Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition
Abstract
:1. Introduction
2. Distribution and Functions of P-gp
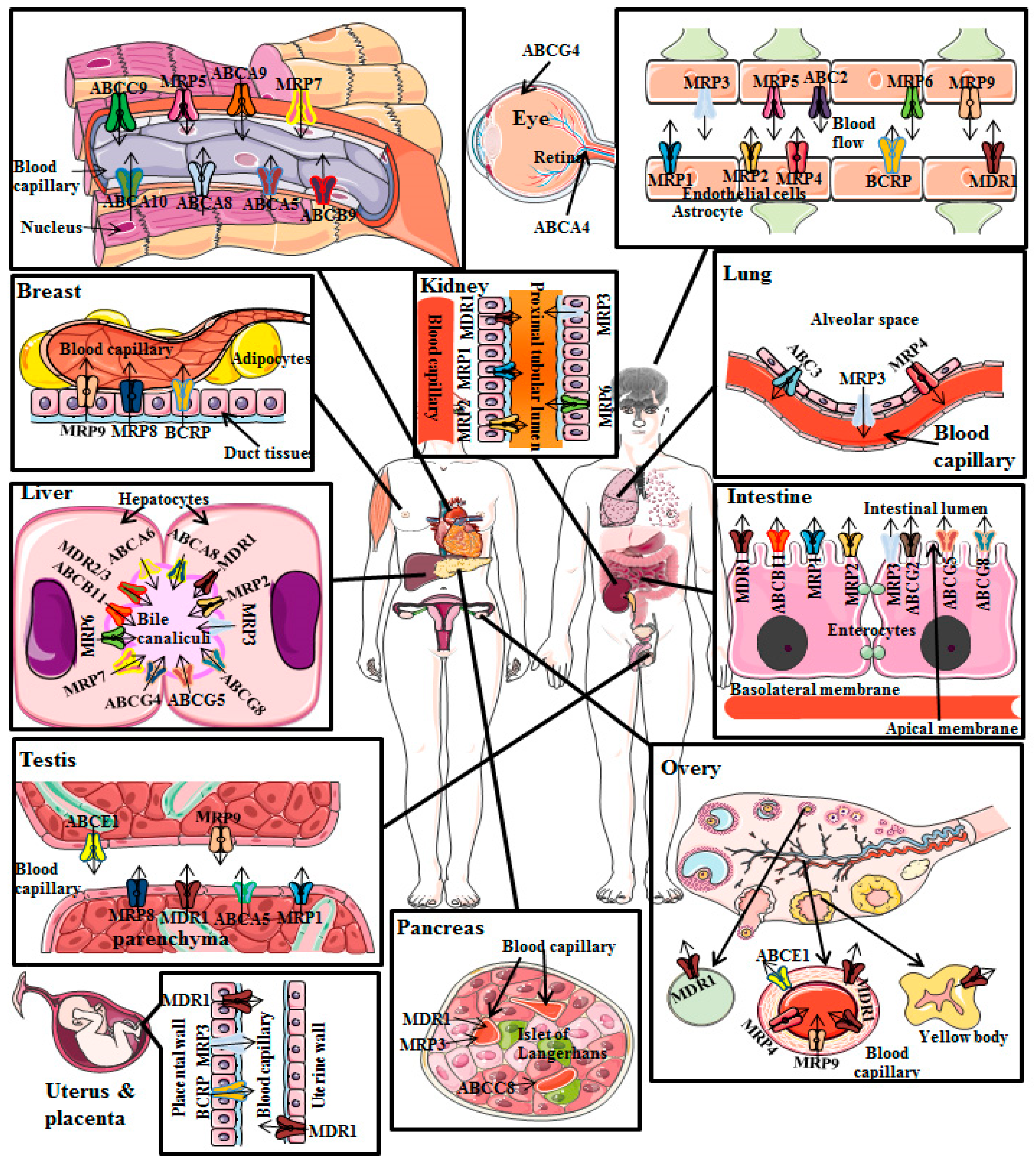
2.1. P-gp Distribution in Cancer Cells
2.2. P-gp Distribution in Normal Human Tissues
- (i)
- P-gp restricts drugs’ entry after oral administration as a result of its presence in the apical membrane of enterocytes of intestine;
- (ii)
- Once the drugs and/or xenobiotics have entered into the systemic circulation, P-gp induces elimination of drugs through urine and bile because of its presence in the canalicular membrane of hepatocytes and in the apical surface of kidney’s proximal convoluted tubular cells, respectively;
- (iii)
- Additionally, P-gp decreases entry of drugs into sensitive tissues particularly in the BBB [26].
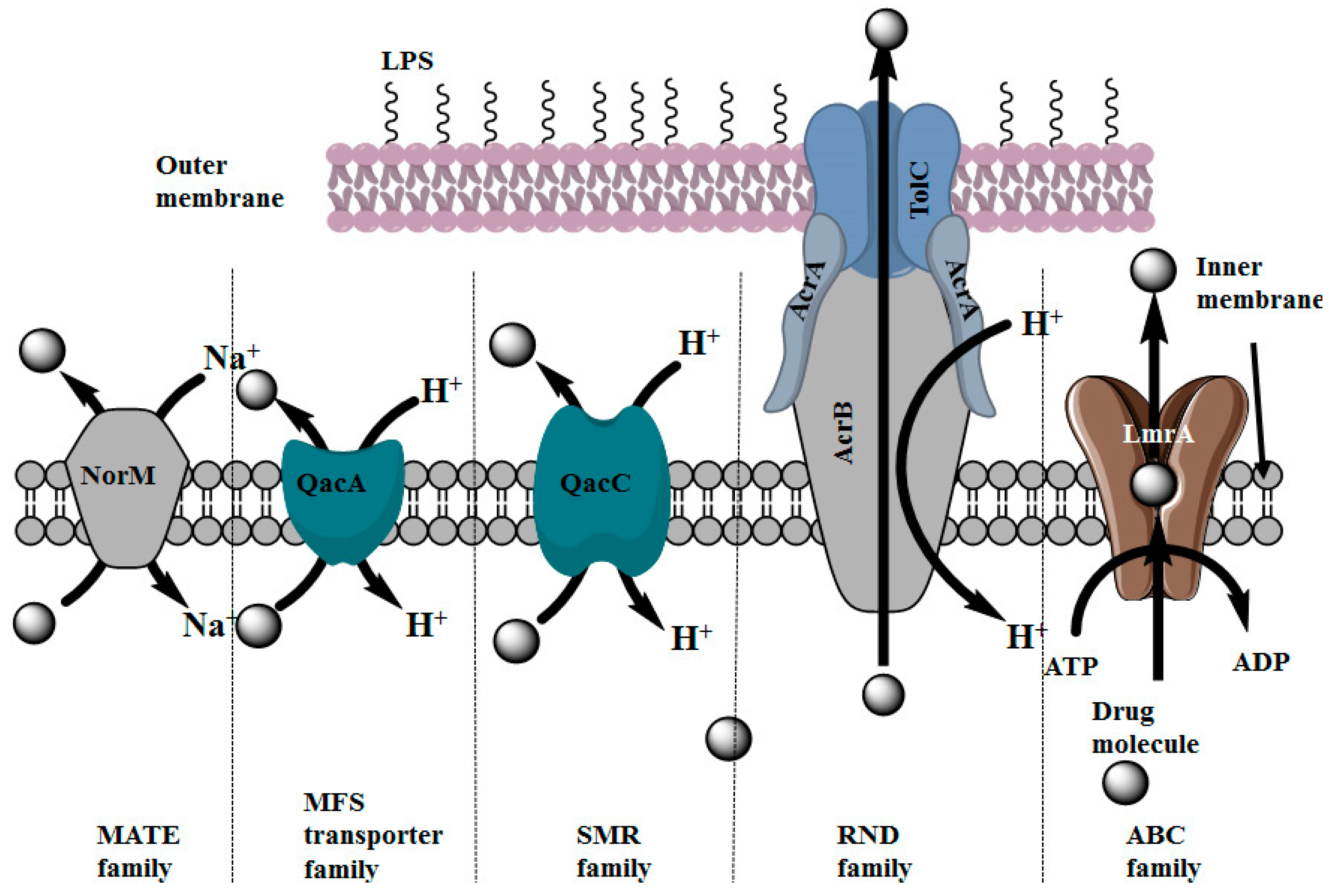
2.3. Distribution of Multidrug Efflux Systems in Microorganisms
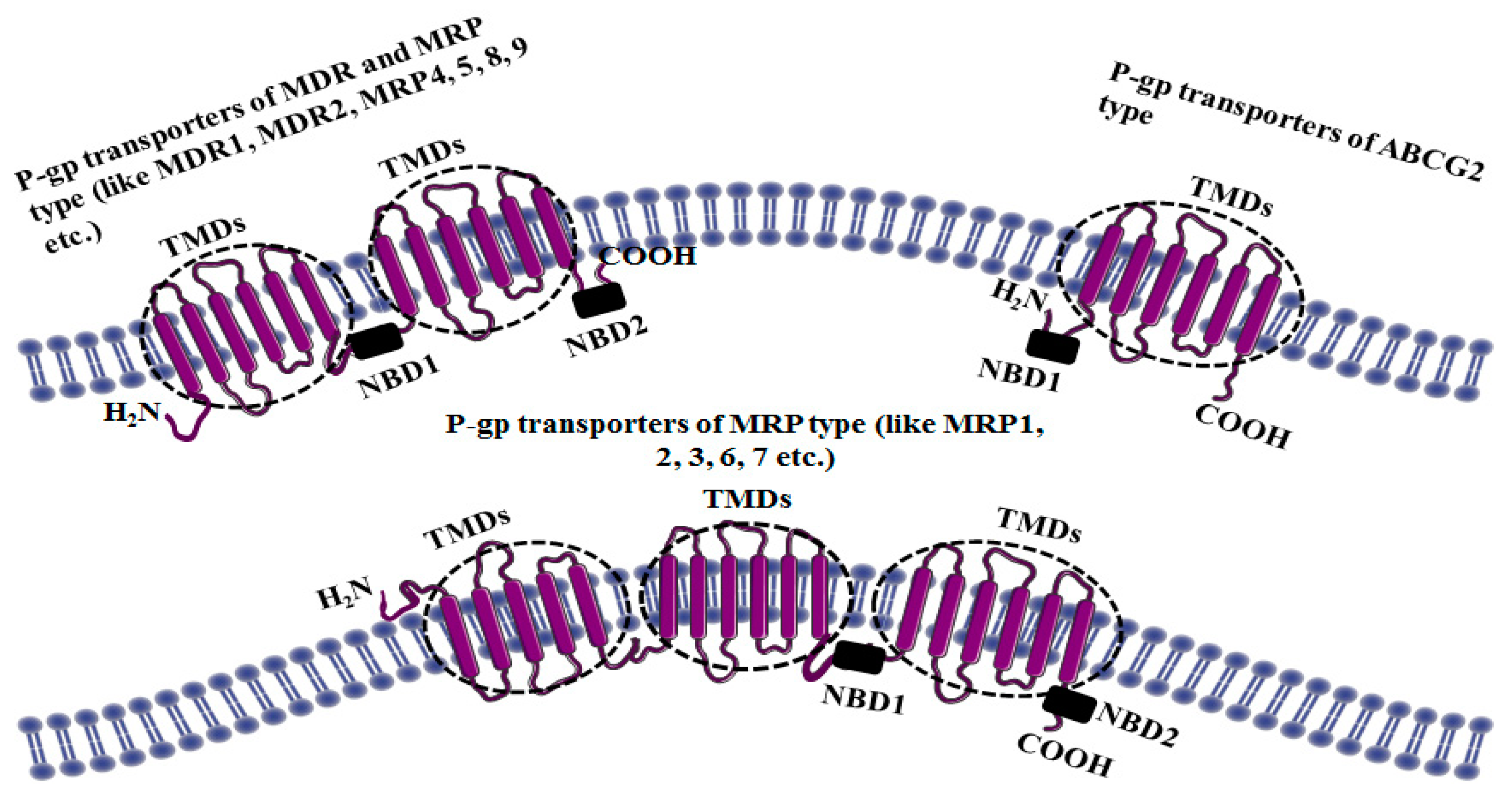
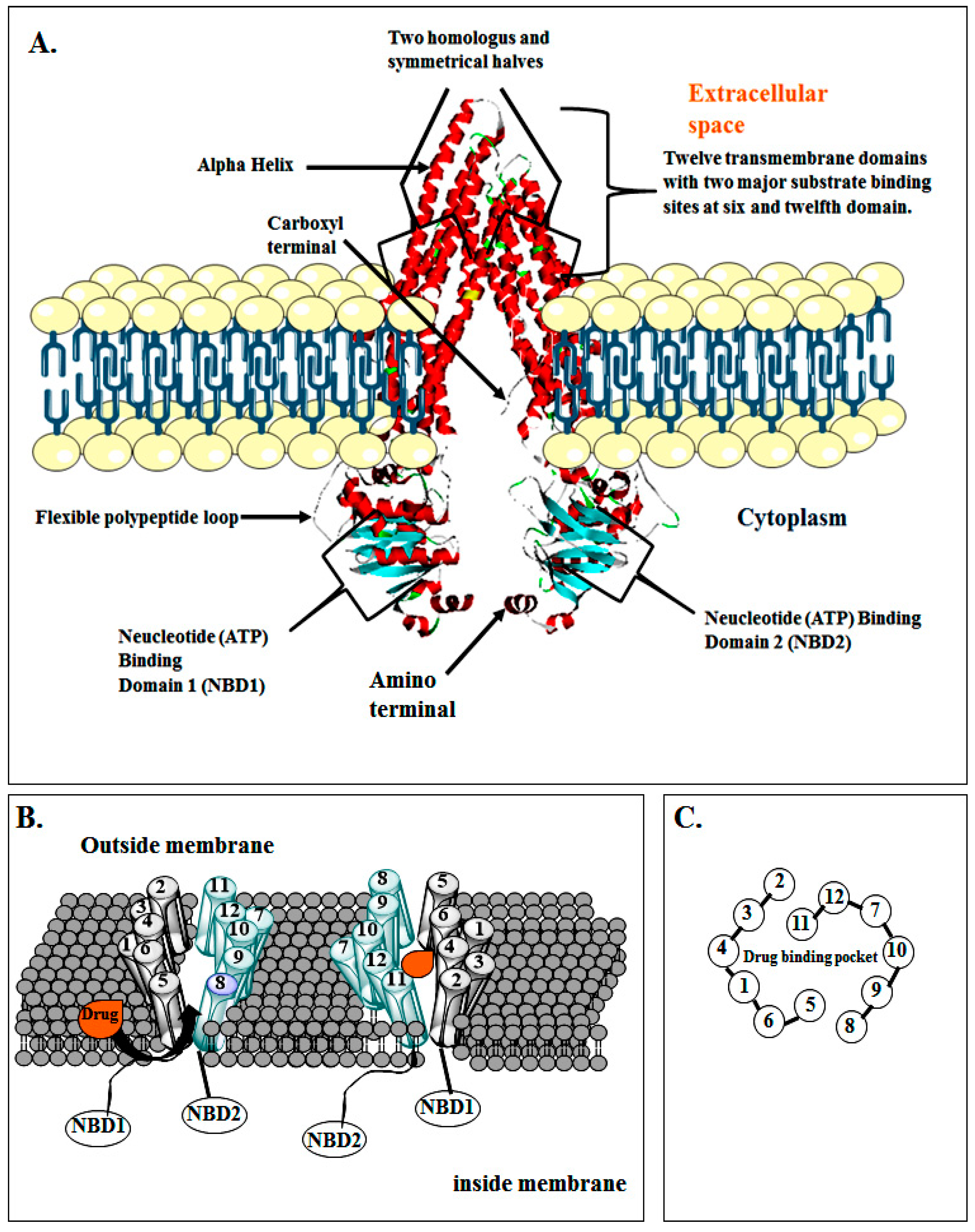
3. Structure of P-gp
4. Mechanism by Which P-gp Induces MDR
4.1. Classical Pore Pump Model
4.2. Hydrophobic Vacuum Cleaner Model
4.3. Flippase Model
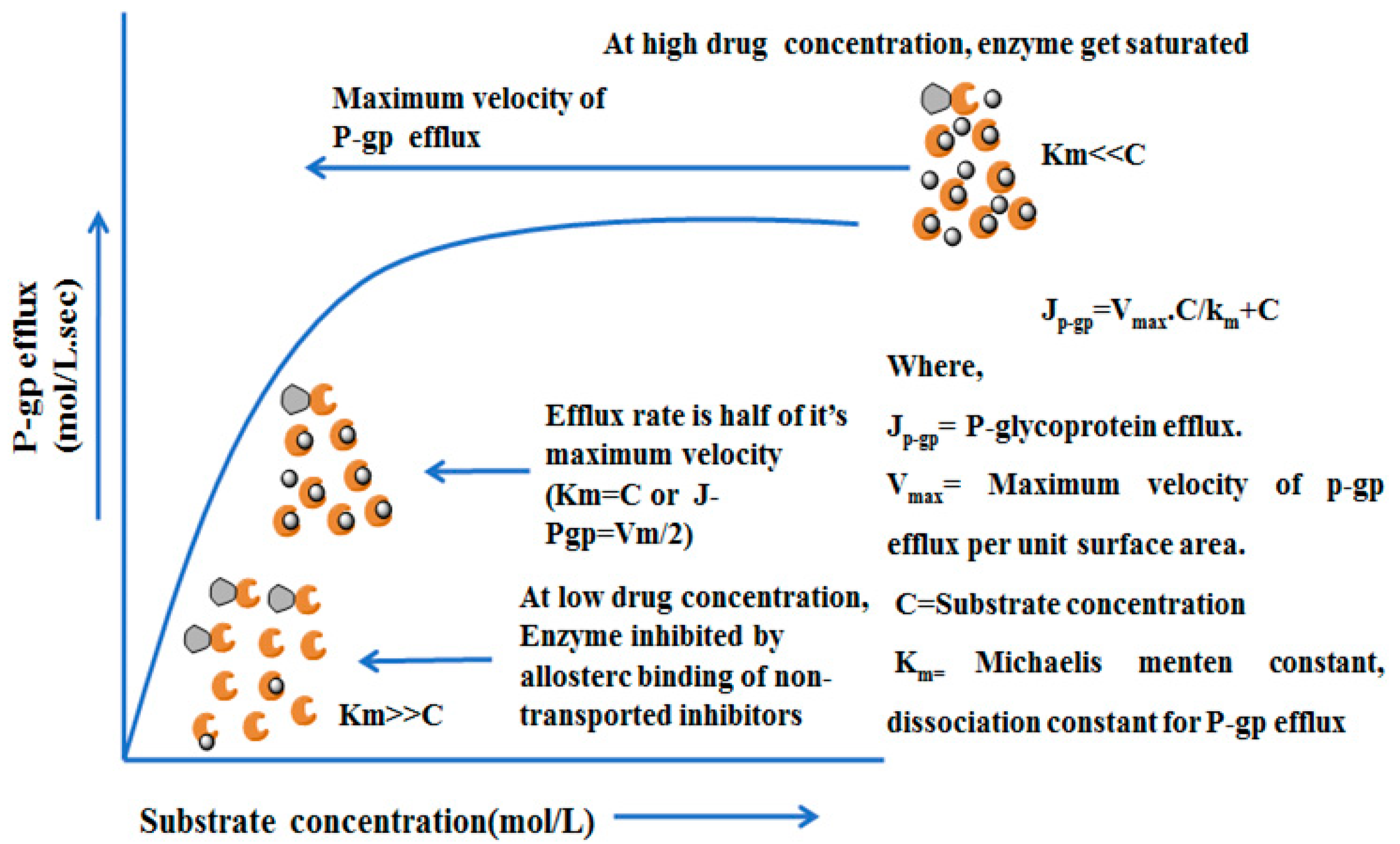
4.4. Mechanisms and Kinetics of P-glycoprotein Efflux
5. P-Glycoprotein Inhibition
5.1. Herbal Modulation of P-gp
5.1.1. Alkaloids
5.1.2. Flavonoids and Phenolics
5.1.3. Terpenoids
5.1.4. Saponins, Sapogenins and Sterols
5.1.5. Coumarins
5.1.6. Peptides
5.1.7. Resins
5.1.8. Miscellaneous Natural Compounds
5.2. Importance of P-gp Inhibitors in Various Therapies
5.2.1. P-gp Inhibitors in Cancer Chemotherapy
5.2.2. P-gp Inhibitors in the Treatment of HIV
5.2.3. P-gp Inhibitors in Antimicrobial Therapy
5.3. Challenges of Selecting Natural Molecules in Place of Existing P-gp Inhibitors
5.4. Toxicity Due to P-gp Inhibition by Phytochemicals
6. Conclusions and Future Prospective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ling, V.; Thompson, L.H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J. Cell. Physiol. 1974, 83, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Linton, K.J.; Higgins, C.F. Structure and function of ABC transporters: The ATP switch provides flexible control. Eur. J. Physiol. 2007, 453, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, T.; Ushigome, F.; Koyabu, N.; Morimoto, S.; Shoyama, Y.; Naito, M.; Tsuruo, T.; Ohtani, H.; Sawada, Y. Inhibition of P-glycoprotein by orange juice components, polymethoxyflavones in adriamycin-resistant human myelogenous leukemia (K562/ADM) cells. Cancer Lett. 2000, 160, 21–28. [Google Scholar] [CrossRef]
- Nabekura, T.; Hiroi, T.; Kawasaki, T.; Uwai, Y. Effects of natural nuclear factor-kappa β inhibitors on anticancer drug efflux transporter human P-glycoprotein. Biomed. Pharmacother. 2015, 70, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Jaggi, M.; Khar, R.; Talegaonkar, S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J. Pharm. Pharm. Sci. 2009, 12, 46–78. [Google Scholar] [CrossRef]
- Yoshida, N.; Koizumi, M.; Adachi, I.; Kawakami, J. Inhibition of P-glycoprotein-mediated transport by terpenoids contained in herbal medicines and natural products. Food Chem. Toxicol. 2006, 44, 2033–2039. [Google Scholar] [CrossRef]
- Lopez, D.; Martinez-Luis, S. Marine natural products with P-glycoprotein inhibitor properties. Mar. Drugs 2014, 12, 525–546. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Einolf, H.; Cohen, D. Efflux transporters and their clinical relevance. Mini Rev. Med. Chem. 2005, 5, 183–195. [Google Scholar] [CrossRef]
- Van Helvoort, A.; Smith, A.J.; Sprong, H.; Fritzsche, I.; Schinkel, A.H.; Borst, P.; van Meer, G. Mdr1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell 1996, 87, 507–517. [Google Scholar] [CrossRef]
- Smith, A.J.; van Helvoort, A.; van Meer, G.; Szabó, K.; Welker, E.; Szakács, G.; Váradi, A.; Sarkadi, B.; Borst, P. MDR3 P-glycoprotein, a phosphatidylcholine translocase, transports several cytotoxic drugs and directly interacts with drugs as judged by interference with nucleotide trapping. J. Biol. Chem. 2000, 275, 23530–23539. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.T.; Dalton, W.S. Multidrug resistance in the laboratory and clinic. Adv. Clin. Chem. 1993, 31, 1–61. [Google Scholar]
- Georges, E.; Bradley, G.; Gariepy, J.; Ling, V. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1990, 87, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Lamy, T.; Drenou, B.; Grulois, I.; Fardel, O.; Jacquelinet, C.; Goasguen, J.; Dauriac, C.; Amiot, L.; Bernard, M.; Fauchet, R. Multi-drug resistance (MDR) activity in acute leukemia determined by rhodamine 123 efflux assay. Leukemia 1995, 9, 1549–1555. [Google Scholar] [PubMed]
- Kramer, R.; Weber, T.; Morse, B.; Arceci, R.; Staniunas, R.; Steele, G.J.; Summerhayes, I. Constitutive expression of multidrug resistance in human colorectal tumours and cell lines. Br. J. Cancer 1993, 67, 959. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Bénard, J.; Hartmann, O.; Boccon-Gibod, L.; Lemerle, J.; Riou, G. Correlation of mdr1 gene expression with chemotherapy in neuroblastoma. J. Natl. Cancer Inst. 1989, 81, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- List, A.F.; Spier, C.M.; Cline, A.; Doll, D.C.; Garewal, H.; Morgan, R.; Sandberg, A.A. Expression of the multidrug resistance gene product (P-glycoprotein) in myelodysplasia is associated with a stem cell phenotype. Br. J. Haematol. 1991, 78, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Drenou, B.; Fardel, O.; Amiot, L.; Fauchet, R. Detection of p glycoprotein activity on normal and leukemic cd34+ cells. Leuk. Res. 1993, 17, 1031–1035. [Google Scholar] [CrossRef]
- Lai, S.-L.; Goldstein, L.J.; Gottesman, M.M.; Pastan, I.; Tsai, C.-M.; Johnson, B.E.; Mulshine, J.L.; Ihde, D.C.; Kayser, K.; Gazdar, A.F. mdr1 gene expression in lung cancer. J. Natl. Cancer Inst. 1989, 81, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Moscow, J.A.; Fairchild, C.R.; Madden, M.J.; Ransom, D.T.; Wieand, H.S.; O’Brien, E.E.; Poplack, D.G.; Cossman, J.; Myers, C.E.; Cowan, K.H. Expression of anionic glutathione-s-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989, 49, 1422–1428. [Google Scholar] [PubMed]
- Marie, J.-P.; Zittoun, R.; Sikic, B.I. Multidrug resistance (mdr1) gene expression in adult acute leukemias: Correlations with treatment outcome and in vitro drug sensitivity. Blood 1991, 78, 586–592. [Google Scholar] [PubMed]
- Cheng, A.-L.; Su, I.-J.; Chen, Y.-C.; Lee, T.-C.; Wang, C.-H. Expression of P-glycoprotein and glutathione-s-transferase in recurrent lymphomas: The possible role of Epstein-Barr virus, immunophenotypes, and other predisposing factors. J. Clin. Oncol. 1993, 11, 109–115. [Google Scholar] [CrossRef]
- Varma, M.V.; Ashokraj, Y.; Dey, C.S.; Panchagnula, R. P-glycoprotein inhibitors and their screening: A perspective from bioavailability enhancement. Pharmacol. Res. 2003, 48, 347–359. [Google Scholar] [CrossRef]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 7735–7738. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Yamashiro, T.; Nagatake, H.; Yamamoto, T.; Watanabe, N.; Tanaka, H.; Shigenobu, K.; Tsuruo, T. Expression and function of multidrug resistance P-glycoprotein in a cultured natural killer cell-rich population revealed by mrk16 monoclonal antibody and AHC-52. Biochem. Pharmacol. 1994, 48, 1641–1646. [Google Scholar] [CrossRef]
- Chaudhary, P.M.; Roninson, I.B. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell 1991, 66, 85–94. [Google Scholar] [CrossRef]
- Bendayan, R.; Ronaldson, P.T.; Gingras, D.; Bendayan, M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J. Histochem. Cytochem. 2006, 54, 1159–1167. [Google Scholar] [CrossRef]
- Chang, G. Multidrug resistance ABC transporters. FEBS Lett. 2003, 555, 102–105. [Google Scholar] [CrossRef]
- Lage, H. ABC-transporters: Implications on drug resistance from microorganisms to human cancers. Int. J. Antimicrob. Agents 2003, 22, 188–199. [Google Scholar] [CrossRef]
- Higgins, C.F. ABC transporters: Physiology, structure and mechanism—An overview. Res. Microbiol. 2001, 152, 205–210. [Google Scholar] [CrossRef]
- Manson, J.M.; Keis, S.; Smith, J.M.; Cook, G.M. Acquired bacitracin resistance in Enterococcus faecalis is mediated by an ABC transporter and a novel regulatory protein, bcrr. Antimicrob. Agents Chemother. 2004, 48, 3743–3748. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.S. Efflux systems in bacterial pathogens: An opportunity for therapeutic intervention? An industry view. Biochem. Pharmacol. 2006, 71, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Pagès, J.-M.; Masi, M.; Barbe, J. Inhibitors of efflux pumps in gram-negative bacteria. Trends Mol. Med. 2005, 11, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Saier, M.H., Jr.; Beatty, J.T.; Goffeau, A.; Harley, K.T.; Heijne, W.; Huang, S.-C.; Jack, D.L.; Jahn, P.; Lew, K.; Liu, J. The major facilitator superfamily. J. Mol. Microbiol. Biotechnol. 1999, 1, 257–279. [Google Scholar] [PubMed]
- Bodor, M.; Kelley, E.J.; Ho, R.J. Characterization of the human mdr1 gene. AAPS J. 2005, 7, E1–E5. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.W.; Clarke, D.M. Do drug substrates enter the common drug-binding pocket of P-glycoprotein through “gates”? Biochem. Biophys. Res. Commun. 2005, 329, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Hrycyna, C.A.; Airan, L.E.; Germann, U.A.; Ambudkar, S.V.; Pastan, I.; Gottesman, M.M. Structural flexibility of the linker region of human P-glycoprotein permits ATP hydrolysis and drug transport. Biochemistry 1998, 37, 13660–13673. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.-W.; Wang, I.X.; Nikaido, K.; Liu, P.-Q.; Ames, G.F.-L.; Kim, S.-H. Crystal structure of the ATP-binding subunit of an ABC transporter. Nature 1998, 396, 703–707. [Google Scholar] [PubMed]
- Booth, C.L.; Pulaski, L.; Gottesman, M.M.; Pastan, I. Analysis of the properties of the n-terminal nucleotide-binding domain of human P-glycoprotein. Biochemistry 2000, 39, 5518–5526. [Google Scholar] [CrossRef] [PubMed]
- Tombline, G.; Bartholomew, L.; Gimi, K.; Tyndall, G.A.; Senior, A.E. Synergy between conserved abc signature ser residues in P-glycoprotein catalysis. J. Biol. Chem. 2004, 279, 5363–5373. [Google Scholar] [CrossRef]
- Yoshida, N.; Takagi, A.; Kitazawa, H.; Kawakami, J.; Adachi, I. Inhibition of P-glycoprotein-mediated transport by extracts of and monoterpenoids contained in zanthoxyli fructus. Toxicol. Appl. Pharmacol. 2005, 209, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, S.V.; Dey, S.; Hrycyna, C.A.; Ramachandra, M.; Pastan, I.; Gottesman, M.M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter 1. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 361–398. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Taguchi, Y.; Morishima, M. How does P-glycoprotein recognize its substrates? Semin. Cancer Biol. 1997, 8, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.W.; Bartlett, M.C.; Clarke, D.M. The dileucine motif at the cooh terminus of human multidrug resistance P-glycoprotein is important for folding but not activity. J. Biol. Chem. 2005, 280, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.W.; Bartlett, M.C.; Clarke, D.M. Disulfide cross-linking analysis shows that transmembrane segments 5 and 8 of human P-glycoprotein are close together on the cytoplasmic side of the membrane. J. Biol. Chem. 2004, 279, 7692–7697. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.W.; Bartlett, M.C.; Clarke, D.M. Val133 and cys137 in transmembrane segment 2 are close to ARG935 and GLY939 in transmembrane segment 11 of human P-glycoprotein. J. Biol. Chem. 2004, 279, 18232–18238. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.; Clarke, D. Recent progress in understanding the mechanism of P-glycoprotein-mediated drug efflux. J. Membr. Biol. 2005, 206, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.; Spiers, J. A primer on the mechanics of P-glycoprotein the multidrug transporter. Pharmacol. Res. 2007, 55, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F.; Gottesman, M.M. Is the multidrug transporter a flippase? Trends Biochem. Sci. 1992, 17, 18–21. [Google Scholar] [CrossRef]
