; Arami, M.; Mahmoodi, N.M.; Bahrami, H.; Khorramfar, S. Novel biocompatible composite (Chitosan-zinc oxide nanoparticle): Preparation, characterization and dye adsorption properties. Colloids Surf. B Biointerfaces 2010, 80, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.D.; Faria, E.A.; De Souza, J.R.; Prado, A.G.S. Preparation of photoactive chitosan–niobium (V) oxide composites for dye degradation. J. Photochem. Photobiol. A Chem. 2006, 182, 202–206. [Google Scholar] [CrossRef]
- Sabar, S.; Nawi, M.A.; Ngah, W.S.W. Photocatalytic removal of Reactive Red 4 dye by immobilised layer-by-layer TiO2/cross-linked chitosan derivatives system. Desalin. Water Treat. 2014, 57, 5851–5857. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Liang, J.; Huang, J.; Tang, C. ZnO Nanofiber and Nanoparticle Synthesized through Electrospinning and Their Photocatalytic Activity under Visible Light. J. Am. Chem. Soc. 2008, 91, 1287–1291. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, D.; Liu, J.; Zhou, J. ZnO Nanocrystallites/Cellulose Hybrid Nanofibers Fabricated by Electrospinning and Solvothermal Techniques and Their Photocatalytic Activity. J. Appl. Polym. Sci. 2011, 121, 1757–1764. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cesano, F.; Bonino, F.; Bordiga, S.; Spoto, G.; Scarano, D.; Zecchina, A. Photoactive TiO2 films on cellulose fibres: Synthesis and characterization. J. Photochem. Photobiol. A Chem. 2007, 189, 286–294. [Google Scholar] [CrossRef]
- Ge, Y.; Xiang, Y.; He, Y.; Ji, M.; Song, G. Preparation of Zn-TiO2/RH/Fe3O4 composite material and its photocatalytic degradation for the dyes in wastewater. Desalin. Water Treat. 2016, 57, 9837–9844. [Google Scholar] [CrossRef]
- Choia, C.; Hwanga, K.J.; Kimb, Y.J.; Kimb, G.; Parkc, J.Y.; Sungho, J. Rice-straw-derived hybrid TiO2–SiO2 structures with enhanced photocatalytic properties for removal of hazardous dye in aqueous solutions. Nano Energy 2016, 20, 76–83. [Google Scholar] [CrossRef]
- Lee, M.; Chen, B.Y.; Walter Den, W. Chitosan as a Natural Polymer for heterogeneous Catalysts Support: A Short Review on Its Applications. Appl. Sci. 2015, 5, 1272–1283. [Google Scholar] [CrossRef]
- Luo, L.; Yang, L.C.; Xiao, M.; Bian, L.; Yuan, B.; Liu, Y.; Jiang, F.; Pan, X. A novel biotemplated synthesis of TiO2/wood charcoal composites for synergistic removal of bisphenol A by adsorption and photocatalytic degradation. Chem. Eng. J. 2015, 262, 1275–1283. [Google Scholar] [CrossRef]
- Ohtani, N.; Tonoi, M. Improved Photoluminescence Lifetime of Organic Emissive Materials Embedded in Organic-Inorganic Hybrid Thin Films Fabricated by Sol-Gel Method Using Tetraethoxysilane. Mol. Cryst. Liq. Cryst. 2014, 599, 132–138. [Google Scholar] [CrossRef]
- Yan, S.C.; Lv, S.B.; Li, Z.S.; Zouabd, Z.G. Organic-inorganic composite photocatalyst of g-C(3)N(4) and TaON with improved visible light photocatalytic activities. Dalton Trans. 2010, 39, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Navarro, M.T.; Rey, F.; Ruiz, V.R.; Sabater, M.J. Direct synthesis of a photoactive inorganic-organic mesostructured hybrid material and its application as a photocatalyst. ChemPhysChem 2010, 10, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Foruzanmehr, M.R.; Vuillaum, P.Y.; Elkoun, S.; Robert, M. Physical and mechanical properties of PLA composites reinforced by TiO2 grafted flax fibers. Mater. Des. 2016, 106, 295–304. [Google Scholar] [CrossRef]


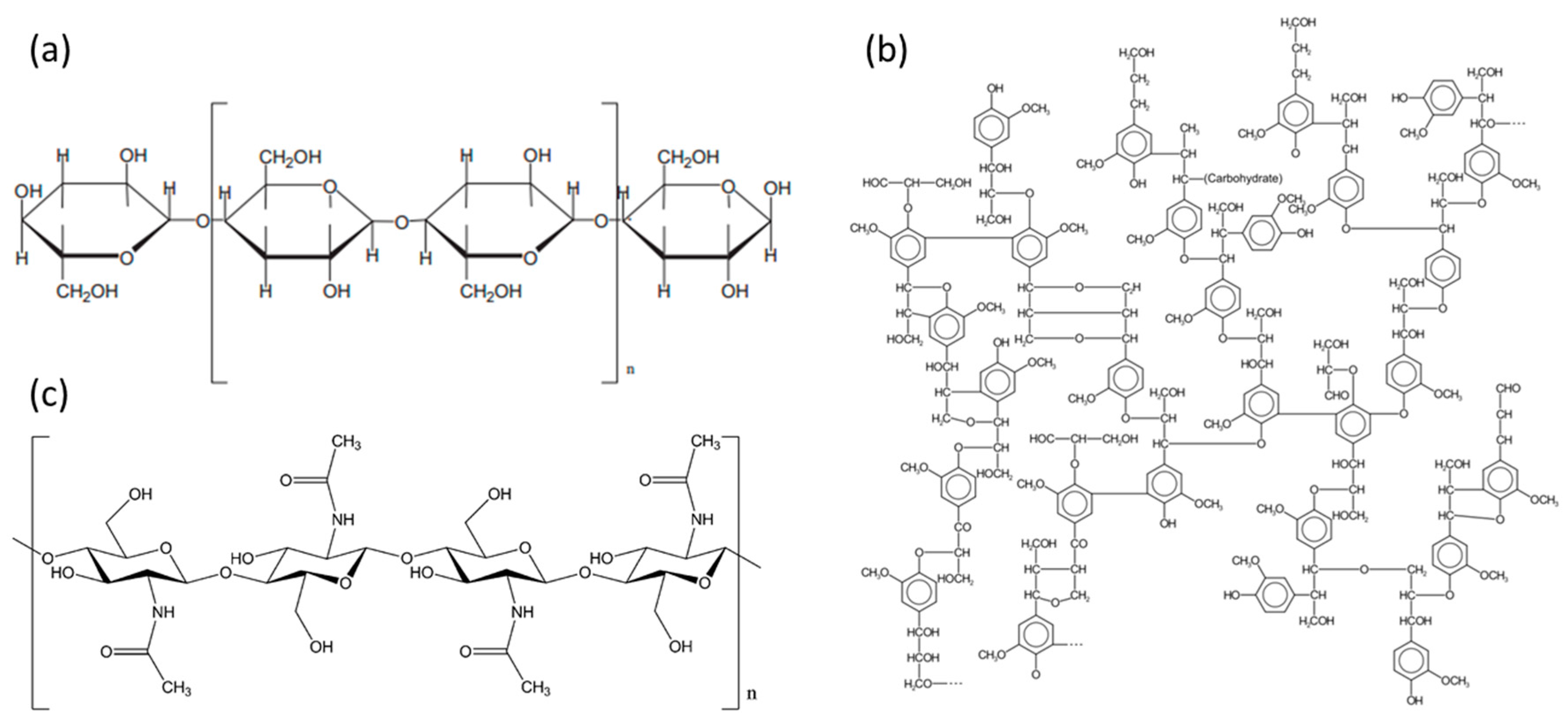