- Srivalli, K.M.R.; Lakshmi, P. Overview of P-glycoprotein inhibitors: A rational outlook. Braz. J. Pharm. Sci. 2012, 48, 353–367. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Bostian, K.A. Practical applications and feasibility of efflux pump inhibitors in the clinic—A vision for applied use. Biochem. Pharmacol. 2006, 71, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Lomovskaya, O.; Zgurskaya, H.I.; Totrov, M.; Watkins, W.J. Waltzing transporters and ‘the dance macabre ‘between humans and bacteria. Nat. Rev. Drug Discov. 2007, 6, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kuppens, I.E.; Witteveen, E.O.; Jewell, R.C.; Radema, S.A.; Paul, E.M.; Mangum, S.G.; Beijnen, J.H.; Voest, E.E.; Schellens, J.H. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (gf120918) and oral topotecan in cancer patients. Clin. Cancer Res. 2007, 13, 3276–3285. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Wagner, P.; Ibrahim, N.; Rivera, E.; Theriault, R.; Booser, D.; Symmans, F.W.; Wong, F.; Blumenschein, G.; Fleming, D.R. Phase ii study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer 2005, 104, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of mdr and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Binkhathlan, Z.; Lavasanifar, A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: Current status and future perspectives. Curr. Cancer Drug Targets 2013, 13, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Ieiri, I. Functional significance of genetic polymorphisms in P-glycoprotein (MDR1, ABCB1) and breast cancer resistance protein (BCRP, ABCG2). Drug Metab. Pharmacokinet. 2012, 27, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Shin, S.C.; Choi, J.S. Effects of oral kaempferol on the pharmacokinetics of tamoxifen and one of its metabolites, 4-hydroxytamoxifen, after oral administration of tamoxifen to rats. Biopharm. Drug Dispos. 2008, 29, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.L.; Jordan, M.A.; Chavez, P.I. Evidence-based drug–herbal interactions. Life Sci. 2006, 78, 2146–2157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-F.; Zhou, Z.-W.; Li, C.-G.; Chen, X.; Yu, X.; Xue, C.C.; Herington, A. Identification of drugs that interact with herbs in drug development. Drug Discov. Today 2007, 12, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.C.; Foster, M.S.; Vandenhoek, S.; Krantis, A.; Budzinski, J.W.; Arnason, J.T.; Gallicano, K.D.; Choudri, S. An in vitro evaluation of human cytochrome p450 3a4 and P-glycoprotein inhibition by garlic. J. Pharm. Pharm. Sci. 2001, 4, 176–184. [Google Scholar] [PubMed]
- Ignacimuthu, S.; Shanmugam, N. Antimycobacterial activity of two natural alkaloids, vasicine acetate and 2-acetyl benzylamine, isolated from Indian shrub Adhatoda vasica Ness. leaves. J. Biosci. 2010, 35, 565–570. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, G.; Gibbons, S. Antibacterial activity of two canthin-6-one alkaloids from Allium neapolitanum. Phytother. Res. 2007, 21, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Pan, O. Modulation of multidrug resistance by three bisbenzyl-isoquinolines in comparison with verapamil. Acta Pharmacol. Sin. 1997, 18, 455–458. [Google Scholar]
- Chou, T.-C.; Depew, K.M.; Zheng, Y.-H.; Safer, M.L.; Chan, D.; Helfrich, B.; Zatorska, D.; Zatorski, A.; Bornmann, W.; Danishefsky, S.J. Reversal of anticancer multidrug resistance by the ardeemins. Proc. Natl. Acad. Sci. USA 1998, 95, 8369–8374. [Google Scholar] [CrossRef] [PubMed]
- Rabindran, S.K.; Ross, D.D.; Doyle, L.A.; Yang, W.; Greenberger, L.M. Fumitremorgin c reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000, 60, 47–50. [Google Scholar] [PubMed]
- Nakatsu, S.; Kondo, S.; Kondo, Y.; Yin, D.; Peterson, J.W.; Kaakaji, R.; Morimura, T.; Kikuchi, H.; Takeuchi, J.; Barnett, G.H. Induction of apoptosis in multi-drug resistant (mdr) human glioblastoma cells by sn-38, a metabolite of the camptothecin derivative cpt-11. Cancer Chemother. Pharmacol. 1997, 39, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Fan, S.; Zhan, Q.; Kohn, K.W.; O’Connor, P.M.; Pommier, Y. Inactivation of p53 increases the cytotoxicity of camptothecin in human colon HCT116 and breast MCF-7 cancer cells. Clin. Cancer Res. 1997, 3, 1653–1660. [Google Scholar] [PubMed]
- Okura, T.; Ibe, M.; Umegaki, K.; Shinozuka, K.; Yamada, S. Effects of dietary ingredients on function and expression of P-glycoprotein in human intestinal epithelial cells. Biol. Pharm. Bull. 2010, 33, 255–259. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, G.-Q. Effects of various principles from Chinese herbal medicine on rhodamine123 accumulation in brain capillary endothelial cells. Acta Pharmacol. Sin. 2002, 23, 591–596. [Google Scholar] [PubMed]
- Lee, S.Y.; Rhee, Y.H.; Jeong, S.J.; Lee, H.J.; Lee, H.J.; Jung, M.H.; Kim, S.H.; Lee, E.O.; Ahn, K.S.; Ahn, K.S. Hydrocinchonine, cinchonine, and quinidine potentiate paclitaxel-induced cytotoxicity and apoptosis via multidrug resistance reversal in MES-SA/DX5 uterine sarcoma cells. Environ. Toxicol. 2011, 26, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Solary, E.; Mannone, L.; Moreau, D.; Caillot, D.; Casasnovas, R.; Guy, H.; Grandjean, M.; Wolf, J.; Andre, F.; Fenaux, P. Phase I study of cinchonine, a multidrug resistance reversing agent, combined with the chvp regimen in relapsed and refractory lymphoproliferative syndromes. Leukemia 2000, 14, 2085. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Lan, L.B.; Sanglard, D.; Furuya, K.; Schuetz, J.D.; Schuetz, E.G. Interaction of cytochrome P450 A inhibitors with P-glycoprotein. J. Pharmacol. Exp. Ther. 2002, 303, 323–332. [Google Scholar]
- Gibbons, S. Phytochemicals for bacterial resistance-strengths, weaknesses and opportunities. Planta Med. 2008, 74, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.D.; Yang, M.C.; Lee, K.H.; Kim, K.R.; Choi, S.U.; Lee, K.R. Protoberberine alkaloids and their reversal activity of P-gp expressed multidrug resistance (MDR) from the rhizome of Coptis japonica Makino. Arch. Pharm. Res. 2006, 29, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tan, J.; Wink, M.; Ma, Y.; Li, N.; Su, G. An isoquinoline alkaloid from the Chinese herbal plant Corydalis yanhusuo wt wang inhibits P-glycoprotein and multidrug resistance-associate protein 1. Food Chem. 2013, 136, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jang, S.-W.; Pak, J.H.; Shim, S. Glaucine inhibits breast cancer cell migration and invasion by inhibiting mmp-9 gene expression through the suppression of NF-κB activation. Mol. Cell. Biochem. 2015, 403, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-H.; Min, H.-Y.; Chung, H.-J.; Song, J.; Park, H.-J.; Kim, S.; Lee, S.K. Anti-proliferative activity and suppression of P-glycoprotein by (−)-antofine, a natural phenanthroindolizidine alkaloid, in paclitaxel-resistant human lung cancer cells. Food Chem. Toxicol. 2012, 50, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.E.; Forleo, D.A.; Gunawardana, G.P.; Gunasekera, S.P.; Koehn, F.E.; McConnel, O. Antitumor tetrahydroisoquinoline alkaloids from the colonial ascidian Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4508–4512. [Google Scholar] [CrossRef]
- Kanzaki, A.; Takebayashi, Y.; Ren, X.-Q.; Miyashita, H.; Mori, S.; Akiyama, S.-I.; Pommier, Y. Overcoming multidrug drug resistance in P-glycoprotein/mdr1-overexpressing cell lines by ecteinascidin 743. Mol. Cancer Ther. 2002, 1, 1327–1334. [Google Scholar] [PubMed]
- Mi, Q.; Cui, B.; Silva, G.L.; Lantvit, D.; Lim, E.; Chai, H.; You, M.; Hollingshead, M.G.; Mayo, J.G.; Kinghorn, A.D. Pervilleine A, a novel tropane alkaloid that reverses the multidrug-resistance phenotype. Cancer Res. 2001, 61, 4030–4037. [Google Scholar] [PubMed]
- Smith, C.D.; Zilfou, J.T.; Stratmann, K.; Patterson, G.; Moore, R.E. Welwitindolinone analogues that reverse P-glycoprotein-mediated multiple drug resistance. Mol. Pharmacol. 1995, 47, 241–247. [Google Scholar] [PubMed]
- Severina, I.I.; Muntyan, M.S.; Lewis, K.; Skulachev, V.P. Transfer of cationic antibacterial agents berberine, palmatine, and benzalkonium through bimolecular planar phospholipid film and Staphylococcus aureus membrane. IUBMB Life 2001, 52, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Dwivedi, G.R.; Darokar, M.P.; Srivastava, S.K. Antibacterial and synergy of clavine alkaloid lysergol and its derivatives against nalidixic acid-resistant Escherichia coli. Chem. Biol. Drug Dis. 2013, 81, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Rho, M.-C.; Toyoshima, M.; Hayashi, M.; Koyano, T.; Subramaniam, G.; Kam, T.-S.; Komiyama, K. Reversal of multidrug resistance by kopsiflorine isolated from Kopsia dasyrachis. Planta Med. 1999, 65, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.-S.; Subramaniam, G.; Sim, K.-M.; Yoganathan, K.; Koyano, T.; Toyoshima, M.; Rho, M.-C.; Hayashi, M.; Komiyama, K. Reversal of multidrug resistance (MDR) by aspidofractinine-type indole alkaloids. Bioorg. Med. Chem. Lett. 1998, 8, 2769–2772. [Google Scholar] [CrossRef]
- Quesada, A.; Grávalos, M.G.; Puentes, J.F. Polyaromatic alkaloids from marine invertebrates as cytotoxic compounds and inhibitors of multidrug resistance caused by P-glycoprotein. Br. J. Cancer 1996, 74, 677. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.B.; Jacobs, R.S. A marine natural product, patellamide d, reverses multidrug resistance in a human leukemic cell line. Cancer Lett. 1993, 71, 97–102. [Google Scholar] [CrossRef]
- Fu, X.; Do, T.; Schmitz, F.J.; Andrusevich, V.; Engel, M.H. New cyclic peptides from the ascidian Lissoclinum patella. J. Nat. Prod. 1998, 61, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- García-Reynaga, P.; VanNieuwenhze, M.S. A new total synthesis of patellamide A. Org. Lett. 2008, 10, 4621–4623. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wink, M. Lobeline, a piperidine alkaloid from Lobelia can reverse P-gp dependent multidrug resistance in tumor cells. Phytomedicine 2008, 15, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Cherigo, L.; Lopez, D.; Martinez-Luis, S. Marine natural products as breast cancer resistance protein inhibitors. Mar. Drugs 2015, 13, 2010–2029. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Shukla, S.; Steadman, K.; Obrzut, T.; Finley, E.M.; Ambudkar, S.V.; Bates, S.E. Inhibition of ABCG2-mediated transport by protein kinase inhibitors with a bisindolylmaleimide or indolocarbazole structure. Mol. Cancer Ther. 2007, 6, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Michalet, S.; Cartier, G.; David, B.; Mariotte, A.-M.; Dijoux-franca, M.-G.; Kaatz, G.W.; Stavri, M.; Gibbons, S. N-caffeoylphenalkylamide derivatives as bacterial efflux pump inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 1755–1758. [Google Scholar] [CrossRef] [PubMed]
- Mohtar, M.; Johari, S.A.; Li, A.R.; Isa, M.M.; Mustafa, S.; Ali, A.M.; Basri, D.F. Inhibitory and resistance-modifying potential of plant-based alkaloids against methicillin-resistant Staphylococcus aureus (mrsa). Curr. Microbiol. 2009, 59, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wink, M. The beta-carboline alkaloid harmine inhibits bcrp and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phytother. Res. 2010, 24, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Eldin, E.E.M.N.; Fatani, S.H.; Wink, M. Influence of combinations of digitonin with selected phenolics, terpenoids, and alkaloids on the expression and activity of P-glycoprotein in leukaemia and colon cancer cells. Phytomedicine 2013, 21, 47–61. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Ma, X.; Mukherjee, R.; Farnsworth, N.R.; Cordell, G.A.; Kinghorn, A.D.; Pezzuto, J.M. Indole alkaloids from peschiera laeta that enhance vinblastine-mediated cytotoxicity with multidrug-resistant cells. J. Nat. Prod. 1994, 57, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Meschini, S.; Marra, M.; Calcabrini, A.; Federici, E.; Galeffi, C.; Arancia, G. Voacamine, a bisindolic alkaloid from peschiera fuchsiaefolia, enhances the cytotoxic effect of doxorubicin on multidrug-resistant tumor cells. Int. J. Oncol. 2003, 23, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Meschini, S.; Marra, M.; Condello, M.; Calcabrini, A.; Federici, E.; Dupuis, M.; Cianfriglia, M.; Arancia, G. Voacamine, an alkaloid extracted from peschiera fuchsiaefolia, inhibits P-glycoprotein action in multidrug-resistant tumor cells. Int. J. Oncol. 2005, 27, 1597–1604. [Google Scholar] [PubMed]
- Min, Y.D.; Kwon, H.C.; Yang, M.C.; Lee, K.H.; Choi, S.U.; Lee, K.R. Isolation of limonoids and alkaloids from Phellodendron amurense and their multidrug resistance (MDR) reversal activity. Arch. Pharm. Res. 2007, 30, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tan, T.M.C.; Lim, L.-Y. In vitro and in vivo evaluation of the effects of piperine on P-gp function and expression. Toxicol. Appl. Pharmacol. 2008, 230, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, M.; Sharma, S.; Nargotra, A.; Koul, S.; Khan, I.A. Piperine as an inhibitor of rv1258c, a putative multidrug efflux pump of mycobacterium tuberculosis. J. Antimicrob. Chemother. 2010, 65, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Stavri, M.; Piddock, L.J.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, S.A.; Efferth, T. Cytotoxicity of the indole alkaloid reserpine from Rauwolfia serpentina against drug-resistant tumor cells. Phytomedicine 2015, 22, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Eliason, J.F.; Ramuz, H.; Yoshikubo, T.; Ishikawa, T.; Yamamoto, T.; Tsuruo, T. Novel dithiane analogues of tiapamil with high activity to overcome multidrug resistance in vitro. Biochem. Pharmacol. 1995, 50, 187–196. [Google Scholar] [CrossRef]
- Gibbons, S.; Udo, E. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet (k) determinant. Phytother. Res. 2000, 14, 139–140. [Google Scholar] [CrossRef]
- Rethy, B.; Hohmann, J.; Minorics, R.; Varga, A.; Ocsovszki, I.; Molnar, J.; Juhász, K.; Falkay, G.; Zupko, I. Antitumour properties of acridone alkaloids on a murine lymphoma cell line. Anticancer Res. 2008, 28, 2737–2743. [Google Scholar] [PubMed]
- Ding, Z.; Tang, S.-C.; Weerasinghe, P.; Yang, X.; Pater, A.; Liepins, A. The alkaloid sanguinarine is effective against multidrug resistance in human cervical cells via bimodal cell death. Biochem. Pharmacol. 2002, 63, 1415–1421. [Google Scholar] [CrossRef]
- Weerasinghe, P.; Hallock, S.; Tang, S.-C.; Trump, B.; Liepins, A. Sanguinarine overcomes P-glycoprotein-mediated multidrug-resistance via induction of apoptosis and oncosis in CEM-VLB 1000 cells. Exp. Toxicol. Pathol. 2006, 58, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.D.; Choi, S.U.; Lee, K.R. Aporphine alkaloids and their reversal activity of multidrug resistance (MDR) from the stems and rhizomes of sinomenium acutum. Arch. Pharm. Res. 2006, 29, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Lavie, Y.; Harel-Orbital, T.; Gaffield, W.; Liscovitch, M. Inhibitory effect of steroidal alkaloids on drug transport and multidrug resistance in human cancer cells. Anticancer Res. 2001, 21, 1189–1194. [Google Scholar] [PubMed]
- Ding, Y.; Xie, X.; Zhao, J.; Yang, P. Reversal of adriamycin resistance by matrine in leukemia multidrug resistance cell line k562/adm. J. Dalian Med. Univ. 2004, 26, 256–258, 279. [Google Scholar]
- Li, X.; Zhang, S.; Zheng, S. Cellular biological effects of matrine on k562 and k562/vin cells. Chin. J. Pathophysiol. 2001, 18, 1233–1237. [Google Scholar]
- Chanmahasathien, W.; Ampasavate, C.; Greger, H.; Limtrakul, P. Stemona alkaloids, from traditional Thai medicine, increase chemosensitivity via P-glycoprotein-mediated multidrug resistance. Phytomedicine 2011, 18, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, R.; Che, X.F.; Yamaguchi, T.; Ushiyama, M.; Zheng, C.L.; Okumura, H.; Takeda, Y.; Shibayama, Y.; Nakamura, K.; Jeung, H.C. Cepharanthine potently enhances the sensitivity of anticancer agents in k562 cells. Cancer Sci. 2005, 96, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Wink, M. Tetrandrine and fangchinoline, bisbenzylisoquinoline alkaloids from stephania tetrandra can reverse multidrug resistance by inhibiting P-glycoprotein activity in multidrug resistant human cancer cells. Phytomedicine 2014, 21, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-P.; Wang, L.; Yang, J.-S.; Nomura, M.; Miyamoto, K.-I. Reversal of P-glycoprotein-dependent resistance to vinblastine by newly synthesized bisbenzylisoquinoline alkaloids in mouse leukemia p388 cells. Biol. Pharm. Bull. 2005, 28, 1979–1982. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-U.; Park, S.-H.; Kim, K.-H.; Choi, E.-J.; Kim, S.; Park, W.-K.; Zhang, Y.-H.; Kim, H.-S.; Jung, N.-P.; Lee, C.-O. The bis benzylisoquinoline alkaloids, tetrandine and fangchinoline, enhance the cytotoxicity of multidrug resistance-related drugs via modulation of P-glycoprotein. Anticancer Drugs 1998, 9, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.-S.; Sim, K.-M.; Pang, H.-S.; Koyano, T.; Hayashi, M.; Komiyama, K. Cytotoxic effects and reversal of multidrug resistance by ibogan and related indole alkaloids. Bioorg. Med. Chem. Lett. 2004, 14, 4487–4489. [Google Scholar] [CrossRef] [PubMed]
- Tournier, N.; Chevillard, L.; Megarbane, B.; Pirnay, S.; Scherrmann, J.-M.; Decleves, X. Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2). Int. J. Neuropsychopharmacol. 2010, 13, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.; Garvey, M.I.; Rahman, M.M.; Gibbons, S. Natural and synthetic compounds such as trimethoprim behave as inhibitors of efflux in gram-negative bacteria. J. Antimicrob. Chemother. 2010, 65, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Serly, J.; Christov, V.; Stamboliyska, B.; Molnar, J. Alkaloids derived from genus Veratrum and Peganum of mongolian origin as multidrug resistance inhibitors of cancer cells. Fitoterapia 2011, 82, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Cabral, V.; Luo, X.; Junqueira, E.; Costa, S.S.; Mulhovo, S.; Duarte, A.; Couto, I.; Viveiros, M.; Ferreira, M.-J.U. Enhancing activity of antibiotics against staphylococcus aureus: Zanthoxylum capense constituents and derivatives. Phytomedicine 2015, 22, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.; Leimkugel, J.; Oluwatuyi, M.; Heinrich, M. Activity of Zanthoxylum clava-herculis extracts against multi-drug resistant methicillin-resistant Staphylococcus aureus (MDR-MRSA). Phytother. Res. 2003, 17, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Gyemant, N.; Tanaka, M.; Antus, S.; Hohmann, J.; Csuka, O.; Mandoky, L.; Molnar, J. In vitro search for synergy between flavonoids and epirubicin on multidrug-resistant cancer cells. In Vivo 2005, 19, 367–374. [Google Scholar] [PubMed]
- Ye, J.; Zheng, Y.; Liu, D. Reversal effect and its mechanism of ampelopsin on multidrug resistance in K562/ADR cells. Zhongguo Zhong Yao Za Zhi 2009, 34, 761–765. [Google Scholar] [PubMed]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef] [PubMed]
- Stermitz, F.R.; Scriven, L.N.; Tegos, G.; Lewis, K. Two flavonols from Artemisa annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Med. 2002, 68, 1140–1141. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Yang, S.-L.; Roberts, M.F.; Elford, B.C.; Phillipson, J.D. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Rep. 1992, 11, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Jodoin, J.; Demeule, M.; BeÂliveay, R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim. Biophys. Acta 2002, 1542, 149–159. [Google Scholar] [CrossRef]
- Knop, J.; Misaka, S.; Singer, K.; Hoier, E.; Müller, F.; Glaeser, H.; König, J.; Fromm, M.F. Inhibitory effects of green tea and (–)-epigallocatechin gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-glycoprotein. PLoS ONE 2015, 10, e0139370. [Google Scholar] [CrossRef] [PubMed]
- Mossa, J.S.; El-Feraly, F.S.; Muhammad, I. Antimycobacterial constituents from Juniperus procera, Ferula communis and Plumbago zeylanica and their in vitro synergistic activity with isonicotinic acid hydrazide. Phytother. Res. 2004, 18, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, E.H.; Grant, M.H. The effect of the flavonoids, quercetin, myricetin and epicatechin on the growth and enzyme activities of MCF7 human breast cancer cells. Chem. Biol. Interact. 1998, 116, 213–228. [Google Scholar] [CrossRef]
- Ofer, M.; Wolffram, S.; Koggel, A.; Spahn-Langguth, H.; Langguth, P. Modulation of drug transport by selected flavonoids: Involvement of P-gp and OCT? Eur. J. Pharm. Sci. 2005, 25, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Cooray, H.C.; Janvilisri, T.; van Veen, H.W.; Hladky, S.B.; Barrand, M.A. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem. Biophys. Res. Commun. 2004, 317, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Van Zanden, J.J.; Wortelboer, H.M.; Bijlsma, S.; Punt, A.; Usta, M.; Bladeren, P.J.; Rietjens, I.M.; Cnubben, N.H. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem. Pharmacol. 2005, 69, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Morris, M.E. Effects of the flavonoids biochanin a, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J. Pharmacol. Exp. Ther. 2003, 304, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Morris, M.E. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol. Pharmacol. 2004, 65, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Lechner, D.; Gibbons, S.; Bucar, F. Plant phenolic compounds as ethidium bromide efflux inhibitors in Mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 62, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; De Castro, W.V.; Manthey, J.A.; Derendorf, H.; Butterweck, V. Polymethoxylated flavones and other phenolic derivates from citrus in their inhibitory effects on P-glycoprotein-mediated transport of talinolol in Caco-2 cells. J. Agric. Food Chem. 2007, 55, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H.; Sun, K.-H.; An, C.-S.; Yoo, J.-C.; Hahm, K.-S.; Lee, I.-H.; Sohng, J.-K.; Kim, Y.-C. Reversal of P-glycoprotein-mediated multidrug resistance by 5,6,7,3′,4′-pentamethoxyflavone (sinensetin). Biochem. Biophys. Res. Commun. 2002, 295, 832–840. [Google Scholar] [CrossRef]
- Romiti, N.; Tramonti, G.; Donati, A.; Chieli, E. Effects of grapefruit juice on the multidrug transporter P-glycoprotein in the human proximal tubular cell line hk-2. Life Sci. 2004, 76, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Surya Sandeep, M.; Sridhar, V.; Puneeth, Y.; Ravindra Babu, P.; Naveen Babu, K. Enhanced oral bioavailability of felodipine by naringenin in wistar rats and inhibition of P-glycoprotein in everted rat gut sacs in vitro. Drug Dev. Ind. Pharm. 2014, 40, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Khantamat, O.; Chaiwangyen, W.; Limtrakul, P.-N. Screening of flavonoids for their potential inhibitory effect on P-glycoprotein activity in human cervical carcinoma kb cells. Chiang Mai Med. Bull. 2004, 43, 45–56. [Google Scholar]
- De Castro, W.V.; Mertens-Talcott, S.; Derendorf, H.; Butterweck, V. Grapefruit juice–Drug interactions: Grapefruit juice and its components inhibit P-glycoprotein (ABCB1) mediated transport of talinolol in Caco-2 cells. J. Pharm. Sci. 2007, 96, 2808–2817. [Google Scholar] [CrossRef] [PubMed]
- De Castro, W.V.; Mertens-Talcott, S.; Derendorf, H.; Butterweck, V. Effect of grapefruit juice, naringin, naringenin, and bergamottin on the intestinal carrier-mediated transport of talinolol in rats. J. Agric. Food Chem. 2008, 56, 4840–4845. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaga, Y.; Takanaga, H.; Matsuo, H.; Naito, M.; Tsuruo, T.; Ohtani, H.; Sawada, Y. Effect of bioflavonoids on vincristine transport across blood–brain barrier. Eur. J. Pharmacol. 2000, 395, 193–201. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Hamdan, D.; Farrag, N.; El-Shazly, A.; Wink, M. Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from citrus species in human colon and leukaemia cell lines. Eur. J. Pharmacol. 2010, 626, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Najar, I.; Sachin, B.; Sharma, S.; Satti, N.; Suri, K.; Johri, R. Modulation of P-glycoprotein atpase activity by some phytoconstituents. Phytother. Res. 2010, 24, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Rameshkumar, K.; Alan Sheeja, D.; Nair, M.S.; George, V. Curcuma ecalcarata–New natural source of pinocembrin and piperitenone. Nat. Prod. Res. 2015, 29, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-H.; Sun, X.; Qi, Y.; Mei, C.; Sun, X.-B.; Du, G.-H. Uptake characteristics of pinocembrin and its effect on P-glycoprotein at the blood–brain barrier in in vitro cell experiments. J. Asian Nat. Prod. Res. 2012, 14, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Anuchapreeda, S.; Buddhasukh, D. Modulation of human multidrug-resistance MDR-1 gene by natural curcuminoids. BMC Cancer 2004, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-J.; Cai, Y.-J.; Ding, J. The short-time treatment with curcumin sufficiently decreases cell viability, induces apoptosis and copper enhances these effects in multidrug-resistant k562/a02 cells. Mol. Cell. Biochem. 2012, 360, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Belofsky, G.; Carreno, R.; Lewis, K.; Ball, A.; Casadei, G.; Tegos, G.P. Metabolites of the “smoke tree”, Dalea spinosa, potentiate antibiotic activity against multidrug-resistant Staphylococcus aureus. J. Nat. Prod. 2006, 69, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Ngameni, B.; Tangmouo, J.G.; Bolla, J.-M.; Alibert-Franco, S.; Ngadjui, B.T.; Pagès, J.-M. Efflux pumps are involved in the defense of gram-negative bacteria against the natural products isobavachalcone and diospyrone. Antimicrob. Agents Chemother. 2010, 54, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Lechner, D.; Gibbons, S.; Jachak, S.; Srivastava, A.; Bucar, F. In Curcuminoids as Efflux Pump Inhibitors (EPIS) in Mycobacterium Smegmatis mc2155. In Proceedings of the 7th Joint Meeting of GA, AFERP, ASP, PSI & SIF, Athens, Greece, 3–8 August 2008; p. 12. [Google Scholar]
- Saczko, J.; Kulbacka, J.; Chwilkowska, A.; Pola, A.; Lugowski, M.; Marcinkowska, A.; Malarska, A.; Banas, T. Cytosolic superoxide dismutase activity after photodynamic therapy, intracellular distribution of photofrin ii and hypericin, and P-glycoprotein localization in human colon adenocarcinoma. Folia Histochem. Cytobiol. 2007, 45, 93–98. [Google Scholar] [PubMed]
- Eagling, V.; Profit, L.; Back, D. Inhibition of the CYP3A4-mediated metabolism and P-glycoprotein-mediated transport of the hiv-1 protease inhibitor saquinavir by grapefruit juice components. Br. J. Clin. Pharmacol. 1999, 48, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, W.; Qu, L.; Wu, J.; Si, J. Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma cells via downregulation of mdr1 and p‑glycoprotein expression. Mol. Med. Rep. 2013, 8, 1883–1887. [Google Scholar] [PubMed]
- Falcão-Silva, V.S.; Silva, D.A.; Souza, M.d.F.V.; Siqueira-Junior, J.P. Modulation of drug resistance in staphylococcus aureus by a kaempferol glycoside from Herissantia tiubae (Malvaceae). Phytother. Res. 2009, 23, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Yarla, N.; Ganapaty, S. Bioactive flavonoids as ABC transporters inhibitors for reversion of multidrug resistance in cancer. J. Mar. Sci. Res. Dev. 2013, 4, 1–2. [Google Scholar]