| Entry | Polymer Hybrid Materials | Target Contaminant | Light Source | Fabrication Method | Photocatalytic Behavior | Ref. |
|---|---|---|---|---|---|---|
| 1 | ZnO nanorods on polybutylene terephthalate (PBT) polymer fiber mats | Azo organic dye (acid red 40) | Ultraviolet (UV) radiation in the range of 320–390 nm providing 79 mW/cm2 of energy flux. | Thin films formed by low temperature vapor phase atomic layer deposition (ALD) and hydrothermal growth of ZnO nanorod crystals on a seed layer. | Degradation ratio ~90% of the dye within 2 h. The combination of ALD and hydrothermal method allow to obtain the best performance of the photocatalyst and may be also used for other crystal growth systems, such as TiO2, Fe2O3, SnO2 and V2O5, where high area and ready solution access are desired. | [55] |
| 2 | ZnO nanoparticles on wool and polyacrylonitrile (PANI) fibers | Methylene blue (MB) and eosin yellowish (EY) dye | High-pressure mercury lamp covers illumination spectrum ranging from ultraviolet to visible (200–800 nm). | Impregnation of polymeric fibers using sol-gel process at ambient temperature. ZnO-sol is based on the method described in the literature with minor changes in details. | There is 77% MB dye degradation after 6 h upon ZnO/PANI and 80% upon ZnO/wool fibers, which is 4-fold more in comparison to bare fibers. Similar results of degradation were obtained for EY dye, where the degradation ratios equal 64% and 50%, respectively. | [57] |
| 3 | CeO2-ZnO-polyvinylpyrrolidone (PVP) | Rhodamine B (RhB) | UV lamp (8 W) with emission wavelengths at 254 nm. | The electrospinning technique was followed by thermal treatment to obtain CeO2–ZnO nanofibers. The nonwoven mat was prepared from the precursor solution of PVP/Ce(NO3)3/Zn(CH3COO)2. | After 3 h of irradiation, only 17.4% and 82.3% of Rhodamine B was decomposed catalyzed by pure CeO2 and ZnO fibers, respectively, whereas almost 98% was decomposed applying the CeO2–ZnO-composite fibers. | [74] |
| 4 | ZnO nanowires on polyethylene (PP) | Methylene blue (MB) | UV light source (6 W) | ZnO nanowires were grown from seed ZnO nanoparticles affixed onto the commercially available fibers by hydrothermal method. | After 2.5 h of irradiation, ZnO/polyethylene fibers degraded 83% of the MB, whereas the fibers without ZnO degradate only 32%. 24% of MB was found undergo self-degradation under the same UV light without using polyethylene fibers. | [75] |
| 5 | ZnO/SnO2-polyvinylpyrrolidone (PVP) | Rhodamine B | High-pressure mercury lamp (50 W) with main emission wavelength at 313 nm. | A simple combination method of sol-gel process and electrospinning technique. The electrospun composite nanofibers was obtained by the precursor solution of PVP/ZnCl2/SnCl2. | After 50 min, the degradation efficiency of RhB was equal to 75, 35, and 85% for ZnO, SnO2, and TiO2 fibers, respectively. However, the time for complete decolorization of dye solution over the ZnO/SnO2-nanofibers was 30 min. | [76] |
| 6 | Reduced graphene oxide/titanium dioxide filter (RGO/TiO2) and reduced graphene oxide/zinc oxide filter (RGO/ZnO) on polypropylene(PP) porous filter | Methylene blue (MB) | Halogen lamp (150 W) | The polypropylene (PP) porous filter was incorporated with reduced graphene oxide (RGO) and metal oxides via a simple hydrothermal approach. | The combination of RGO and the metal oxide compounds on the filters shows more than 70% of MB adsorption in 20 min compared with those consisting of individual materials, degradation after 120 min 99%. | [50] |