- Wesołowska, O.; Wiśniewski, J.; Środa, K.; Krawczenko, A.; Bielawska-Pohl, A.; Paprocka, M.; Duś, D.; Michalak, K. 8-prenylnaringenin is an inhibitor of multidrug resistance-associated transporters, P-glycoprotein and MRP1. Eur. J. Pharmacol. 2010, 644, 32–40. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Wu, F.; Morris, M.E. 5,7-dimethoxyflavone and multiple flavonoids in combination alter the ABCG2-mediated tissue distribution of mitoxantrone in mice. Pharm. Res. 2011, 28, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yang, Q.; Wang, T.; Cao, Y.; Jiang, Q.-Y.; Sun, H.-W.; Hou, M.-X.; Yang, Y.-P.; Feng, F. Rhamnetin induces sensitization of hepatocellular carcinoma cells to a small molecular kinase inhibitor or chemotherapeutic agents. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Leng, P.; Yang, Y.; Yu, L.G.; Lou, H.X. Plagiochin E, a botanic-derived phenolic compound, reverses fungal resistance to fluconazole relating to the efflux pump. J. Appl. Microbiol. 2008, 104, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.S.; Laddha, K. Isolation, characterization and quantification of isoflavone in Momordica dioica roxb. Ex wild (cucurbitaceae) fruits. Int. J. Appl. Res. Nat. Prod. 2012, 5, 28–36. [Google Scholar]
- Choi, S.-J.; Shin, S.-C.; Choi, J.-S. Effects of myricetin on the bioavailability of doxorubicin for oral drug delivery in rats: Possible role of CYP3A4 and P-glycoprotein inhibition by myricetin. Arch. Pharm. Res. 2011, 34, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, O.; Hendrich, A.B.; Łania-Pietrzak, B.; Wiśniewski, J.; Molnar, J.; Ocsovszki, I.; Michalak, K. Perturbation of the lipid phase of a membrane is not involved in the modulation of MRP1 transport activity by flavonoids. Cell. Mol. Biol. Lett. 2009, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Sabina, H.; Aliya, R. Seaweed as a new source of flavone, scutellarein 4′-methyl-ether. Pak. J. Bot. 2009, 41, 1927–1930. [Google Scholar]
- Han, Y.-L.; Li, D.; Yang, Q.-J.; Zhou, Z.-Y.; Liu, L.-Y.; Li, B.; Lu, J.; Guo, C. In vitro inhibitory effects of scutellarin on six human/rat cytochrome p450 enzymes and P-glycoprotein. Molecules 2014, 19, 5748–5760. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhao, C.; Yan, M.; Zhang, L.Y.; Xia, Y.Z. Inhibition of P-glycoprotein function by procyanidine on blood–brain barrier. Phytother. Res. 2009, 23, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-X.; Sun, Y.-B.; Wang, S.-Q.; Duan, L.; Huo, Q.-L.; Ren, F.; Li, G.-F. Grape seed procyanidin reversal of P-glycoprotein associated multi-drug resistance via down-regulation of NF-κB and MAPK/ERK mediated YB-1 activity in a2780/t cells. PLoS ONE 2013, 8, e71071. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Tsukahara, S.; Asada, S.; Sugimoto, Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004, 64, 4346–4352. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Tian, Y.; Tan, F.; Jiang, X.H.; Wang, L. Intra-herb pharmacokinetics interaction between quercetin and isorhamentin1. Acta Pharmacol. Sin. 2008, 29, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Chung, S.Y.; Han, A.R.; Sung, M.K.; Jang, D.S.; Lee, J.; Kwon, Y.; Lee, H.J.; Seo, E.K. P-glycoprotein inhibitory activity of two phenolic compounds, (−)-syringaresinol and tricin from Sasa borealis. Chem. Biodivers. 2007, 4, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, R.; Koshiba, C.; Suzuki, C.; Lee, E. Wogonin potentiates the antitumor action of etoposide and ameliorates its adverse effects. J. Cancer Chemother. Pharmacol. 2011, 67, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kim, M.; Choi, H.; Choi, J. Effects of baicalein on the pharmacokinetics of tamoxifen and its main metabolite, 4-hydroxytamoxifen, in rats: Possible role of cytochrome P450 3A4 and P-glycoprotein inhibition by baicalein. Arch. Pharm. Res. 2011, 34, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Zhang, S.; Morris, M.E. Effect of flavonoids on MRP1-mediated transport in panc-1 cells. J. Pharm. Sci. 2003, 92, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Singh, R.P.; Agarwal, C.; Chan, D.C.; Agarwal, R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G2-M arrest, and apoptosis. Clin. Cancer Res. 2002, 8, 3512–3519. [Google Scholar] [PubMed]
- Mahringer, A.; Karamustafa, S.; Klotz, D.; Kahl, S.; Konkimalla, V.B.; Wang, Y.; Wang, J.; Liu, H.-Y.; Boechzelt, H.; Hao, X. Inhibition of P-glycoprotein at the blood–brain barrier by phytochemicals derived from traditional chinese medicine. Cancer Genom. Proteom. 2010, 7, 191–205. [Google Scholar]
- Choi, J.-S.; Choi, B.-C.; Kang, K.W. Effect of resveratrol on the pharmacokinetics of oral and intravenous nicardipine in rats: Possible role of P-glycoprotein inhibition by resveratrol. Die Pharm. Int. J. Pharm. Sci. 2009, 64, 49–52. [Google Scholar]
- Nabekura, T.; Kamiyama, S.; Kitagawa, S. Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem. Biophys. Res. Commun. 2005, 327, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.; Pan, C.; Ma, Q.; Zhang, S.; Yan, L. Reversal effect of resveratrol on multidrug resistance in kbv200 cell line. Biomed. Pharmacother. 2008, 62, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Bedada, S.K.; Yellu, N.R.; Neerati, P. Effect of resveratrol on the pharmacokinetics of fexofenadine in rats: Involvement of P-glycoprotein inhibition. Pharmacol. Rep. 2016, 68, 338–343. [Google Scholar] [CrossRef] [PubMed]
- da Graça Rocha, G.; Simoes, M.; Lúcio, K.A.; Oliveira, R.R.; Kaplan, M.A.C.; Gattass, C.R. Natural triterpenoids from Cecropia lyratiloba are cytotoxic to both sensitive and multidrug resistant leukemia cell lines. Bioorg. Med. Chem. 2007, 15, 7355–7360. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.; Caviedes, L.; Aponte, J.C.; Vaisberg, A.J.; Lewis, W.H.; Lamas, G.; Sarasara, C.; Gilman, R.H.; Hammond, G.B. Aegicerin, the first oleanane triterpene with wide-ranging antimycobacterial activity, isolated from Clavija procera. J. Nat. Prod. 2006, 69, 845–846. [Google Scholar] [CrossRef] [PubMed]
- Wortelboer, H.M.; Usta, M.; van Zanden, J.J.; van Bladeren, P.J.; Rietjens, I.M.; Cnubben, N.H. Inhibition of multidrug resistance proteins MRP1 and MRP2 by a series of α,β-unsaturated carbonyl compounds. Biochem. Pharmacol. 2005, 69, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Corea, G.; Di Pietro, A.; Dumontet, C.; Fattorusso, E.; Lanzotti, V. Jatrophane diterpenes from euphorbia spp. As modulators of multidrug resistance in cancer therapy. Phytochem. Rev. 2009, 8, 431–447. [Google Scholar] [CrossRef]
- Reis, M.; Ferreira, R.J.; Santos, M.M.; Dos Santos, D.J.; Molnár, J.; Ferreira, M.-J.U. Enhancing macrocyclic diterpenes as multidrug-resistance reversers: Structure–Activity studies on jolkinol D derivatives. J. Med. Chem. 2013, 56, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Duarte, N.; Varga, A.; Cherepnev, G.; Radics, R.; Molnár, J.; Ferreira, M.-J.U. Apoptosis induction and modulation of P-glycoprotein mediated multidrug resistance by new macrocyclic lathyrane-type diterpenoids. Bioorg. Med. Chem. 2007, 15, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Valente, I.S.; Reis, M.; Duarte, N.L.; Serly, J.; Molnár, J.P.; Ferreira, M.-J.U. Jatrophane diterpenes from Euphorbia mellifera and their activity as P-glycoprotein modulators on multidrug-resistant mouse lymphoma and human colon adenocarcinoma cells. J. Nat. Prod. 2012, 75, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Molnár, J.; Gyémánt, N.; Tanaka, M.; Hohmann, J.; Bergmann-Leitner, E.; Molnár, P.; Deli, J.; Didiziapetris, R.; Ferreira, M.J. Inhibition of multidrug resistance of cancer cells by natural diterpenes, triterpenes and carotenoids. Curr. Pharm. Des. 2006, 12, 287–311. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.M.; Gyémánt, N.; Ascenso, J.R.; Abreu, P.M.; Molnár, J.; Ferreira, M.-J.U. Euphoportlandols a and b, tetracylic diterpene polyesters from euphorbia portlandica and their anti-mdr effects in cancer cells. J. Nat. Prod. 2006, 69, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.-J.U.; Gyemant, N.; Madureira, A.M.; Molnar, J. Inhibition of P-glycoprotein transport activity in a resistant mouse lymphoma cell line by diterpenic lactones. Anticancer Res. 2005, 25, 3259–3262. [Google Scholar] [PubMed]
- Duarte, N.; Járdánházy, A.; Molnár, J.; Hilgeroth, A.; Ferreira, M.-J.U. Synergistic interaction between P-glycoprotein modulators and epirubicine on resistant cancer cells. Bioorg. Med. Chem. 2008, 16, 9323–9330. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, J.; Wang, B.; Sheng, L.; Liu, Z.; Yang, S.; Li, Y. Inhibitory effects of herbal constituents on P-glycoprotein in vitro and in vivo: Herb–drug interactions mediated via P-gp. Toxicol. Appl. Pharmacol. 2014, 275, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Castilho, R.O.; da Costa, M.R.; Wagner-Souza, K.; Kaplan, M.A.C.; Gattass, C.R. Pentacyclic triterpenes from Chrysobalanaceae sp.: Cytotoxicity on multidrug resistant and sensitive leukemia cell lines. Cancer Lett. 2003, 190, 165–169. [Google Scholar] [CrossRef]
- Wibowo, M.; Wang, Q.; Holst, J.; White, J.M.; Hofmann, A.; Davis, R.A. Dihydro-β-agarofurans from the australian endemic rainforest plant Denhamia pittosporoides inhibit leucine transport in prostate cancer cells. Asian J. Org. Chem. 2016, 5, 1461–1466. [Google Scholar] [CrossRef]
- Torres-Romero, D.; Muñoz-Martínez, F.; Jiménez, I.A.; Castanys, S.; Gamarro, F.; Bazzocchi, I.L. Novel dihydro-β-agarofuran sesquiterpenes as potent modulators of human P-glycoprotein dependent multidrug resistance. Org. Biomol. Chem. 2009, 7, 5166–5172. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Selva, F.; Campillo, M.; Reyes, C.P.; Jiménez, I.A.; Castanys, S.; Bazzocchi, I.L.; Pardo, L.; Gamarro, F.; Ravelo, A.G. Sar studies of dihydro-β-agarofuran sesquiterpenes as inhibitors of the multidrug-resistance phenotype in a Leishmania tropica line overexpressing a P-glycoprotein-like transporter. J. Med. Chem. 2004, 47, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.L.; Yu, H.L.; Li, D.; Meng, X.L.; Zhou, Z.Y.; Yu, Q.; Zhang, X.Y.; Wang, F.J.; Guo, C. Inhibitory effects of limonin on six human cytochrome P450 enzymes and P-glycoprotein in vitro. Toxicol. In Vitro 2011, 25, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Williamson, E.; Zloh, M.; Gibbons, S. Isopimaric acid from pinus nigra shows activity against multidrug-resistant and emrsa strains of Staphylococcus aureus. Phytother. Res. 2005, 19, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.C.; Kaatz, G.W.; Seo, S.M.; Wareham, N.; Williamson, E.M.; Gibbons, S. The phenolic diterpene totarol inhibits multidrug efflux pump activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 4480–4483. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Nishimura, K.; Kurimoto, S.; Takaishi, Y. New 29-nor-cycloartanes with a 3,4-seco-and a novel 2,3-seco-structure from the leaves of Sinocalycanthus chinensis. Bioorg. Med. Chem. 2011, 19, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Abraham, I.; Carvalho, P.; Kuang, Y.-H.; Shaala, L.A.; Youssef, D.T.; Avery, M.A.; Chen, Z.-S.; El Sayed, K.A. Sipholane triterpenoids: Chemistry, reversal of ABCB1/P-glycoprotein-mediated multidrug resistance, and pharmacophore modeling. J. Nat. Prod. 2009, 72, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Laphookhieo, S.; Shi, Z.; Fu, L.-W.; Akiyama, S.-I.; Chen, Z.-S.; Youssef, D.T.; van Soest, R.W.; El Sayed, K.A. Reversal of P-glycoprotein-mediated multidrug resistance by sipholane triterpenoids. J. Nat. Prod. 2007, 70, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, D.; Xia, Q.; Wang, P.; Rong, C.; Su, Y. Reversal of P-glycoprotein-mediated multidrug resistance of human hepatic cancer cells by astragaloside II. J. Pharm. Pharmacol. 2012, 64, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.B.; Darbour, N.; Bayet, C.; Doreau, A.; Raad, I.; Phung, B.H.; Dumontet, C.; Di Pietro, A.; Dijoux-Franca, M.G.; Guilet, D. Selective modulation of P-glycoprotein activity by steroidal saponines from Paris polyphylla. Fitoterapia 2009, 80, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Manda, V.K.; Dale, O.R.; Awortwe, C.; Ali, Z.; Khan, I.A.; Walker, L.A.; Khan, S.I. Evaluation of drug interaction potential of Labisia pumila (kacip fatimah) and its constituents. Front. Pharmacol. 2014, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-J.; Shen, X.-L.; Lu, H.-L.; Zhang, Y.-H.; Huang, X.-A.; Fu, L.-C.; Fong, W.-F. Tenacigenin B derivatives reverse P-glycoprotein-mediated multidrug resistance in HepG2/dox cells. J. Nat. Prod. 2008, 71, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Ramalhete, C.; Spengler, G.; Martins, M.; Viveiros, M.; Mulhovo, S.; Ferrira, M.J.V.; Amaral, L. Inhibition of efflux pumps in Methicillin-resistant Staphylococcus aureus and Enterococcus faecalis resistant strains by triterpenoids from Momordica balsamina. Int. J. Antimicrob. Agents 2011, 37, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Ramalhete, C.; Molnár, J.; Mulhovo, S.; Rosário, V.E.; Ferreira, M.-J.U. New potent P-glycoprotein modulators with the cucurbitane scaffold and their synergistic interaction with doxorubicin on resistant cancer cells. Bioorg. Med. Chem. 2009, 17, 6942–6951. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H.; Kang, G.; Min, Y.-D. Reversal of P-glycoprotein-mediated multidrug resistance by protopanaxatriol ginsenosides from Korean red ginseng. Planta Med. 2003, 69, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Risinger, A.L.; Jackson, E.M.; Polin, L.A.; Helms, G.L.; LeBoeuf, D.A.; Joe, P.A.; Hopper-Borge, E.; Ludueña, R.F.; Kruh, G.D.; Mooberry, S.L. The taccalonolides: Microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res. 2008, 68, 8881–8888. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Yoshioka, Y.; Miyamoto, Y.; Higuchi, K.; Setiawan, A.; Murakami, N.; Chen, Z.-S.; Sumizawa, T.; Akiyama, S.-I.; Kobayashi, M. Agosterol A, a novel polyhydroxylated sterol acetate reversing multidrug resistance from a marine sponge of Spongia sp. Tetrahedron Lett. 1998, 39, 6303–6306. [Google Scholar] [CrossRef]
- Rubis, B.; Polrolniczak, A.; Knula, H.; Potapinska, O.; Kaczmarek, M.; Rybczynska, M. Phytosterols in physiological concentrations target multidrug resistant cancer cells. Med. Chem. 2010, 6, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Chae, J.-W.; Lee, K.-R.; Lee, B.H.; Choi, E.J.; Ahn, S.H.; Kwon, K.-I.; Bae, M.A. Pharmacokinetic characterization of decursinol derived from Angelica gigas Nakai in rats. Xenobiotica 2011, 41, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Ito, C.; Jyoko, N.; Segawa, H.; Kuroda, J.; Okada, M.; Adachi, S.; Nakahata, T.; Yuasa, T.; Furukawa, H. Inhibition of leukemic cell growth by a novel anti-cancer drug (GUT-70) from Calophyllum brasiliense that acts by induction of apoptosis. Int. J. Cancer 2005, 113, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, A.; Matsuo, H.; Yamada, S.; Takanaga, H.; Morimoto, S.; Shoyama, Y.; Ohtani, H.; Sawada, Y. Effect of furanocoumarin derivatives in grapefruit juice on the uptake of vinblastine by caco-2 cells and on the activity of cytochrome p450 3a4. Br. J. Pharmacol. 2000, 130, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Deferme, S.; Augustijns, P. The effect of food components on the absorption of P-gp substrates: A review. J. Pharm. Pharmacol. 2003, 55, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kasaian, J.; Mosaffa, F.; Behravan, J.; Masullo, M.; Piacente, S.; Ghandadi, M.; Iranshahi, M. Reversal of P-glycoprotein-mediated multidrug resistance in MCF-7/ADR cancer cells by sesquiterpene coumarins. Fitoterapia 2015, 103, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Hanafi-Bojd, M.Y.; Iranshahi, M.; Mosaffa, F.; Tehrani, S.O.; Kalalinia, F.; Behravan, J. Farnesiferol A from Ferula persica and galbanic acid from Ferula szowitsiana inhibit P-glycoprotein-mediated rhodamine efflux in breast cancer cell lines. Planta Med. 2011, 77, 1590–1593. [Google Scholar] [CrossRef] [PubMed]
- Barthomeuf, C.; Demeule, M.; Grassi, J.; Saidkhodjaev, A.; Beliveau, R. Conferone from Ferula schtschurowskiana enhances vinblastine cytotoxicity in MDCK-MDR1 cells by competitively inhibiting P-glycoprotein transport. Planta Med. 2006, 72, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, B.S.F.; Memariani, Z.; Khashiarmanesh, Z.; Iranshahi, M.; Naderinasab, M. Effect of galbanic acid, a sesquiterpene coumarin from Ferula szowitsiana, as an inhibitor of efflux mechanism in resistant clinical isolates of staphylococcus aureus. Braz. J. Microbiol. 2010, 41, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Sarkhail, P.; Shafiee, A.; Sarkheil, P. Biological activities and pharmacokinetics of praeruptorins from Peucedanum species: A systematic review. BioMed. Res. Int. 2013, 2013, 343808. [Google Scholar] [CrossRef] [PubMed]
- Barthomeuf, C.; Grassi, J.M.; Demeule, M.; Fournier, C.; Boivin, D.; Beliveau, R. Inhibition of P-glycoprotein transport function and reversion of MDR1 multidrug resistance by cnidiadin. Cancer Chemother. Pharmacol. 2005, 56, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.J.; Giannakakou, P.; Gunasekera, S.P.; Longley, R.E.; Day, B.W.; Hamel, E. The microtubule-stabilizing agent discodermolide competitively inhibits the binding of paclitaxel (taxol) to tubulin polymers, enhances tubulin nucleation reactions more potently than paclitaxel, and inhibits the growth of paclitaxel-resistant cells. Mol. Pharmacol. 1997, 52, 613–622. [Google Scholar] [PubMed]
- Aoki, S.; Cao, L.; Matsui, K.; Rachmat, R.; Akiyama, S.I.; Kobayashi, M. Kendarimide A, a novel peptide reversing P-glycoprotein-mediated multidrug resistance in tumor cells, from a marine sponge of Haliclona sp. Tetrahedron 2004, 60, 7053–7059. [Google Scholar] [CrossRef]
- Stratmann, K.; Burgoyne, D.L.; Moore, R.E.; Patterson, G.M.; Smith, C.D. Hapalosin, a cyanobacterial cyclic depsipeptide with multidrug-resistance reversing activity. J. Org. Chem. 1994, 59, 7219–7226. [Google Scholar] [CrossRef]
- Raju, R.; Piggott, A.M.; Huang, X.-C.; Capon, R.J. Nocardioazines: A novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits P-glycoprotein. Org. Lett. 2011, 13, 2770–2773. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Deng, R.; Lu, Y.; Xu, Q.; Yan, M.; Ye, D.; Chen, W. Gambogic acid as a non-competitive inhibitor of ATP-binding cassette transporter B1 reverses the multidrug resistance of human epithelial cancers by promoting ATP-binding cassette transporter B1 protein degradation. Basic Clin. Pharmacol. Toxicol. 2013, 112, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Pereda-Miranda, R.; Kaatz, G.W.; Gibbons, S. Polyacylated oligosaccharides from medicinal Mexican morning glory species as antibacterials and inhibitors of multidrug resistance in Staphylococcus aureus. J. Nat. Prod. 2006, 69, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Kourtesi, C.; Ball, A.R.; Huang, Y.-Y.; Jachak, S.M.; Vera, D.M.A.; Khondkar, P.; Gibbons, S.; Hamblin, M.R.; Tegos, G.P. Microbial efflux systems and inhibitors: Approaches to drug discovery and the challenge of clinical implementation. Open Microbiol. J. 2013, 7, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Lai, H.; Chen, J.; Li, L.; Wong, Y.-S.; Chen, T.; Li, X. Natural borneol, a monoterpenoid compound, potentiates selenocystine-induced apoptosis in human hepatocellular carcinoma cells by enhancement of cellular uptake and activation of ROS-mediated DNA damage. PLoS ONE 2013, 8, e63502. [Google Scholar] [CrossRef] [PubMed]
- Soenen, D.R.; Hwang, I.; Hedrick, M.P.; Boger, D.L. Multidrug resistance reversal activity of key ningalin analogues. Bioorg. Med. Chem. Lett. 2003, 13, 1777–1781. [Google Scholar] [CrossRef]
- Musumeci, R.; Speciale, A.; Costanzo, R.; Annino, A.; Ragusa, S.; Rapisarda, A.; Pappalardo, M.; Iauk, L. Berberis aetnensis C. Presl. extracts: Antimicrobial properties and interaction with ciprofloxacin. Int. J. Antimicrob. Agents 2003, 22, 48–53. [Google Scholar] [CrossRef]
- Spitaler, M.; Utz, I.; Hilbe, W.; Hofmann, J.; Grunicke, H. Pkc-independent modulation of multidrug resistance in cells with mutant (v185) but not wild-type (g185) P-glycoprotein by bryostatin 1. Biochem. Pharmacol. 1998, 56, 861–869. [Google Scholar] [CrossRef]
- Zhu, H.-J.; Wang, J.-S.; Markowitz, J.S.; Donovan, J.L.; Gibson, B.B.; Gefroh, H.A.; DeVane, C.L. Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J. Pharmacol. Exp. Ther. 2006, 317, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Feinshtein, V.; Erez, O.; Ben-Zvi1, Z.; Erez, N.; Eshkoli, T.; Sheizaf, B.; Sheiner, E.; Huleihel, M.; Holcberg, G. Cannabidiol changes P-gp and BCRP expression in trophoblast cell lines. Peer J. 2013, 1, e153. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Chai, L.; Zhang, H.; Wang, Y.; Zhang, B.; Gao, X. Borneol Depresses P-Glycoprotein Function by a NF-κB Signaling Mediated Mechanism in a Blood Brain Barrier In Vitro Model. Int. J. Mol. Sci. 2015, 16, 27576–27588. [Google Scholar] [CrossRef] [PubMed]
- Saab, A.M.; Guerrini, A.; Sacchetti, G.; Maietti, S.; Zeino, M.; Arend, J.; Gambari, R.; Bernardi, F.; Efferth, T. Phytochemical analysis and cytotoxicity towards multidrug-resistant leukemia cells of essential oils derived from lebanese medicinal plants. Planta Med. 2012, 78, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Stermitz, F.R.; Cashman, K.K.; Halligan, K.M.; Morel, C.; Tegos, G.P.; Lewis, K. Polyacylated neohesperidosides from Geranium caespitosum: Bacterial multidrug resistance pump inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 1915–1918. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Cui, L.; Kuroda, M.; Ito, T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001, 9, 486–493. [Google Scholar] [CrossRef]
- Zhang, X.; Ao, Z.; Bello, A.; Ran, X.; Liu, S.; Wigle, J.; Kobinger, G.; Yao, X. Characterization of the inhibitory effect of an extract of Prunella vulgaris on ebola virus glycoprotein (gp)-mediated virus entry and infection. Antivir. Res. 2016, 127, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.-K.; Zhu, G.-Y.; Shen, X.-L.; Chattopadhyay, A.; Dey, S.; Fong, W.-F. Gomisin A alters substrate interaction and reverses P-glycoprotein-mediated multidrug resistance in HepG2-DR cells. Biochem. Pharmacol. 2006, 72, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Han, A.R.; Sung, M.K.; Jung, H.J.; Nam, J.W.; Seo, E.K.; Lee, H.J. Potent modulation of P-glycoprotein activity by naturally occurring phenylbutenoids from Zingiber cassumunar. Phytother. Res. 2009, 23, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Cordon-Cardo, C.; O’Brien, J.P.; Casals, D.; Rittman-Grauer, L.; Biedler, J.L.; Melamed, M.R.; Bertino, J.R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. USA 1989, 86, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.M.; Volkman, S.K.; Thaithong, S.; Martin, R.K.; Kyle, D.E.; Milhous, W.K.; Wirth, D.F. Amplification of pfmdr1 associated with mefloquine and halofantrine resistance in Plasmodium falciparum from thailand. Mol. Biochem. Parasitol. 1993, 57, 151–160. [Google Scholar] [CrossRef]
- Descoteaux, S.; Ayala, P.; Samuelson, J.; Orozco, E. Increase in mRNA of multiple EH P-gp genes encoding P-glycoprotein homologues in emetine-resistant Entamoeba histolytica parasites. Gene 1995, 164, 179–184. [Google Scholar] [CrossRef]
- Gamarro, F.; Chiquero, M.J.; Amador, M.V.; Légaré, D.; Ouellette, M.; Castanys, S. P-glycoprotein overexpression in methotrexate-resistant Leishmania tropica. Biochem. Pharmacol. 1994, 47, 1939–1947. [Google Scholar] [CrossRef]
- Munagala, S.; Sirasani, G.; Kokkonda, P.; Phadke, M.; Krynetskaia, N.; Lu, P.; Sharom, F.J.; Chaudhury, S.; Abdulhameed, M.D.M.; Tawa, G. Synthesis and evaluation of strychnos alkaloids as MDR reversal agents for cancer cell eradication. Bioorg. Med. Chem. 2014, 22, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, F.; Ratain, M.J. Irinotecan treatment in cancer patients with ugt1a1 polymorphisms. Oncology 2003, 17, 52–55. [Google Scholar] [PubMed]
- Okyar, A.; Piccolo, E.; Ahowesso, C.; Filipski, E.; Hossard, V.; Guettier, C.; La Sorda, R.; Tinari, N.; Iacobelli, S.; Lévi, F. Strain-and sex-dependent circadian changes in ABCC2 transporter expression: Implications for irinotecan chronotolerance in mouse ileum. PLoS ONE 2011, 6, e20393. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Charman, W.N.; Porter, C.J. Application of compartmental modeling to an examination of in vitro intestinal permeability data: Assessing the impact of tissue uptake, P-glycoprotein, and CYP3A. Drug Metab. Dispos. 2003, 31, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Kemper, E.M.; van Zandbergen, A.E.; Cleypool, C.; Mos, H.A.; Boogerd, W.; Beijnen, J.H.; van Tellingen, O. Increased penetration of paclitaxel into the brain by inhibition of P-glycoprotein. Clin. Cancer Res. 2003, 9, 2849–2855. [Google Scholar] [PubMed]
- Filipski, E.; Berland, E.; Ozturk, N.; Guettier, C.; van der Horst, G.T.; Lévi, F.; Okyar, A. Optimization of irinotecan chronotherapy with P-glycoprotein inhibition. Toxicol. Appl. Pharmacol. 2014, 274, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Seral, C.; Michot, J.-M.; Chanteux, H.; Mingeot-Leclercq, M.-P.; Tulkens, P.M.; Van Bambeke, F. Influence of P-glycoprotein inhibitors on accumulation of macrolides in J774 murine macrophages. Antimicrob. Agents Chemother. 2003, 47, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, P.; Asenso, J.; Yang, X.-D.; Wang, C.; Wei, W. Advances in plant-based inhibitors of Pglycoprotein. J. Enzyme Inhib. Med. Chem. 2016, 31, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M.; Poore, C.M.; Lopaczynska, J.; Yeh, G.C. Flavonol stimulated efflux of 7,12-dimethylbenz(a)-anthracene in multidrug-resistant breast cancer cells. Cancer Res. 1993, 53, 5977–5981. [Google Scholar] [PubMed]
- Critchfield, J.W.; Welsh, C.J.; Phang, J.M.; Yeh, G.C. Modulation of Adriamycin accumulation and efflux by flavonoids in HCT-15 colon cells: Activation of Pglycoprotein as a putative mechanism. Biochem. Pharmacol. 1994, 48, 1437–1445. [Google Scholar] [CrossRef]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front. Microbiol. 2012, 23, 3–130. [Google Scholar] [CrossRef] [PubMed]


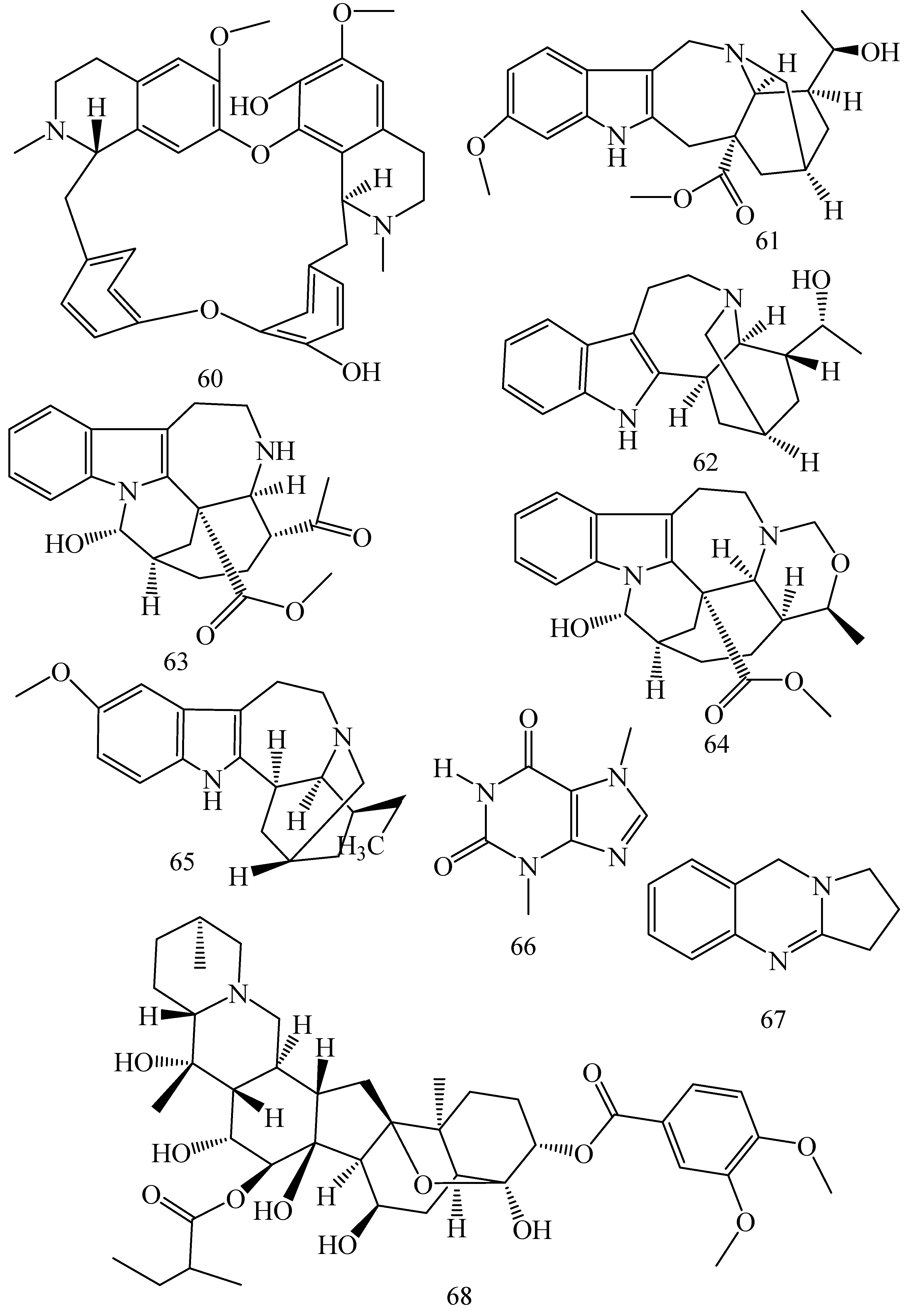
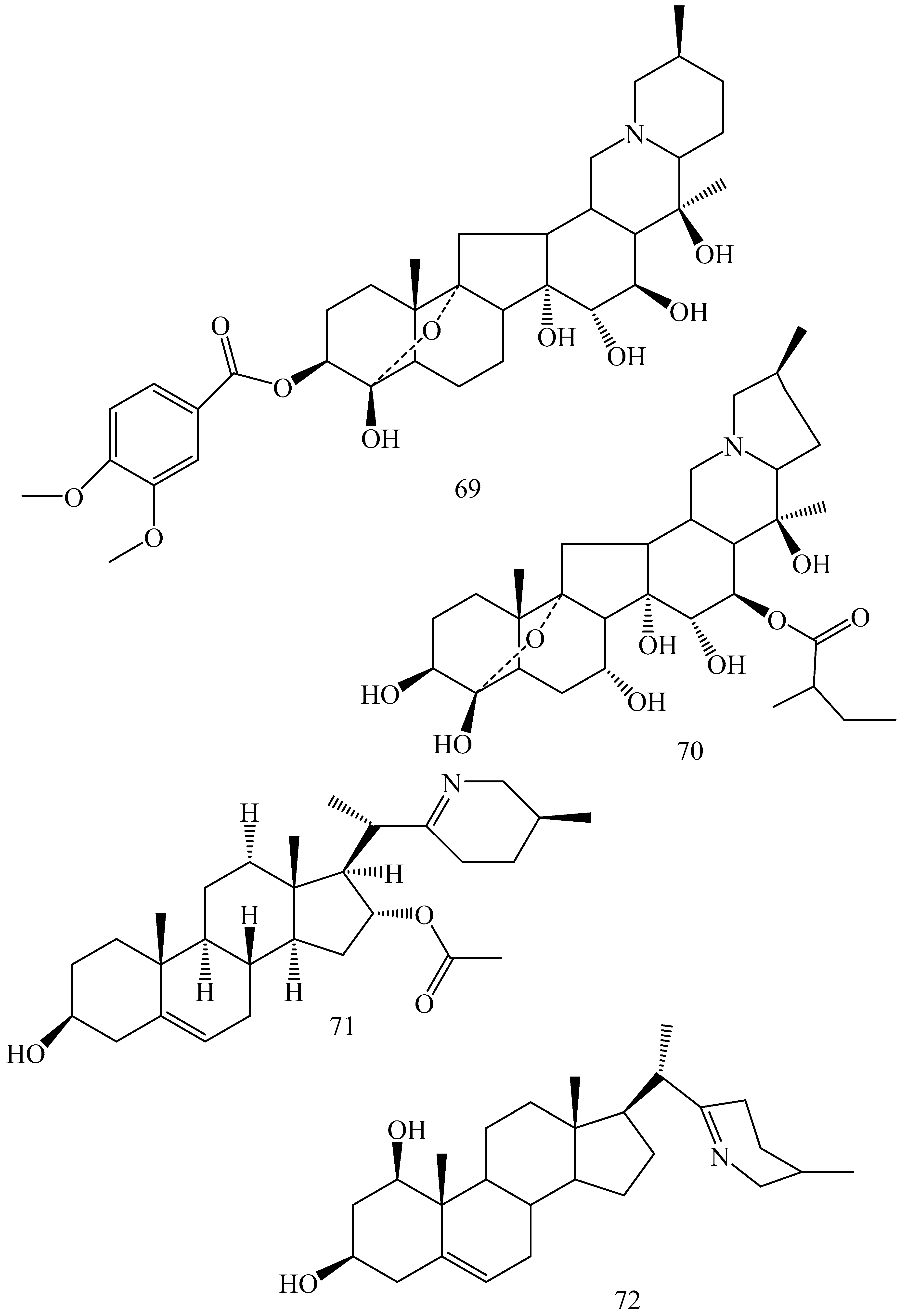
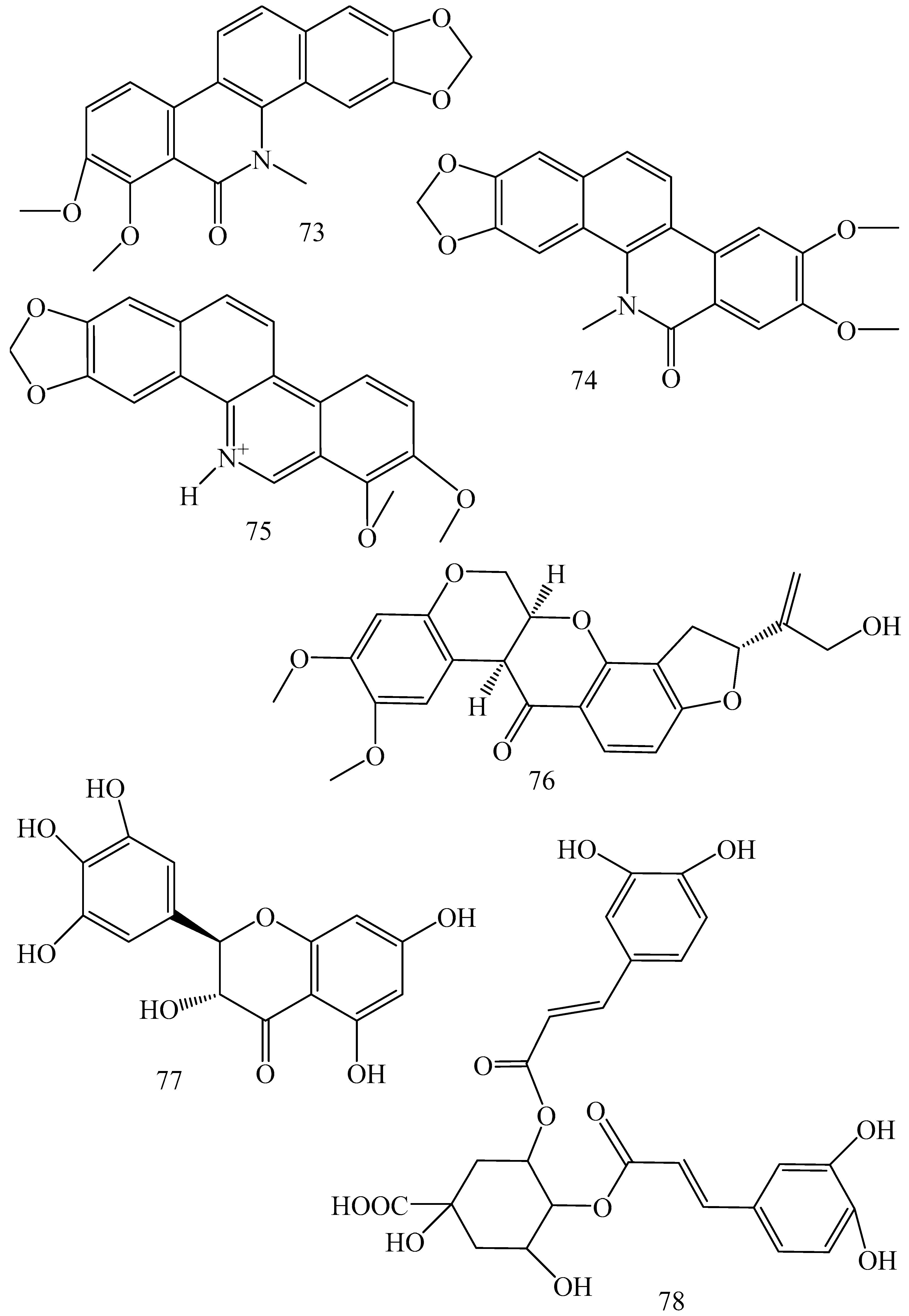

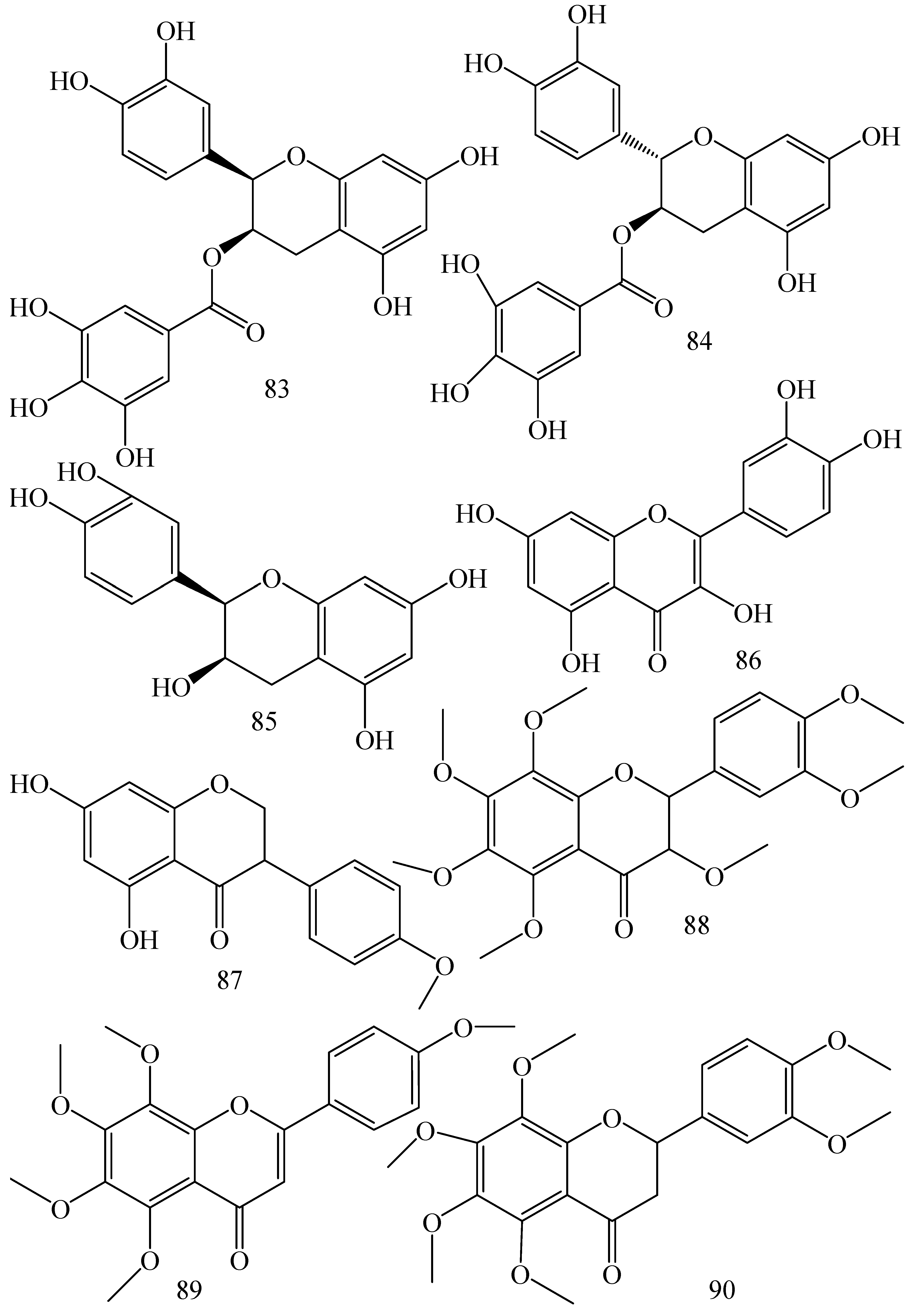