| Entry | Polymer Hybrid Materials | Target Contaminant | Light Source | Fabrication Method | Photocatalytic Behavior | Ref. |
|---|---|---|---|---|---|---|
| 1 | Titanium dioxide (TiO2) immobilized in cellulose matrix | Phenol | UV (6 W) light at wavelength of 254 nm was used. The mean light intensity equal to 0.56 mW/cm2. | Composite films have been prepared via a sol-gel method. | The composite films exhibited high degradation ratio (90% after 2 h of irradiation) without remarkable loss of photocatalytic activity after three times. | [85] |
| 2 | ZnAc/cellulose acetate (CA) composite nanofibers | Rhodamine B and phenol | Ultraviolet lamps (PHILIPS 365 nm) as the irradiation source. | Electrospinning technique in combination with calcination. | Almost 100% of Rhodamine B and 85% phenol (after 24 h) was decomposed in the presence of TiO2/ZnO composite nanofibers under mild conditions. | [104] |
| 3 | ZnO/cellulose hybrid nanofibers | Methylene blue (MB) and eosin yellowish (EY) dye | Tungsten lamp (500 W) was used as the visible light source. | A novel method that combines electrospinning and solvothermal techniques | Nearly 50% of Rhodamine B was decomposed after 24 h of irradiation under visible light. | [105] |
| 4 | Photoactive TiO2 films on cellulose fibers | Methylene blue (MB) and heptane-extracted bitumen fraction (BF) containing a mixture of heavy aromatic hydrocarbons | Reproducible solar light (50 mW/cm2). | Sol-gel method | The degradation ratio of MB reached 90% after 20 h and 90% for BF fraction after 9 h without loss of activity after three illumination cycles. | [106] |
| 5 | Rice-straw-derived hybrid TiO2–SiO2 structures | Methylene blue (MB) | UV-A (8 W) lamps (300–450 nm) providing an irradiation power flux of 2.0 mW/cm2. | Impregnation method. | The photocatalytic decomposition of methylene blue after 90 min obtained was 100%. | [107] |
| 6 | Chitosan (CS)-encapsulated TiO2 nanohybrid | Methylene blue (MB) | UV light at a wavelength of 365 nm. | Nanohybrid materials was prepared by chemical precipitation method. | The catalyst showed high photocatalytic activity of 90% degradation after 3 h of irradiation and without losing photocatalytic activity after five recycle tests. | [100] |
| 7 | Fe3O4/chitosan/TiO2 nanocomposites | Methylene blue (MB) | Illumination with UV light. | Facile and low-cost method by solvents thermal reduction. | The degradation rate of methyl blue was 93% after 30 min for Fe3O4/CTS/TiO2 nanocomposites. | [108] |
| Synthetic Polymers | Biopolymers | |
|---|---|---|
| Availability | Decreasing | High |
| Physicochemical resistance | High | Low |
| Thermal stability | High | Low |
| Large-scale applications | Possible | Difficult |
| Environmental-friendly | No | Yes |
| Cost of production | Low | High |
| Sustainability | Low | High |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colmenares, J.C.; Kuna, E. Photoactive Hybrid Catalysts Based on Natural and Synthetic Polymers: A Comparative Overview. Molecules 2017, 22, 790. https://doi.org/10.3390/molecules22050790
Colmenares JC, Kuna E. Photoactive Hybrid Catalysts Based on Natural and Synthetic Polymers: A Comparative Overview. Molecules. 2017; 22(5):790. https://doi.org/10.3390/molecules22050790
Chicago/Turabian StyleColmenares, Juan Carlos, and Ewelina Kuna. 2017. "Photoactive Hybrid Catalysts Based on Natural and Synthetic Polymers: A Comparative Overview" Molecules 22, no. 5: 790. https://doi.org/10.3390/molecules22050790