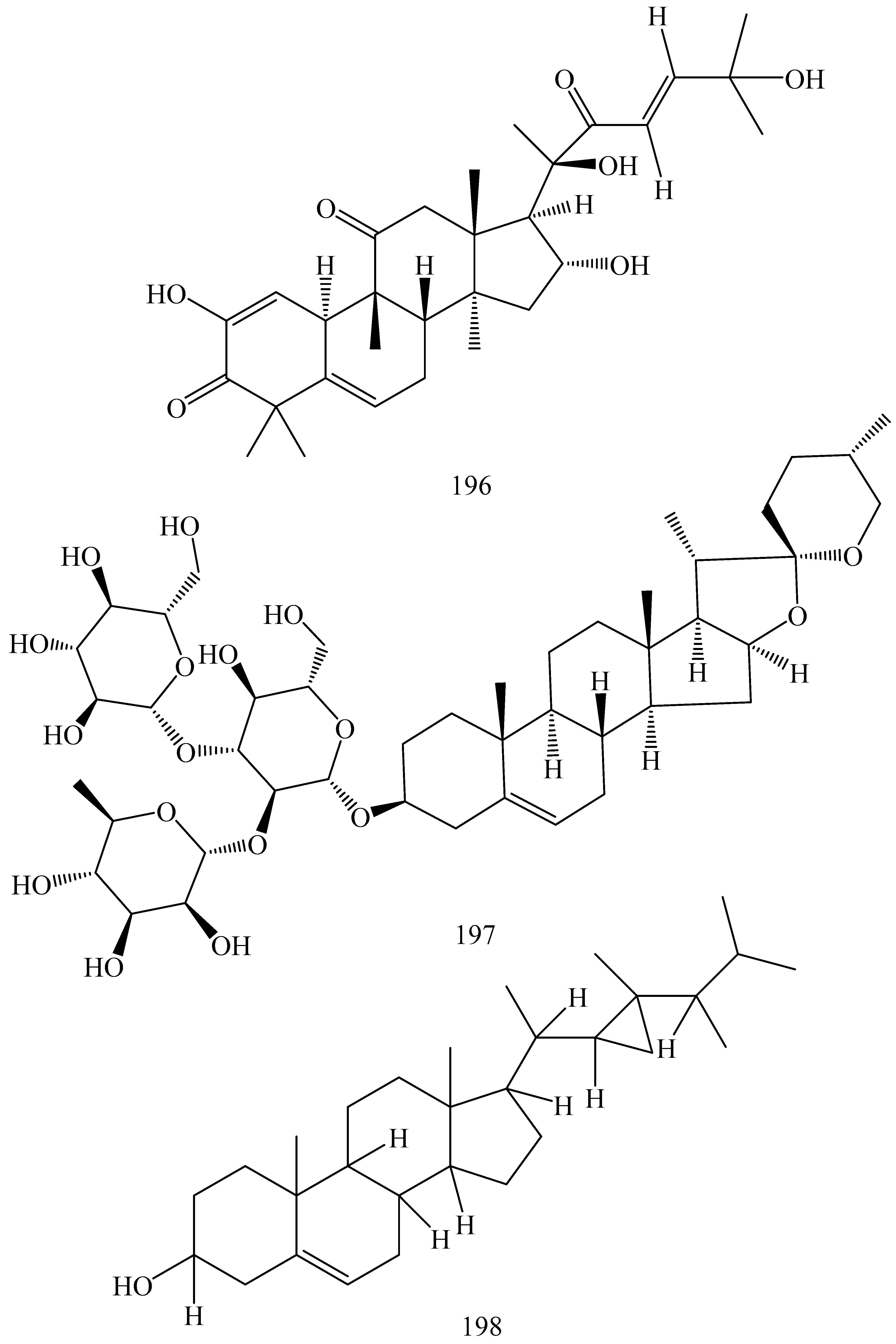
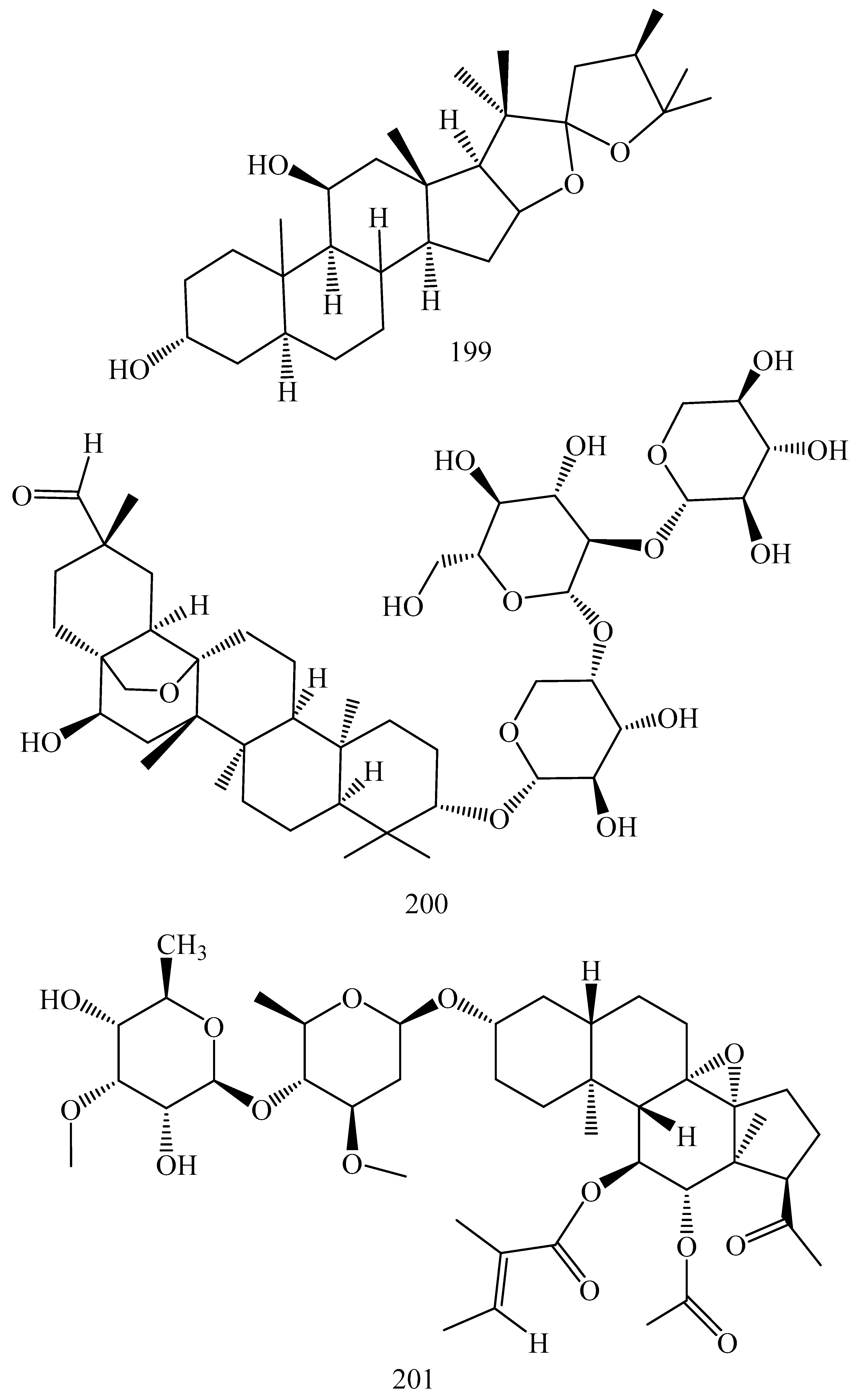
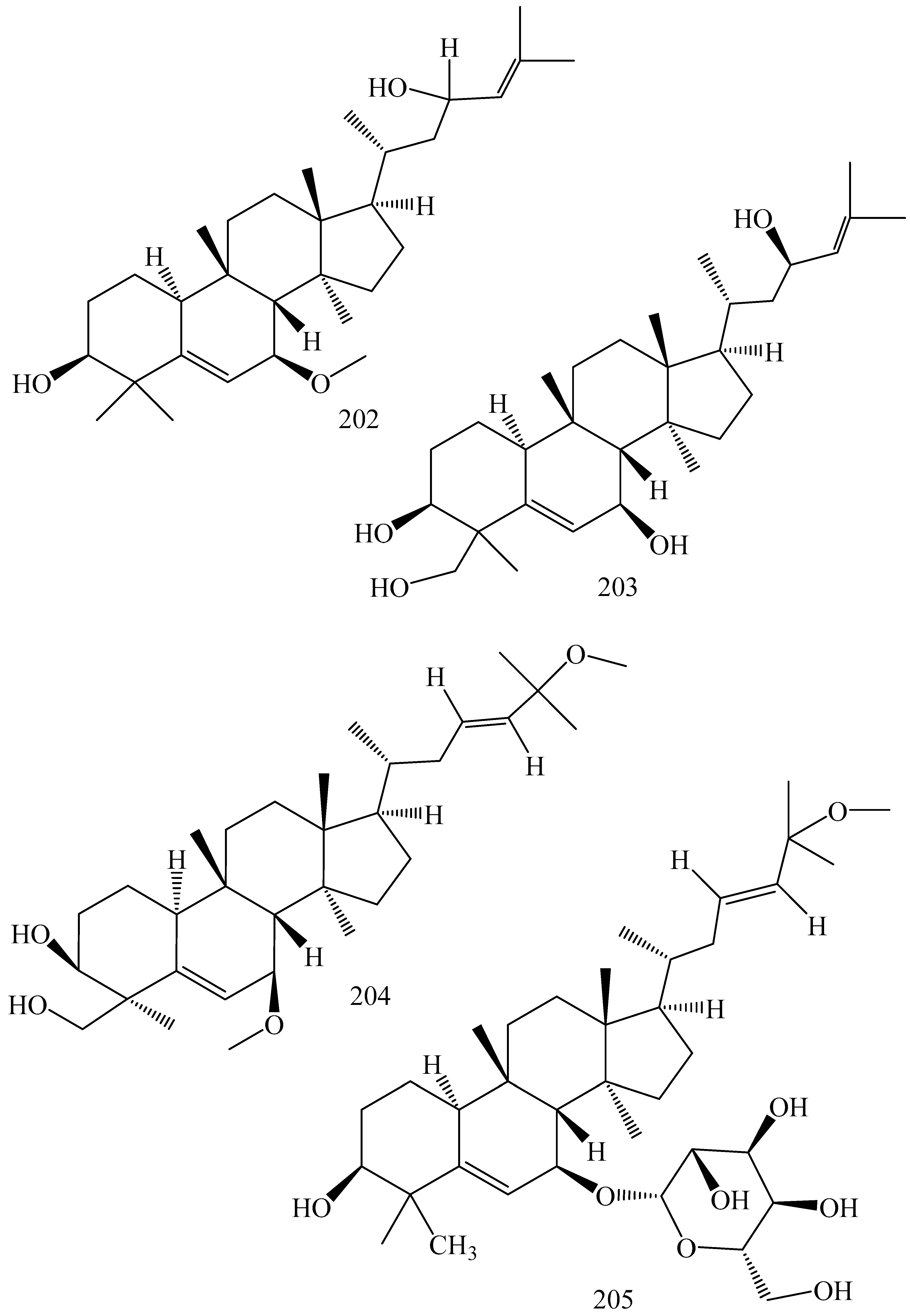
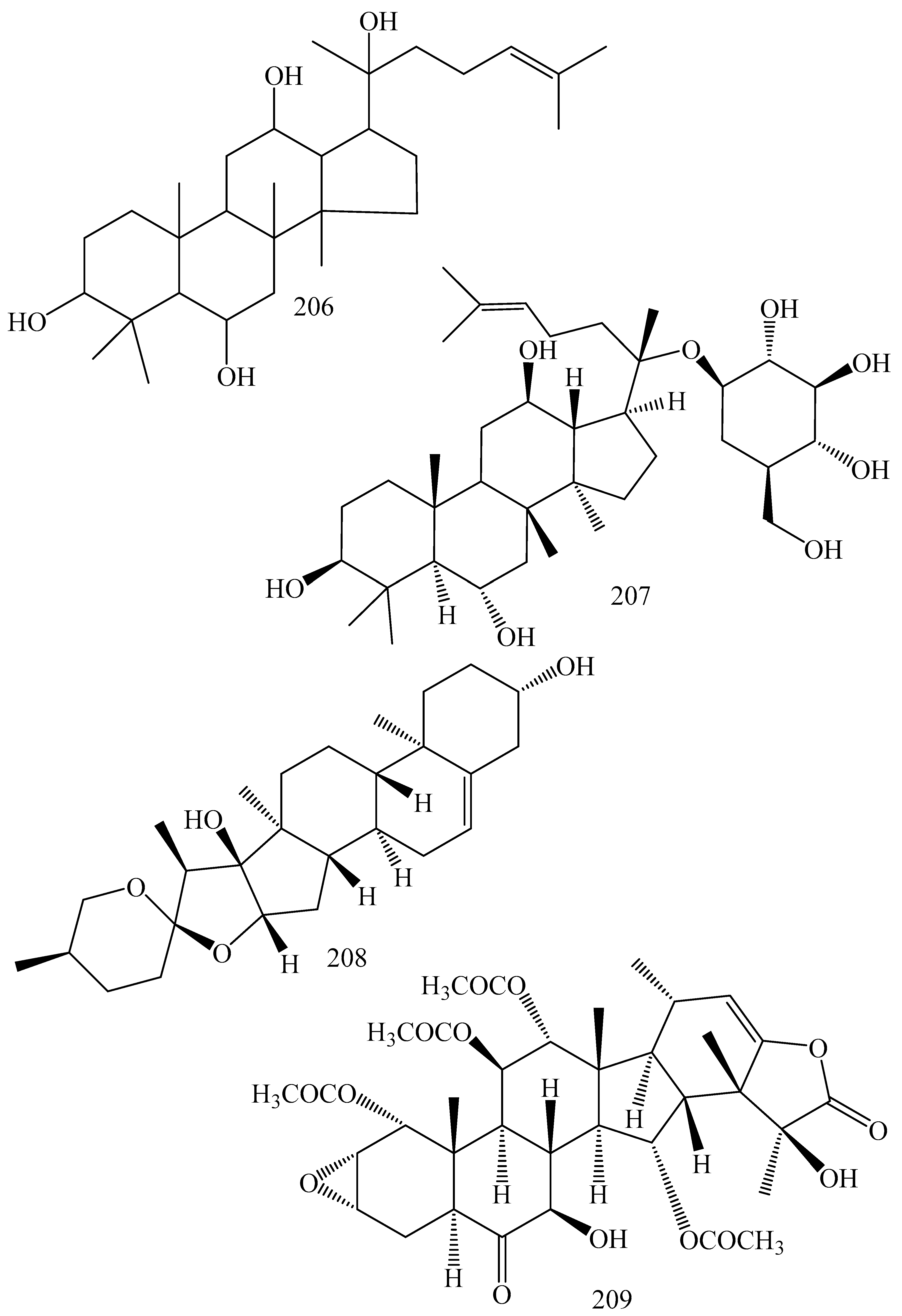
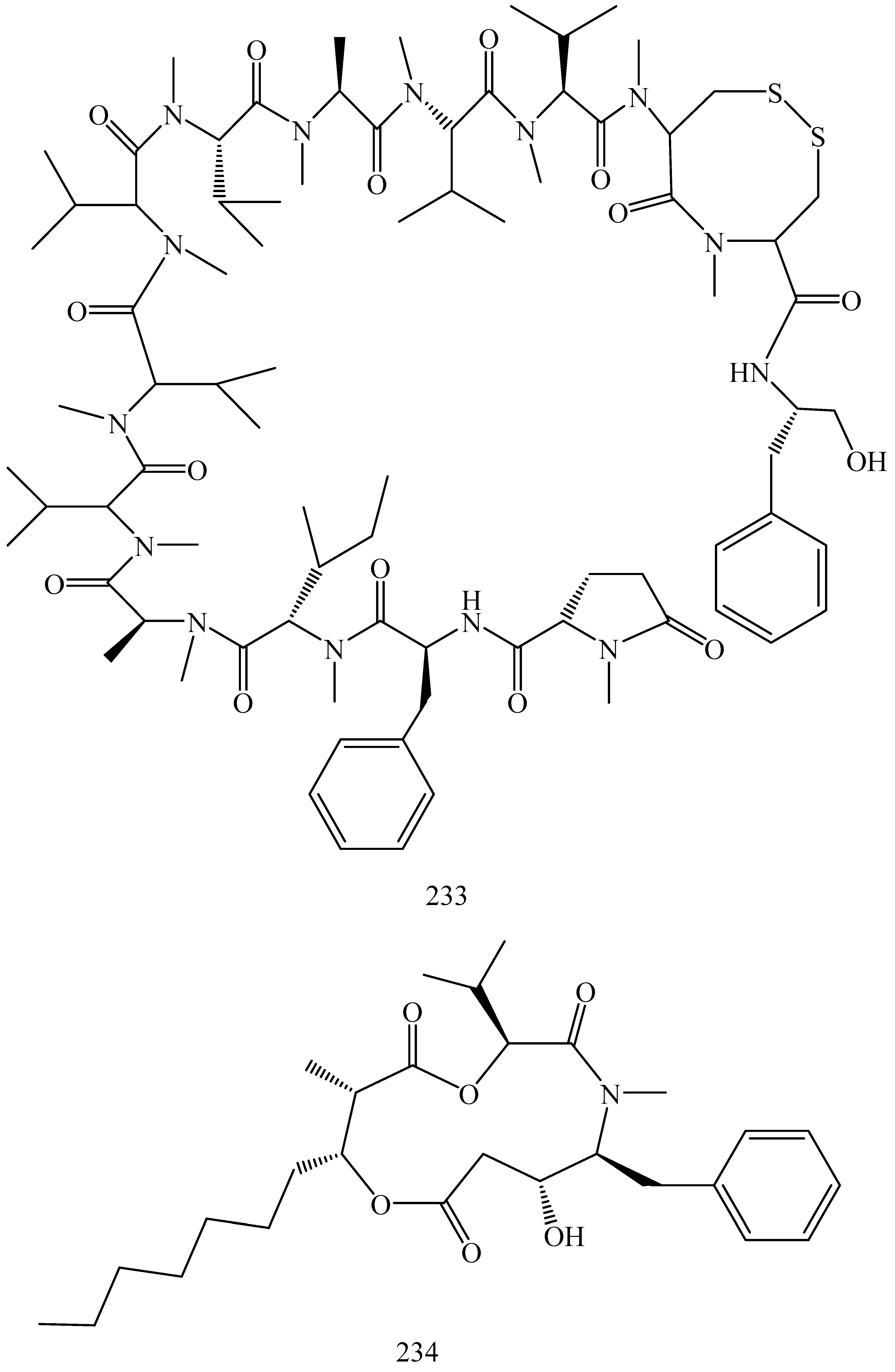
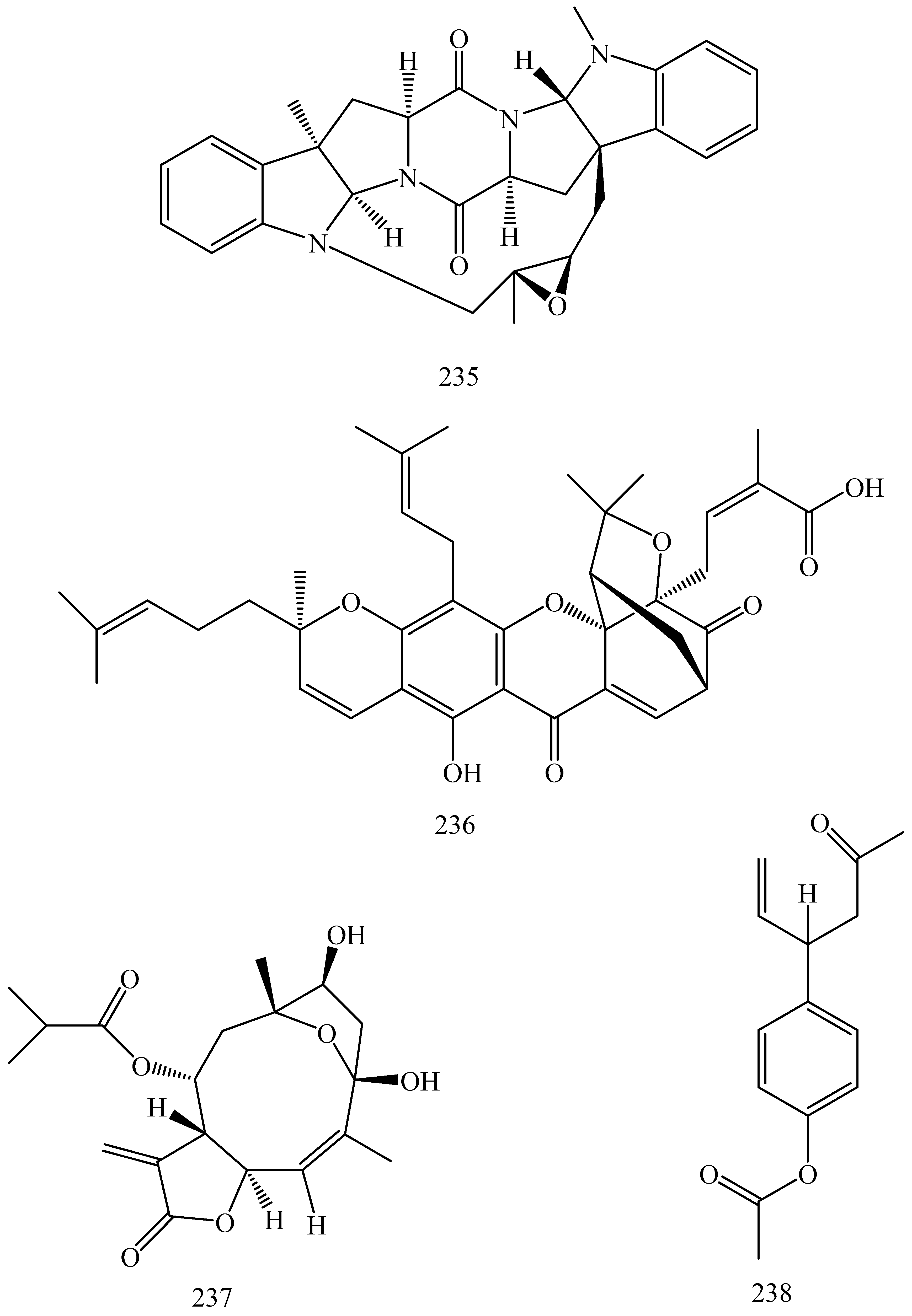

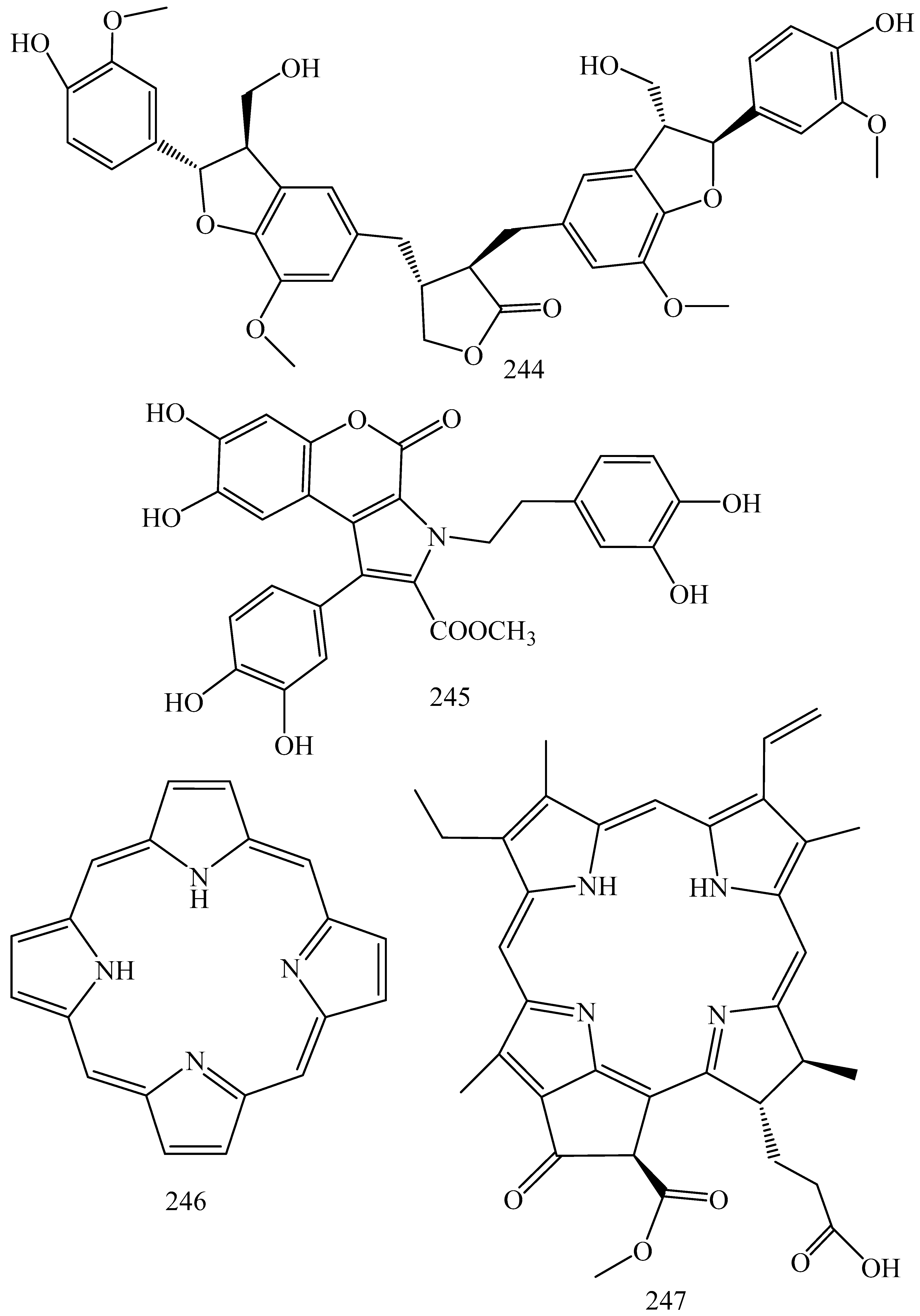
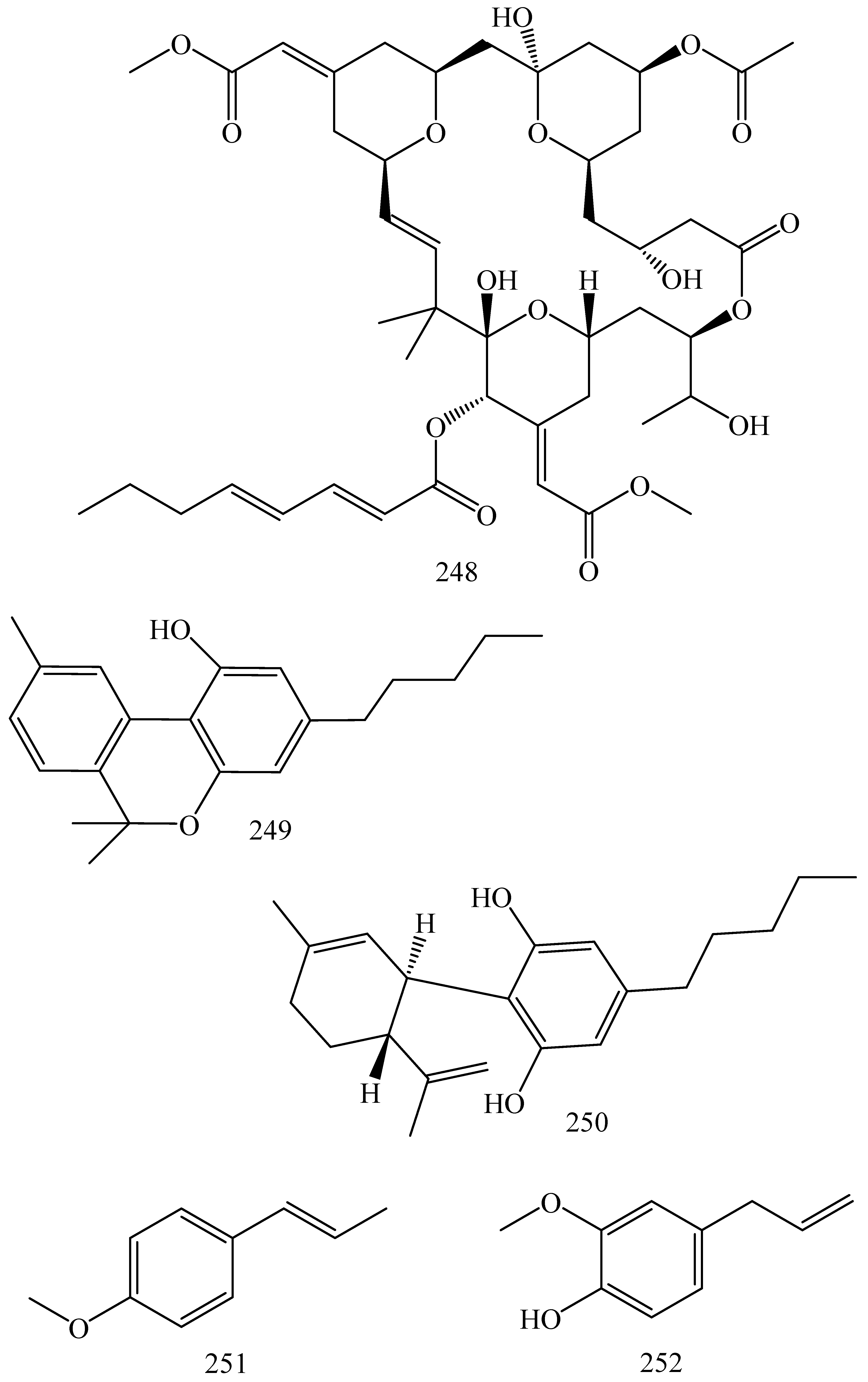

| First Generation | Second Generation | Third Generation |
|---|---|---|
| Verapamil | (R)-Verapamil | Tariquidar (XR9576) |
| Cyclosporine A | Dexniguldipine | Zosuquidar (LY335979) |
| Vincristine | Elacridar (GF-120918) | Laniquidar (R101933) |
| Reserpine | Biricodar | ONT-093 (OC-144-093) |
| Quinidine | Dofequidar | Mitotane (NSC-38721) |
| Tamoxifen | Trifluoperazine | Annamycin |
| Trifluoperazine | Valspodar (PSC-833) |
| ATPase Activity | P-gp Expression | Competition for Binding Sites | ||
|---|---|---|---|---|
| Inhibitors | Stimulators | Down-Regulators | Up-Regulators | |
| Valspodar | Verapamil | Verapamil | Vincristine | Verapamil |
| Tariquidar | Cyclosporine A | Cyclosporine A | Cyclosporine A | |
| Elacridar | Vincristine | Reserpine | Vincristine | |
| ONT-093 | Quinidine | Dexverapamil | Reserpine | |
| Tamoxifen | Toremifene | Quinidine | ||
| Toremifene | Trifluoperazine | Valspodar | ||
| Dexverapamil | Valspodar | Dexniguldipine | ||
| Biricodar | Biricodar | |||
| Elacridar | ||||
| Dofequidar | ||||
| Sources | Compounds | Mechanisms of Action | Reported Literatures | Inhibitory Concentrations | Reference |
|---|---|---|---|---|---|
| Alkaloids | |||||
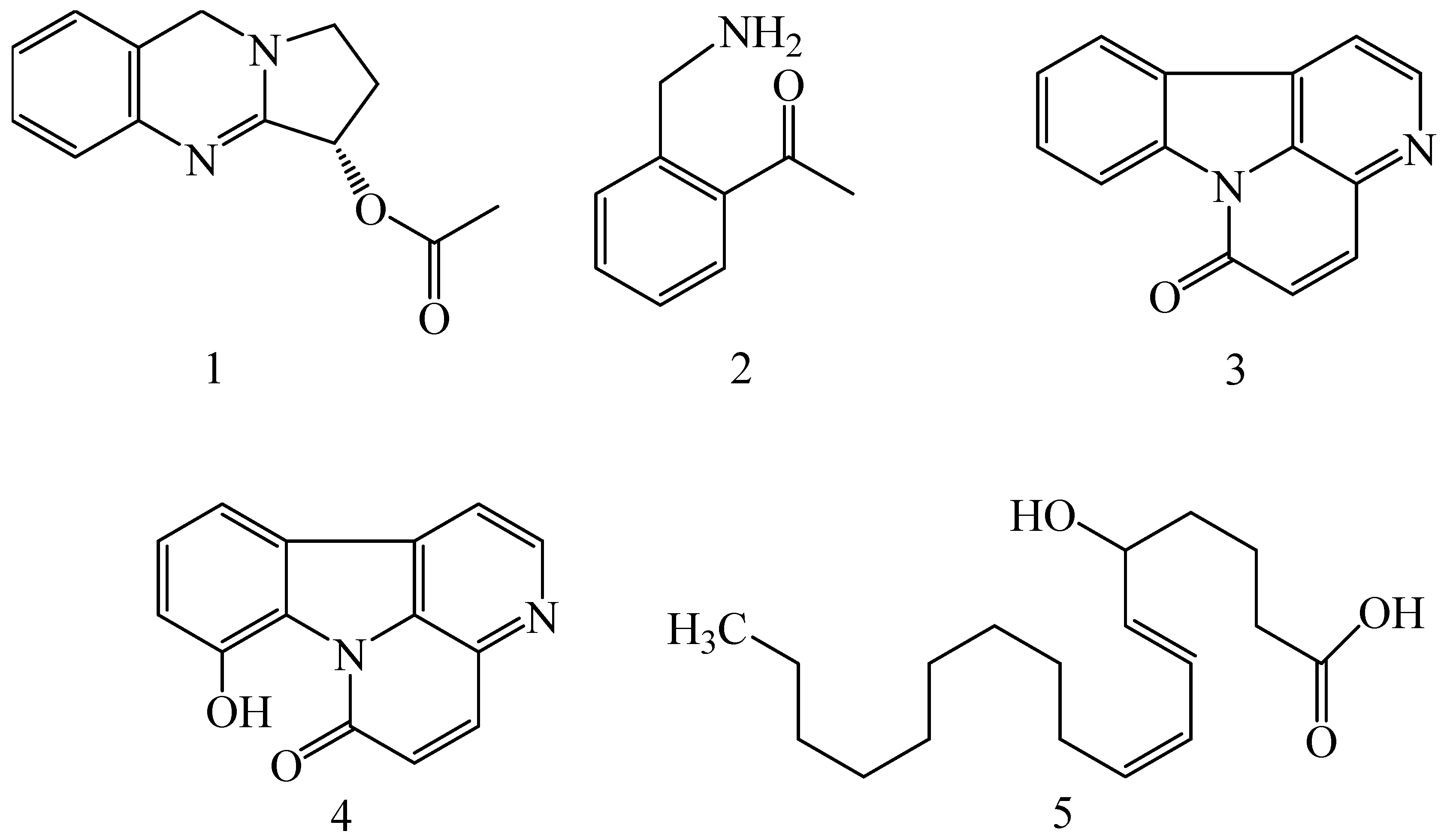
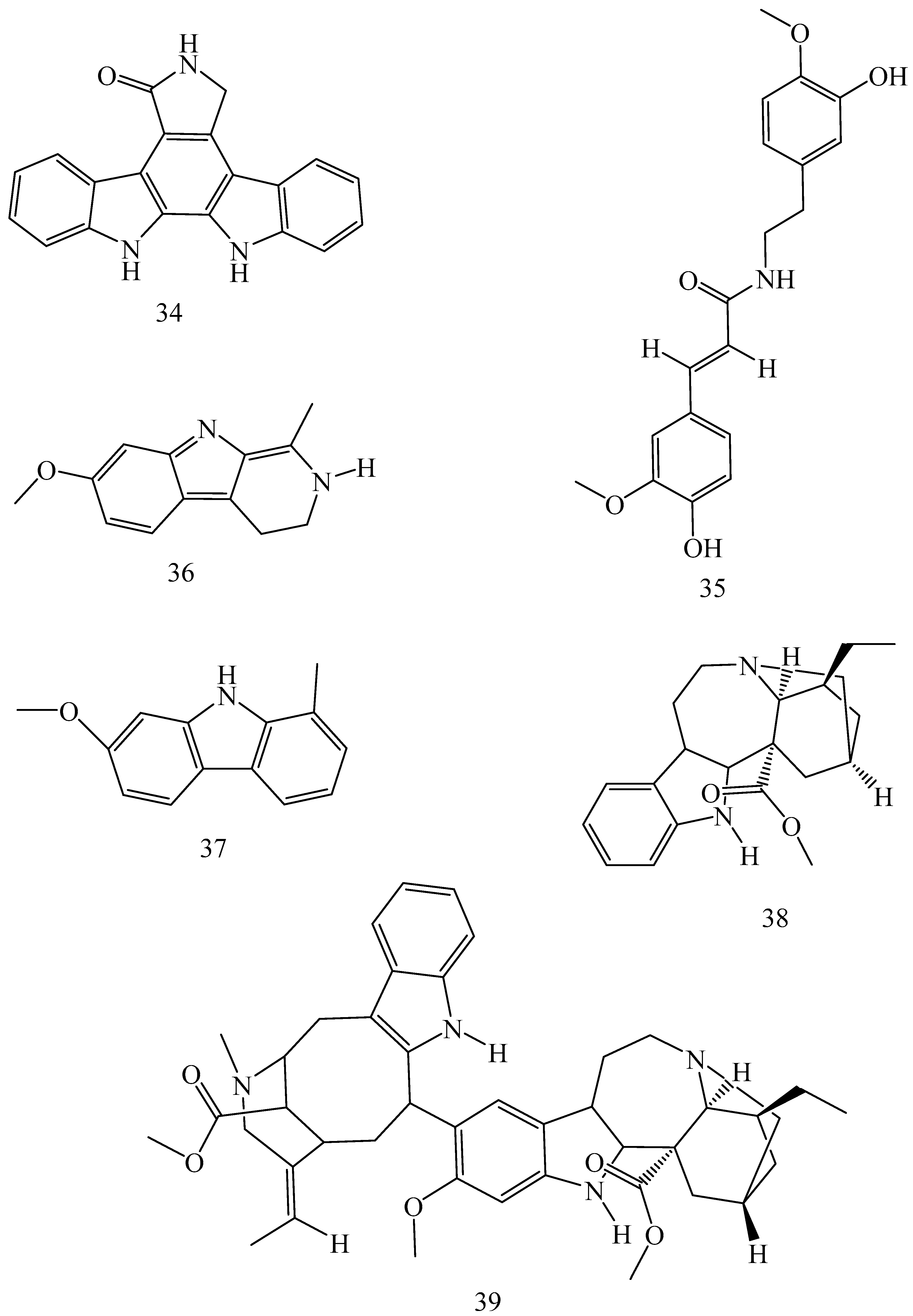
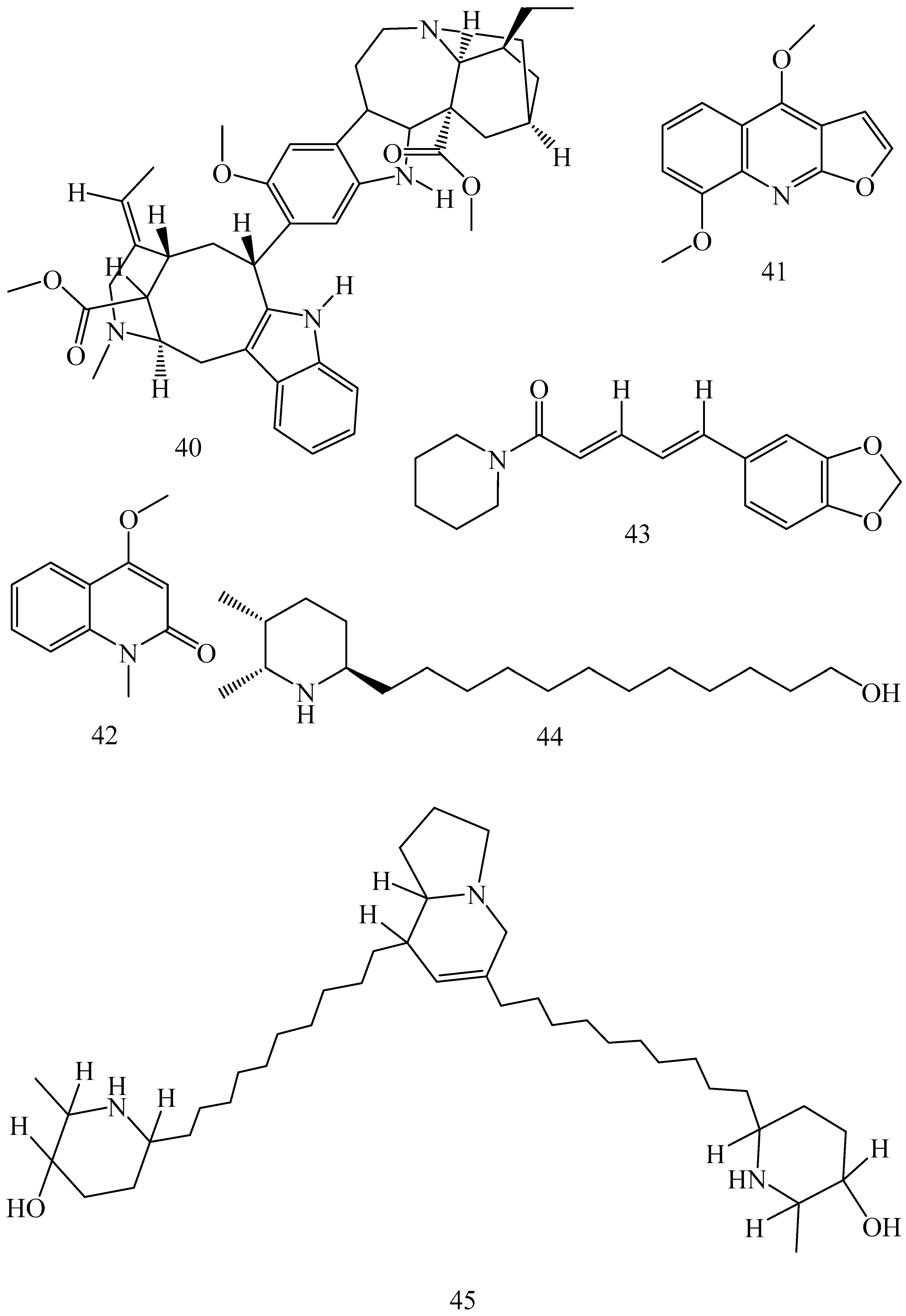
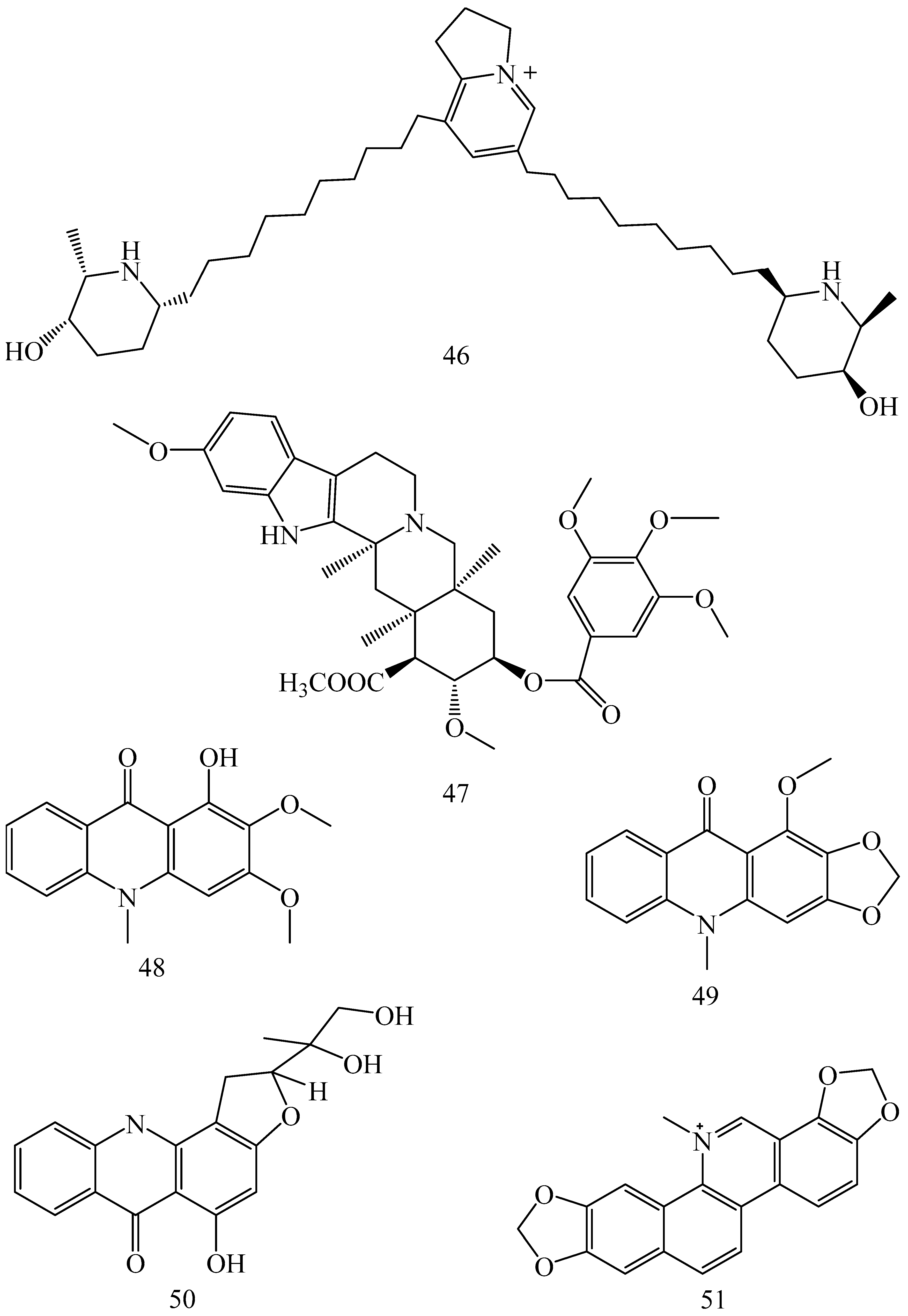
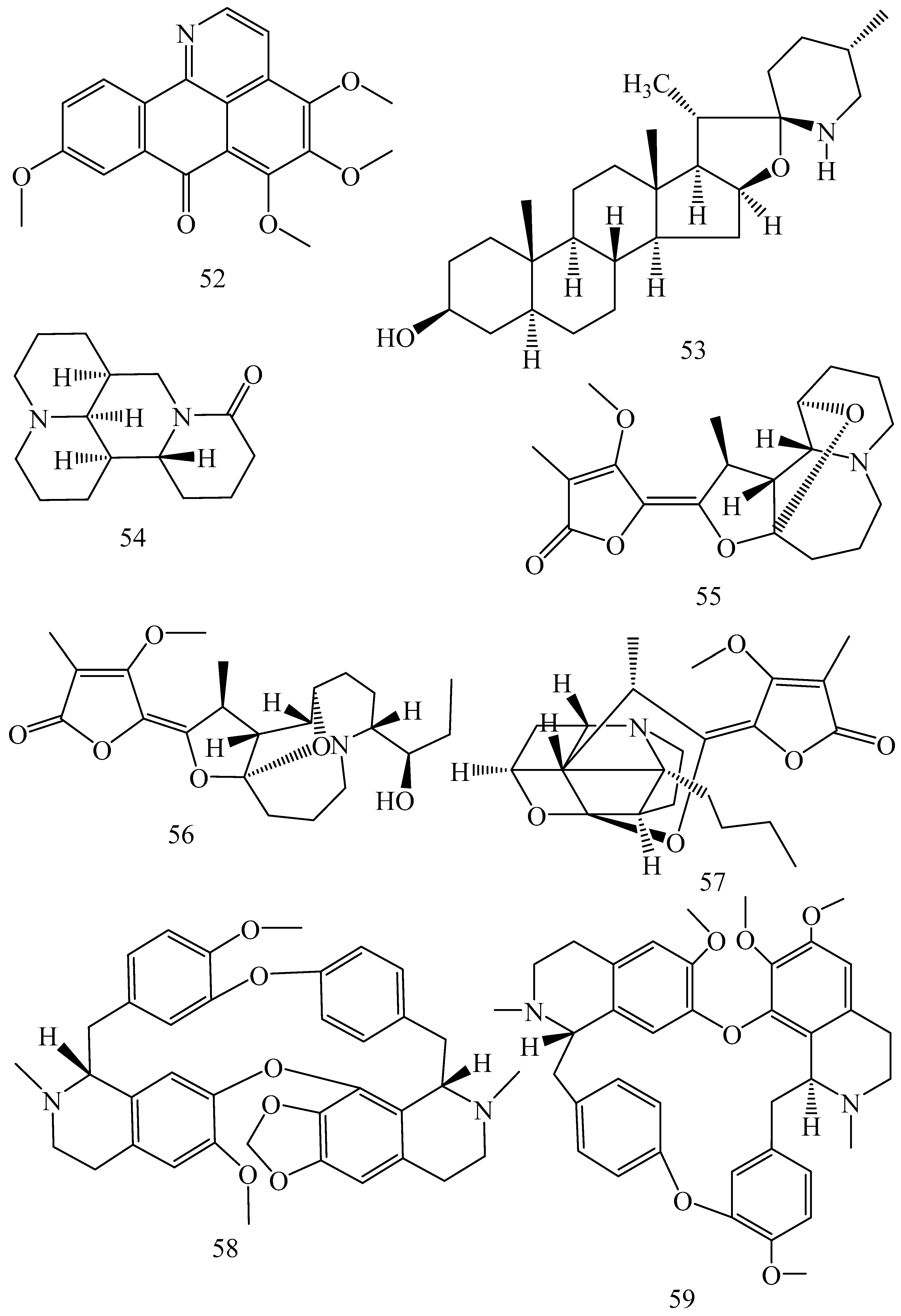
| Adhatoda vasica (Family: Acanthaceae) | Vasicine acetate (1), 2-acetyl benzylamine (2) | Not found. | Inhibits MDR strain of Mycobacterium tuberculosis. | Not found. | [61] |
| Allium neapolitanum (Family: Liliaceae) | Canthin-6-one (3), 8-hydroxy-canthin-6-one (4) | Inhibition of norA gene encoding the NorA MDR efflux protein, TetK tetracycline efflux protein and mecA gene. | Active against a panel of fast growing Mycobacterium species and MDR and MRSA strains of Staphylococcus aureus. | MIC ranges for Mycobacterium and Staphylococcus aureus are 8–32 and 8–64 µg/mL, respectively. | [62] |
| 5(Zeta)-hydroxy-octadeca-6(E)-8(Z)-dienoic acid (5) | Not found. | Active against MDR and MRSA strains of Staphylococcus aureus. | MIC range of 16–32 µg/mL. | ||
| Antizoma miersiana (Family: Menispermaceae) | Cycleanin (6), Insularine (7), Insulanoline (8) | Inhibition of MDR activity is due to favorable structure activity relationship of these compounds (like presence of-OH group) which provide better solubility and attachment with target proteins. | All three compounds increase intracellular doxorubicin accumulation in MCF-7/Adr cell via reversal of MDR. | 10 µM of all three inhibitors produces IC50 values for doxorubicin are 0.40, 0.38, 0.65 µM, respectively. | [63] |
| Aspergillus fischeri (Family: Trichocomaceae) | 5-N-acetylardeemin (9) | Inhibition of MDR-[P-gp+ and MDR-associated protein (MRP)+], MDR-P-gp+, lung resistance protein (LRP)+-expressions. | Reverses resistance to doxorubicin in lung cancer (NSCLC) cells SW2R160 (MDR+) and SW2R120 (LRP+). It also reverses vinblastine and taxol resistance to CCRF-CEM/VBL100 cell lines via P-gp inhibition. | IC50 value for vinblastine and taxol are reduced to 0.00011 and 0.0018 μM, respectively in presence of 5-N-acetylardeemin. | [64] |
| Aspergillus sydowii, Aspergillus fumigates (Family: Trichocomaceae) | Fumitremorgin C (10) | Inhibits BCRP via competitive manner. This molecule has a planar, multi-ring structure like mitoxantrone and doxorubicin and therefore may compete with other cytotoxic drugs for the binding sites on the transporter. | It almost completely reverses resistance mediated by BCRP in MCF-7 cells transfected with this protein. | Not found. | [65] |
| Camptotheca acuminate (Family: Nyssaceae) | Camptothecin (11) | Not found. | Shows activity against P-gp on mouse L1210 leukemia cells. | Not found. | [66,67] |
| Capsicum frutescens (Family: Solanaceae) | Capsaicin (12) | Inhibits mRNA expressions of MDR1 and MRP1. | Increases the amount of Rh 123 accumulation in vinblastine-resistant colon carcinoma LS-180 cells via P-gp inhibition. | Not found. | [68] |
| Catharanthus roseus (Family: Apocynaceae) | Vincristine (13) | Inhibits P-gp function in BBB. | Acts as a P-gp reversal agent in the BBB tested using Rh 123 uptake in cultured bovine brain capillary endothelial cells (BCEC). | Not found. | [69] |
| Cinchona pubescens (Family: Rubiaceae) | Cinchonine (14), Hydrocinchonine (15), Quinidine (16) | Inhibits mRNA expression of P-gp. | Hydrocinchonine, cinchonine, and quinidine significantly increased the cytotoxicity of paclitaxol in P-gp-positive MES-SA/DX5. Cinchonine potentiated anticancer drug accumulation in vivo in phase I trials. | Not found. | [70,71] |
| Claviceps purpurea (Family: Clavicipitaceae) | Ergotamine (17), Ergometrine (18) | Directly inactivate P-gp function via acting as P-gp substrates and inhibit MDR1 and mdr1a expressions. | Inhibit human MDR1 and the mouse ortholog MDR1a. Ergotamine inhibited the NorA efflux pump of Staphylococcus aureus and potentiated the activity of norfloxacin on it. | Not found. | [72,73] |
| Coptis japonica (Family: Ranunculaceae) | 8-Oxocoptisine (19) | Not found. | Shows P-gp mediated MDR reversal activity in MES-SA/DX5 and HCT15 cells and enhances cytotoxicity of paclitaxel. | ED50 values of paclitaxel are reduced to 0.018 and 0.0005 µg/mL in MES-SA/DX5 and HCT15 cell lines, respectively. | [74] |
| Corydalis yanhusuo, Corydalis turtschaninovii (Family: Papaveraceae) | Glaucine (20) | Inhibits P-gp and MRP1-mediated efflux and activates ATPase activities of the transporters. So, acts as a substrate and inhibits P-gp and MRP1 competitively. Suppresses the activity of ABC transporter gene. | Inhibits MRP1 and P-gp mediated efflux tested in human breast cancer cells, MCF-7. | Not found. | [75] |
| Inhibits MMP-9 gene expression through the suppression of NF-κB. | Directly inhibits the migration and invasion of human breast cancer cells. | 15 and 30 μM inhibited 48% and 63% of cell viabilities, respectively. | [76] | ||
| Cynanchum paniculatum (Family: Apocynaceae) | (−)-Antofine (21) | Down-regulates of P-gp mRNA and protein expressions. | Increases intracellular Rh 123 accumulation in paclitaxel resistant human lung cancer cells (A549-PA). | Not found. | [77] |
| Ecteinascidia turbinata (Family: Perophoridae) | Trabectedin (ET-743) (22) | Down-regulates MDR1 gene expression. Inhibits P-gp gene expression. | Shows good anti-cancer activity in vitro against mouse lymphocytic leukemia (L1210) cells. Inhibits P-gp expression in overian cancer and epidermal carcinoma (KB-C2 and KB-8-5, respectively). | 0.5 ng/mL. Not found. | [78,79] |
| Erythroxylum pervillei (Family: Erythroxylaceae) | Pervilleine A (23) | Inhibits P-gp gene expression. | Restores the vinblastine sensitivity of cultured multidrug resistant KB-VI cells through P-gp inhibition. | 0.36 µM. | [80] |
| Vinblastine sensitivity is also restored on CEM/VLB100 cells. | 0.02 µM. | [80] | |||
| Chemosensitivity of KB-8-5 cells to colchicine is restored by pervilleine A. | 0.61 µM. | [80] | |||
| Pervilleine B (24), Pervilleine C (25) | Inhibit of P-gp gene expression. | Both of these are found to restore the vinblastine sensitivity of cultured MDR KB-VI cells. | 0.17 µM for each compound. | [80] | |
| Hepalosiphon welwitschii (Family: Hepalosiphonaceae) | N-methyl welwitindolinon C-isothiocyanate (26) | Not found. | Enhances the cytotoxicity of actinomycin D and daunomycin in vinblastine-resistant ovarian carcinoma (SK-VLB-1) cells. Increases the activity of vinblastine, taxol, actinomycin D, colchicine and daunomycin in breast carcinoma (MCF-7/ADR) cells. | Not found. | [81] |
| Hydrastis canadensis (Family: Ranunculaceae) | Berberine (27) | Not found. Berberine acts as a substrate for NorA pump. | Increases Rh 123 accumulation in cultured bovine brain capillary endothelial cells (BCEC) via inhibition of P-gp. Berberine inhibits NorA pump (MDR pump) in wild-type Staphylococcus aureus RN 4222. | Not found. | [69,82] |
| Ipomoea muricata (Family: Convolvulaceae) | Lysergol (28) | ATPase inhibition and down-regulation of MDR ABC transporter ATP-binding yojI gene. | Inhibits the ABC pump YojI of E. coli (MTCC1652 and KG4). | Not found. | [83] |
| Kopsia dasyrachis (Family: Apocynaceae) | Kopsiflorine (29) | Inhibits mRNA expression of MDR1 gene. | Enhances cytotoxicity of vincristine in MDR KB cells. | 2.3 µg/mL. | [84,85] |
| Lamellaria spp. (Family: Velutinidae) | Lamellarin I (30) | Directly binds with active drug binding sites of P-gp and reverses its function. | Increases the intracellular concentration of Rh 123 in human colon adeno carcinoma cell line (Lo Vo/Dx). 2 µM lamellarin I has MI (fold decrease in resistance/modulator µM concentration) values of 53, 99 and 105 for doxorubicin, daunorubicin and vinblastine in MDR P388/Schabel cells, respectively. These values are 9 to 16 folds > than those obtained with 2 µM of verapamil. | Mentioned in previous column. | [86] |
| Lissoclinum patella (Family: Didemnidae) | Patellamide D (31) | Not found. Acts as a selective antagonist in multidrug resistance. | Directly acts as cytotoxic agent and acts against L1210 murine leukemia cells. Reverses the MDR in the human leukemic cells (CEM/VLB100). Patellamide D at 3.3 µM was compared with 5.1 µM verapamil in modulating drug resistance in vitro. | 2–4 µg/mL. IC50 for vinblastine, colchicine and adriamycin was reduced from 100 to 1.5 ng/mL, 140 to 50–100 ng/mL and 1000 ng/mL to 110 ng/mL, respectively. | [87,88,89] |
| Lobelia inflata (Family: Campanulaceae) | Lobeline (32) | Inhibits P-gp function probably by substrate competition. | Inhibits P-gp activity by sensitize resistant tumor cells at nontoxic concentration. Tested on Caco-2 cells. Also in CEM ADR5000 cells. | 168.3 ± 23.68 µM. 219.3 ± 5.59 µM. | [90] |
| Marine actinomycetes | Arcyriaflavin (33), Staurosporin aglycone (34) | Directly interact with BCRP and ABCG2 proteins. | Show the most potent effect of BCRP inhibition in the BCRP-transferred HEK-293 cell line, with low toxicity in BCRP-transfected cells, and reduce the relative resistance of ABCG2-transfected cells. | Not found. | [91,92] |
| Mirabilis jalapa (Family: Nyctaginaceae) | N-trans-feruloyl 4′-O-methyldopamine (35) | Inhibits NorA efflux protein and works as a substrate. | Shows efflux transporter inhibitory activity in MDR Staphylococcus aureus overexpressing the multidrug efflux transporter NorA, and causes an 8-fold reduction of norfloxacin MIC. | Not found. | [93] |
| Peganum harmala (Family: Nitrariaceae) | Harmaline (36) | Inhibits NorA efflux pump. | Inhibits NorA pump in Staphylococcus aureus and enhances the activity of antibacterial agent, Ethidium bromide. | Not found. | [94] |
| Harmine (37) | Inhibits BCRP via acting as substrate. | Removes resistance to the anticancer drugs mitoxantrone and camptothecin. | Not found. | [95,96] | |
| Decreases mRNA levels of the MDR1 gene. | Shows P-gp reversal activity on Caco-2 and CEM/ADR5000 cells using Rh 123 and calcein as P-gp substrate. | Not found. | |||
| Peschiera laeta, Peschiera fuchsiaefolia (Family: Apocynaceae) | Coronaridine (38), Conoduramine (39), Voacamine (40) | Inhibits ATP dependent P-gp binding of substrates. | Enhances vinblastine accumulation and cytotoxicity in MDR KB cells. | EC50 value for vinblastine is reduced to 1.9, 0.6, 2.0 µM. | [97,98,99] |
| Phellodendron amurense (Family: Rutaceae) | γ-fagarine (41) | Inhibits P-gp mediated MDR. | Both of the compounds show MDR reversal activity in P-gp expressed MDR cells, MES-SA/DX5 and HCT15 and enhances cytotoxicity of paclitaxel. | ED50 values of paclitaxel with γ-fagarine in MES-SA/DX5 and HCT15 cells are 0.264 and 0.0100 µM, respectively. | [100] |
| 4-methoxy-N-methyl-2-quinolone (42) | ED50 values of paclitaxel with 4-methoxy-N-methyl-2-quinolone ED50 values of paclitaxel are 0.335 and 0.0170 µM, respectively. | ||||
| Piper nigrum (Family: Piperaceae) | Piperine (43) | In low dose, it inhibits P-gp expression and function but higher dose can enhance P-gp protein and MDR1 mRNA levels. | Inhibits and modulates P-gp function in dose dependent manner. In low dose, it inhibits p-gp and enhances digoxin potential but in higher dose it enhances p-gp function and reduces digoxin uptake observed in Caco-2 cell line. Piperine enhances anti-microbial activity of rifampicin in Mycobacterium tuberculosis H37Rv and rifampicin resistant Mycobacterium tuberculosis via inhibition of clinically over expressed mycobacterial putative efflux protein (Rv1258c). | Not found. | [101,102] |
| Prosopis juliflora (Family: Fabaceae) | Julifloridine (44), Juliflorine (45), Juliprosine (46) | Inhibits NorA efflux pump by directly inhibiting its function. | Inhibits NorA efflux pump of Staphylococcus aureus and potentiated norfloxacin activity. | Not found. | [103] |
| Rauwolfia serpentina (Family: Apocynaceae) | Reserpine (47) | Not found. Directly binds with NorA MDR pump and inactivates it. | Produces significant enhancement of doxorubicin sensitivity in CEM-ADR5000 and KB cell cell line via P-gp inhibition. | 13.2 ± 1.02 µM and 10 ± 3 µM, respectively. | [104,105,106] |
| Active agaist ABC transporter pump (NorA MDR pump) in methicillin-resistant Staphylococcus aureus (MRSA) strains and enhances tetracycline sensitivity. | Not found. | ||||
| Ruta graveolens (Family: Rutaceae) | Arborinine (48), Evoxanthine (49), Gravacridonediol (50) | Reduces mRNA level of P-gp. | Induces intracellular Rh 123 accumulation in L5178 MDR cells via MDR reversal activity at 40 µM concentration. Provides synergistic activity with doxorubicine in L5178 MDR. Arborinine and evoxanthine also provide MDR reversal activity in human MDR1 gene-transfected mouse lymphoma cells at 400 μM. | IC50 for gravacridonediol + doxorubicine is 33.97 µM | [107] |
| Sanguinaria canadensis (Family: Papaveraceae) | Sanguinarine (51) | Acts via bimodal cell death mechanism or overcome the phenomenon of P-gp-mediated MDR by inducing apoptosis through increasing the Bax/Bcl 2 ratio and activating caspase 3. | Sanguinarine shows P-gp reversal activity on Caco-2 and CEM/ADR5000 cells using Rh 123 and calcein as P-gp substrate. Reversal of P-gp mediated MDR. | Not found. | [108,109] |
| Sinomenium acutum (Family: Menispermaceae) | Dauriporphine (52) | Inhibits P-gp mediated MDR. | Inhibits P-gp mediated MDR in MES-SA/DX5 and HCT15 cells and enhances cytotoxicity of paclitaxel. | ED50 values with paclitaxel are reduced to 0.03 and 0.00010 µg/mL in MES-SA/DX5 and HCT15 cells, respectively. | [110] |
| Solanum lycopersicum (Family: Solanaceae) | Tomatidine (53) | Inhibits P-gp mediated drug transport via direct binding with efflux pump. | Increase uptake of tetramethylrosamine in MDR-NCI Adr R human adenocarcinoma cells via inhibition of P-gp mediated drug transport and MDR. | Not found. | [111] |
| Sophora alopecuroides (Family: Fabaceae) | Matrine (54) | Not found. | Enhances the cytotoxicity of vincristine in resistant K562/VCR cells. Increases the intracellular accumulation of doxorubicin in resistant K562/DOX cell line. | Not found. | [112,113] |
| Stemona aphylla, Stemona burkillii (Family: Stemonaceae) | Stemocurtisine (55), Oxystemokerrine (56), Stemofoline (57) | Not found. | Act as P-gp reversing agent in KB-V1 cells at a concentration of 50 µM and increase the sensitivity toward the cytotoxic drug vinblastine. | Not found. | [114] |
| Act as P-gp reversing agent in KB-V1 cells at the concentrations of 1, 3 and 5 µM and increase sensitivity to cytotoxic drugs vinblastine, paclitaxel and doxorubicin. | Not found | ||||
| Stephania cepharantha (Family: Menispermacea) | Cepharanthine (58) | Inhibits the function of P-gp by directly interacting with the drug binding site of P-gp | Acts on human KB carcinoma cells. | 6.7 ± 4.3 µM. | [115] |
| Stephania tetrandra (Family: Menispermaceae) | Tetrandrine (59), Fangchinoline (60) | Reversal of P-gp-mediated MDR via direct inhibition of P-gp function. | Enhances anticancer activity of daunorubicin, etoposide and cytarabine in acute myeloid leukemia patients via P-gp inhibition. Produce reversal activity on P-gp mediated resistance to paclitaxel in vitro and in vivo in human MDR tumor cell line (KBv200) and resistant KBv200 tumors. Fangchinoline reduces resistance to paclitaxel and actinomycin D in HCT15 cells via MDR-reversal activity. Both the compounds increase the intracellular accumulation of the fluorescent P-gp substrate Rh 123 and inhibited its efflux in Caco-2 and CEM/ADR5000 cells. | Not found. | [116,117,118] |
| Tabernanthe iboga (Family: Apocynaceae) | Heyneanine (61), 19-epi-Heyneanine (62), Dippinine B (63), Dippinine C (64) | Not found. | All of these compounds show P-gp mediated MDR reversal activity in vincristine-resistant KB cells (KB/VJ300) in presence of vincristine 0.1 µg/mL. | IC50 values of vincristine in the presence of heyneanine, 19-epi-heyneanine, dippinine B and dippinine C (0.1 µg/mL) are 8.5, 3.5, 2 and 4 µg/mL, respectively. | [119] |
| Ibogaine (65) | It significantly inhibits P-gp activity via suppressing MDR1 and BCRP expressions. | In hMDR1- and hBCRP-transfected HEK293 cells, it enhances mitoxantrone accumulation. | Not found. | [120] | |
| Theobroma cacao (Family: Sterculiaceae) | Theobromine (66) | Inhibits AcrAB-TolC efflux pump. | Enhances the activity of ciprofloxacin on Enterobacter cloacae, Klebsiella pneumonia, Salmonella typhimurium. | Not found. | [121] |
| Veratrum lobelianum Bernh. (Family: Liliaceae) | Deoxypeganine (67) | Not found. | Reduces MDR in human MDR1-gene-transfected mouse lymphoma cells (L5178Y). | 20.76 µg/mL. | [122] |
| Veratrum nigrum (Family: Liliaceae) | Verabenzoamine (68), Veratroilzigadenine (69), 15-O-(2-Methyl butyroyl)germine (70), Veralosinine (71), Veranigrine (72) | Not found. | Reduce MDR in human MDR1-gene-transfected mouse lymphoma cells (L5178Y). | 21.76, 26.07, 24.86, 22.69 µg/mL, respectively. | [122] |
| Zanthoxylum capense (Family: Rutaceae) | Oxychelerythrine (73), Oxynitidine (74) | Inhibit bacterial NorA MDR pump. | Enhance activity of antibacterial agents like erythromycin, ethidium bromide, tetracycline and oxacillin in Staphylococcus aureus. | Not found. | [123] |
| Zanthoxylum clava-herculis (Family: Rutaceae) | Chelerythrine (75) | Not found. | Reverses the MDR in mdr-MRSA stain of Staphylococcus aureus via inhibiting the efflux mechanism. | Not found. | [124] |
| Flavonoids and Phenolics | |||||
| Amorpha fruticosa (Family: Fabaceae) | Amorphigenin (76) | Inhibits P-gp via synergism with substrate. | Potentiates the activity of epirubicin in human MDR1 gene-transfected mouse lymphoma cells. | Not found. | [125] |
| Ampelopsis spp. (Family: Vitaceae) | Ampelopsin (77) | Not found. | It reverses the MDR to adriamycin in K562/ADR cells. | Not found. | [126] |
| Artemisia absinthium (Family: Asteraceae) | 4′,5′-O-dicaffeoyl quinic acid (78), 3′,5′-O-dicaffeoyl quinic acid (79) | Inhibits NorA efflux pump. | Enhances antimicrobial activity of berberine in resistant strains of Staphylococcus aureus and Enterococcus faecalis. | MIC of berberine + 4′,5′-o-dicaffeoyl quinic acid is 64 µg/mL. | [127] |
| Not found | Enhances the activity of antimicrobial effects against resistant strains of microorganisms. | Not found | |||
| Artemisia annua (Famiy: Asteraceae) | Chrysosplenol D (80), Chrysoplenetin (81) | Inhibit NorA MDR pump and plasmodial efflux pump. | Potentiate berberine and norfloxacin activity against resistant strains of Staphylococcus aureus. Also potentiate antimicrobial activity of artemisinin against Plasmodium falciparum. | Not found. | [128,129] |
| Camellia sinensis (Family: Theaceae) | Epigallocatechin gallate (82), Epicatechin gallate (83), Catechin gallate (84), Epicatechin (85) | Not found. Epigallocatechin gallate inhibits TetK efflux pump and potentiate the activity of isoniazide. | All of these compounds inhibit Rh 123 transport in CHRC5 cells via P-gp efflux inhibition. Epigallocatechin gallate is more effective than others. It is also effective against P-gp mediated transport of vinblastine in Caco-2 cells. | Not found. | [130,131,132] |
| Epigallocatechin gallate inhibits P-gp mediated digoxin transport in Caco-2 cell line and it also inhibited metformin uptake in human embryonic kidney 293 (HEK) cells via inhibiting OATP1B1 (HEK-OATP1B1), OATP1B3 (HEK-OATP1B3), OCT1 (HEK-OCT1), OCT2 (HEK-OCT2), and MATE1 (HEK--MATE1) uptake transporters. | Not found. | ||||
| Inhibits TetK efflux pump in Mycobacterium intracellulare, Mycobacterium smegmatis, Mycobacterium xenopei, and Mycobacterium chelonei in a combination with isoniazide | Not found. | ||||
| Quercetin (86) | Inhibits transport of talinolol via replacing it from P-gp substrate binding sites. Quercetin could competitively inhibit the members of MDR family, P-gp, MRP1 and BCRP and the metabolizing enzyme, CYP3A4. Inhibits efflux function via non-competitive binding with P-gp and MRP1. | Inhibits talinolol transport in Caco-2 cell line via P-gp inhibition. | Observed IC50 values are 97 and 41 µM of talinolol in absence and presence of quercetin. | [133,134,135,136] | |
| Quercetin non-competitively inhibits the function of P-gp in K562/adr and MRP1 in GLC4/adr cells and increases pirarubicin’s cytotoxicity. | IC50 value s of pirarubicin are reduced to 23.0 ± 3.0 and 18.0 ± 8.5 µM, respectively in these two cell lines. | ||||
| Cicer pinnatifidum (Family: Fabaceae) | Biochanin A (87) | Modulates P-gp by interacting bi-functionally with the vicinal ATP-binding site and the steroid binding sites as well as inhibition of P-gp ATPase by binding to the ATP-binding site. | Potentiates cytotoxicity of doxorubicine in P-gp positive MDA435/LCC6MDR1 cells. Inhibits the activity of P-gp in recombinant human P-gp membrane | IC50 value of doxorubicin in MDA435/LCC6MDR1 cell line is reduced to 0.80 ± 0.20 µM in presence of Biochanin A. | [137,138,139] |
| Inhibits BCRP protein expression. | In combination with mitoxantrone shows significant potentiation of cytotoxicity in MCF-7 MS100 cells. | Not found. | |||
| Inhibits TetK efflux pump. | Potentiates antimicrobial activity of ethidium bromide on Mycobacterium smegmatis. | Not found. | |||
| Citrus aurantium (Family: Rutaceae) | 3,3′,4′,5,6,7,8-heptamethoxy flavones (88), Tangeratin (89), Nobiletin (90) | Not found. | Enhance transport across Caco-2 cell monolayer via P-gp inhibition. | Not found. | [140] |
| Citrus reticulata (Family:Rutaceae) | 5,6,7,3′,4′-pentamethoxy flavones (Sinensetin) (91) | Reverses P-gp mediated MDR. | Enhances cytotoxicity of vincristine in P-gp over expressing AML-2/D100 cells. | IC50 value for vincristine is reduced to 1.14 µM. | [141] |
| Citrus paradisi (Family: Rutaceae) | Kaempferol (92), Kaempheride (93), Naringenin (94) | Kaempferol and Naringenin both inhibit MDR-1 mRNA expression. | Kaempferol and Naringenin both decrease P-gp levels in the human immortalized proximal tubular cells (HK-2). | Not found. | [142,143,144,145,146] |
| Naringenin enhances bioavailability of felodipine in whister rats via P-gp inhibition. | Not found. | ||||
| Kaempferol and kaempheride both show P-gp inhibition in K562/BCRP cells. | Not found | ||||
| Kaempferol decreases resistance of vinblastine and doxorubicin in vinblastine resistant KB-VI cell lines. | Kaempferol reduces IC50 values for vinblastine and doxorubicin to 1233 ± 202 and 47533 ± 2145 nM, respectively. | ||||
| Naringenin is a potent inhibitor of P-gp observed via talinolol transport across Caco-2 cell monolayer. | Naringenin reduces IC50 values of talinolol to 236 0 values of mitoxan µM. | ||||
| Kampherol in combination with mitoxantrone in mitoxantrone specific MDR MCF-7 MS100 cell line shows significant inhibition of P-gp function. | IC50 value of mixtrantrone (alone) is 199 ± 19.3 µM, while in combination with kampherol IC50 value is reduced to 3.36 ± 1.84 µM. | ||||
| Naringenin shows P-gp inhibition in MDR MCF-7 MS100 cell line inhibition with mitoxantrone. | IC50 values of mitoxantrone (alone) is 199 ± 19.3 µM, while in combination with naringenin IC50 value is reduced to 1.23 ± 0.16 µM. | ||||
| Citrus sinensis (Family: Rutaceae) | Hesperidin (95), Hesperitin (96) | Not found. | Hesperidin reverses doxorubicin resistance in Caco-2 Cell line via p-gp inhibition. | IC50 value of doxorubicin is reduced to 194.89 ± 43.87 µM in presence of hesperidin. | [147,148] |
| Hesperitin enhances vincristine uptake in BBB via P-gp modulation and it was tested in mouse brain capillary endothelial cells (MBEC4 cells). | Not found. | ||||
| Coffea Arabica (Family: Rubiaceae) | Chlorogenic acid (97) | Inhibits P-gp ATPase activity. | Inhibits P-gp function in jejunal mucosa of rats. | Not found. | [149] |
| Curcuma ecalcarata (Family: Zingiberaceae) | Pinocembrine (98) | Inhibits P-gp protein expression. | Iinhibits P-gp expression in BBB tested in cultured rat brain microvascular endothelial cells (rBMECs). | Not found. | [150,151] |
| Curcuma longa (Family:Zingiberaceae) | Tetrahydro-curcumin (99) | Shows concentration dependent decreased P-gp protein and MDR1 gene expressions. | Inhibits the efflux function of P-gp, MXR and MRP1 in drug resistance KB-V-1, MCF7AdrVp 3000 and MRP1-HEK 293 cell lines and enhances the cytotoxicity of vinblastine, mitoxantrone and etoposide. | IC50 values for vinblastine, mitoxantrone and etoposide in combination with Tetrahydro-curcumin are reduced to 0.7 ± 0.2, 14.6 ± 2.8 and 11.9 ± 2.8 µM, respectively. | [152,153] |
| Curcumin (100) | Curcumin inhibits P-gp function in MDR K562/A02 cells. | Not found. | |||
| Dalea spinosa (Family: Fabaceae) | Pterocarpan (101) | Inhibits NorA efflux pump. | Enhances antimicrobial activity of berberine in resistant strains of Staphylococcus aureus. | Not found. | [154] |
| Dorstenia barteri (Family: Moraceae) | Isobavachalcone (102) | Inhibits AcrAB and TolC efflux pumps. | Inhibits MDR efflux pumps in gram negative bacteria. | Not found. | [155] |
| Eriodictyon californicum (Family: Boraginaceae) | Eriodictoyl (103) | Not found. | Acts as P-gp inhibitor | Not found. | [5] |
| Fragaria ananassa (Family: Rosaceae), Dimorphandra mollis (Family: Fabaceae) | Rutin (104) | Inhibits bacterial TetK efflux pump. | Enhances isoniazid activity against Mycobacterium smegmatis mc2155. | Not found. | [156] |
| Genista tinctoria (Family: Fabaceae) | Genistein (105) | Inhibits BCRP protein expression. | In combination with mitoxantrone shows significant inhibition of mitoxantrone efflux in MCF-7 MS100 cells. Inhibit the labeling of P-gp with its photoactive substrate. | IC50 values of mitoxantrone (alone) is 199 ± 19.3 µM, while in combination with genistein IC50 value is reduced to 2.29 ± 0.86 µM. | [138,157] |
| Ginkgo biloba (Family: Ginkgoaceae), Citrus paradisi (Family: Rutaceae) | Bergamottin (106) 6′,7′-dihydroxy bergamottin (107) | Not found. | Inhibit the P-gp substrate saquinavir transport in human liver microsomes. | IC50 values for saquinavir along with the compounds are 0.74 ± 0.13 µM and 0.33 ± 0.23 µM, respectively. | [158] |
| Epimedium grandiflorum (Family: Barberidaceae) | Icaritin (108) | Down-regulates the expression of P‑gp via decreasing the expression of the MDR1 gene. | Significantly increases the cytotoxicity of adriamycin, vincristine, cisplatin and 5‑fluorouracil in MDR HepG2/ADR (liver cancer cell line). | IC50 values for adriamycin, vincristine, cisplatin and 5‑fluorouracil are reduced to 0.596 ± 0.063, 0.267 ± 0.034, 1.285 ± 0.125 and 63.092 ± 2.174 µg/L, respectively. | [159] |
| Herissantia tiubae (Family: Malvaceae) | Tiliroside (109) | Inhibits NorA efflux protein expression. | Potentiates antimicrobial activities of norfloxacin, ciprofloxacin, lomefloxacin and ofloxacin in Staphylococcus aureus (SA1199B). | Not found. | [160] |
| Humulus lupulus (Family: Cannabaceae) | 8-prenyl naringenin (110) | Inhibits transport of MRP1 in human erythrocytes. | Inhibits MRP1 mediated transport of fluorescent substrate BCECF. It also acts as an effective inhibitor of Rh 123 transport in doxorubicin resistant human adenocarcinoma cell line (LoVo/Dx cells). | IC50 value for 8-prenyl naringenin for MRP1 is 5.76 ± 1.80 µM. | [161,162] |
| Hypericum perforatum (Family: Clusiaceae) | Hypericin (111) | Not found. | Inhibits P-gp function on human doxorubicin-resistant adenocarcinoma cell line (LoVo DX). | Not found. | [157] |
| Kaempferia parviflora (Family: Zingiberaceae) | 5,7-Dimethoxy flavone (112) | Inhibits BCRP protein expression. | Intracellular concentration of mitoxantrone is significantly increased in MDCK/Bcrp1 and MDCK/BCRP cells when co administered with 5,7-dimethoxyflavone. | Not found. | [163] |
| Larix gmelinii (Family: Pinaceae), Sophora japonica (Family: Fabaceae) | Taxifolin (113) | Not found. | Enhances isoniazid activity in Mycobacterium smegmatis mc2155 via inhibiting bacterial TetK efflux pump. | Not found. | [156] |
| Maclura pomifera (Family: Moraceae), Psidium guajava (Family: Myrtaceae) | Morin (114) | Inhibits P-gp ATPase via binding to the ATP-binding site. | Increases accumulation of daunomycin in P-gp overexpressing MCF-7/Adr cells. | Not found. | [137] |
| Malus domestica (Family: Rosaceae) | Phloretin (115), Phloridzin (116) | Inhibit P-gp ATPase via binding to the ATP-binding site. | Increases accumulation of daunomycin in P-gp overexpressing MCF-7/Adr cells. | Not found. | [137] |
| Mangifera indica (Family: Anacardiaceae) | Rhamnetin (117) | Inhibits Notch-1 signaling pathway and P-gp related protein expression. | Enhances the performance of adriamycin, etoposide, paclitaxel and sorafenib in MDR hepatocellular carcinoma cell line (HepG2/ADR). | IC50 values of these drugs in presence of rhamanetin are reduced to 1.74 ± 0.14, 0.12 ± 0.03, 0.05 ± 0.01, 0.82 ± 0.15 μM, respectively. | [164] |
| Marchantia polymorpha (Family: Marchantiaceae) | Plagiochin E (118) | Inhibits Cdr1p efflux pump and mRNA expression of efflux transporter gene (CDR1). | Inhibits azole resistance in Candida albicans and potentiate the antimicrobial activity of fluconazole. | Not found. | [165] |
| Mentha piperita (Family: Lamiaceae) | Spiraeoside (119) | Not found. | Inhibits talinolol efflux out of the Caco-2 cell monolayers via P-gp inhibition. | Not found. | [134] |
| Momordica dioica (Family: Cucurbitaceae) | Daidzin (120) | Stimulates ATPase activity and inhibits BCRP expression. | Enhances the accumulation of two BCRP substrates, mitoxantrone and bodipy-FL-prazosin in mitoxantrone selected BCRP-overexpressing epithelial breast cancer cell line (MCF/MR) via inhibiting P-gp function. | Not found. | [135,166] |
| Myristica fragrans (Family: Myristiaceae) | Myricetin (121) | Stimulates ATPase activity and inhibits MRP1 expression. | Enhances the cellular accumulation of Rh 123 in MCF-7/Adr cells and enhances doxorubicin oral bioavailability in rats. | Not found. | [167,168] |
| Inhibits MRP1 mediated BCECF efflux out of human erythrocytes. | IC50 value of doxorubicin along with myricetin is reduced to 52.6 ± 2 µM. | ||||
| Osmundea pinnatifida (Family: Rhodomelaceae) | Scutellarein (122), Scutellarein 4′-methyl ether (123) | Not found. | Inhibitory effect of scutellarin on P-gp activity were examined on a human metastatic malignant melanoma cell line, WM-266-4, by calcein-AM fluorometry screening assay. | Not found. | [168,169,170] |
| Passiflora caerulea (Family: Passifloraceae) | Chrysin (124) | Inhibits BCRP protein expression. | In combination with mitoxantrone shows significant P-gp inhibition in MCF-7MS-100 cells. | IC50 values of mitoxantrone (alone) is 199 ± 19.3µM, while in combination with chrysin IC50 value is reduced to 1.13 ± 1.11 µM. | [138] |
| Pinus massoniana (Family: Pinaceae), Citrus paradise (Family: Rutaceae) | Procyanidine (125) | Inhibits P-gp ATPase in BBB. Reverses P-gp associated MDR by inhibiting the function and expression of P-gp through down-regulation of NF-κB activity and MAPK/ERK pathway mediated YB-1 nuclear translocation. | Inhibits P-gp in BBB and acts on cerebral tumors. | Not found. | [171,172] |
| Procyanidine potentiates paclitaxel and adriamycin concentration in MDR human ovarian cancer cell line (A2780/T). | IC50 values of paclitaxel and adriamycin are reduced to 11.36 ± 1.13 and 6.30 ± 0.38 µM, respectively. | ||||
| Prunus armeniaca (Family: Rosaceae) | Isoquercetin (126) | Reduces mRNA expression of P-gp. | Reduces P-gp expression. | Not found. | [5] |
| Robinia pseudoacacia (Family: Fabaceae) | Acacetin (127), Robinin (128) | Stimulate ATPase activity and inhibits MRP1 expression. Also act as natural substrates of BCRP and competitively inhibits BCRP-mediated drug efflux. Inhibits P-gp via synergism with substrate. | Inhibit MRP1 mediated BCECF efflux in human erythrocytes. Show P-gp inhibition in K562/BCRP cell line and potentiate mitoxantrone, SN-38, topotecan accumulation. Potentiate activity of epirubicin in MDR protein-expressing human breast cancer cell line (MDA-MB-231). | IC50 value for acacetin in human erythrocyte is 6.5 ± 4 µM/L. ID50 values for robinin + epirubicin is 0.02 µg/mL | [125,168,173] |
| Salicornia herbacea (Family: Amaranthaceae), Hippophae rhamnoides (Family: Elaeagnacae) | Isorhamnetin (129) | Co-transporting of isorhamnetin across Caco-2 cells monolayer may cause competitive substrate inhibition of P-gp, MRP-2 and BCRP. Also inhibits bacterial TetK efflux pump. | It inhibited P-gp function in Caco-2 cells. It also enhanced isoniazid activity in Mycobacterium smegmatis mc2155 | Not found. | [156,174] |
| Sasa borealis (Family: Poaceae) | Tricin (130) | Not found. | Shows inhibitory effects on the P-gp in adriamycin-resistant human breast cancer cells, MCF-7/Adr. | Not found. | [175] |
| Several plant species under Fabaceae family | Rotenone (131), Formononetin (132), Afrormosin (133) | Inhibit P-gp via synergism with substrate. | Rotenone potentiates the activity of epirubicin in human mdr1 gene-transfected mouse lymphoma cell line. | ID50 value for rotenone + epirubicin is 0.006 µg/mL. | [125] |
| Formononetin potentiates the activity of epirubicin in MRP-expressing human breast cancer cell line (MDA-MB-231). | ID50 value for formononetin + epirubicin is 0.02 µg/mL. | ||||
| Afrormosin potentiates the activity of epirubicin in MRP-expressing human breast cancer cell line (MDA-MB-231). | ID50 value for afrormosin + epirubicin is 0.06 µg/mL. | ||||
| Scutellaria baicalensis (Family: Lamiaceae) | Wogonin (134) | Not found. | Potentiates antitumor action of etoposide through inhibition of its efflux via P-gp tranporters in Jurkat cells and A549 cells. | Not found. | [176] |
| Baicalein (135) | Not found. | Inhibits P-gp efflux pump in the small intestine. | Not found. | [139,177] | |
| Inhibits TetK efflux pump. | Potentiates antimicrobial activity of ethidium bromide on. Mycobacterium smegmatis. | Not found. | |||
| Silybum marianum (Family: Asteraceae) | Silymarin (136), Silybin (137) | Not found. | Silymarin increases daunomycin accumulation in P-gp positive cells. | Not found. | [137,178,179] |
| Silymarin also inhibits P-gp mediated digoxin and vinblastine transport in Caco-2 cell line. | Not found. | ||||
| Doxorubicin cytotoxicity in MDA435/LCC6MDR1 cell line was increased by silymarin via inhibition of P-gp. | IC50 value of doxorubicin is reduced to 8.74 ± 5.88 µM in MDA435/LCC6MDR1 cells. | ||||
| In human prostate carcinoma DU145 cells, silybin potentiates doxorubicin induced toxicity. | Not found. | ||||
| Thalassia testudinum (Family: Hydrocharitaceae) | Luteolin (138), Luteolin-4′-O-glucoside (139) | Not found. | Show P-gp inhibition in K562/BCRP cells and potentiate mitoxantrone, SN-38, topotecan accumulation. | Not found. | [173] |
| Various plant species | Apigenin (140) | Inhibits BCRP protein expression. | Shows significant inhibition of mitoxantrone efflux in MCF-7 MS100 cells. | In combination IC50 value of mitoxantrone is 1.73 ± 1.42 µM | [138] |
| Vicia orobus (Family: Fabaceae) | Diosmetin (141) | Not found. | Inhibits P-gp in BBB and is determined in PBCECs (porcine brain capillaries and capillary endothelial cells) by the calcein assay. It also produces cytotoxicity in CEM/Adr5000 cell line via P-gp inhibition. | EC50 value of calcein in PBCEC is reduced to 16.3 ± 8.2 µg/L.IC50 value in CEM/ADR5000 cell is reduced to 3.5 ± 1.3 µg/L. | [180] |
| Vitis vinífera (Family: Vitaceae) | Resveratrol (142) | Not found. | Promotes fexofenadin absorption in rat intestine and bioavailability of nicardipine via P-gp inhibition. It increases accumulation of daunorubicin in KB-C2 cells. It inhibits the P-gp activity in P-gp overexpressing MCF-7/Adr cells. Cytotoxicity of vincristine, adriamycin and paclitaxel was enhanced by reveratrol on KBv200 cells. | Not found. | [181,182,183,184] |
| Terpenoids | |||||
| Bipolaris leersiae (Family: Pleosparaceae) | Ophiobolin A (143) | Not found. | Potentially Inhibites P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] |
| Cecropia lyratiloba (Family: Moraceae) | Euscaphic acid (144), tormentic acid (145), 2-alpha-acetyl tormentic acid (146), 3-beta-acetyl tormentic acid (147) | Inhibit expression of P-gp and reverse MDR. | Show reversal of MDR activity in MDR leukemia cells (K562/VCR). | Not found. | [185] |
| Clavija procera (Family: Theophrastaceae) | Aegicerin (148) | Not found. | Shows reversal of MDR activity in resistant Mycobacterium tuberculosis. | Not found. | [186] |
| Crocus sativus (Family: Iridaceae) | Safranal (149) | Not found. | Potentially Inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] |
| Cymbopogon citrus (Family: Poaceae) | Citral (150) | Directly inhibits MRP1 and MRP2 via binding with their active sites. | A significant inhibition is observed in both MRP1 and MRP2 in isolated Sf9-MRP1- and Sf9-MRP2-membrane vesicles. | Not found. | [187] |
| Eucalyptus dives (Family: Myrtaceae) | Piperitone (151) | Not found. | Inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] |
| Euphorbia dendroides (Family: Euphorbiaceae) | Euphodendroidin D (152) | Inhibits P-gp activity via binding with its active sites. | Prevents daunomycin efflux from K562/R7 human leukemic cells via P-gp inhibition. | Not found. | [188] |
| Euphorbia lagascae (Family: Euphorbiaceae) | Jolkinol D (153) | Inhibits P-gp activity via binding with its active sites. | Enhances doxorubicin cytotoxicity synergistically on human MDR-1 gene transfected mouse lymphoma cells. | IC50 value of doxorubicin is reduced to 0.26 ± 0.05 µM. | [189] |
| Euphorbia lagascae (Family: Euphorbiaceae) | Latilagascene B (154), Latilagascene E (155), Latilagascene D (156) | All compounds inhibit P-gp mediated MDR via directly blocking its active sites. | Latilagascene B, latilagascene E and latilagascene D show synergistic activity with doxorubicine on MDR-1 gene-transfected L1210 mouse lymphoma cells. | Not found. | [190] |
| Euphorbia mellifera (Family: Euphorbiaceae) | Euphomelliferine A (157), Euphomelliferine (158) | Inhibit P-gp activity via binding with its active sites. | Successfully inhibit P-gp activity on human MDR colon adenocarcinoma (MDR COLO-320) and human MDR1 gene transferred mouse (L5178Y MDR) cells. | Not found. | [191] |
| Euphorbia paralias (Family: Euphorbiaceae) | Paraliane (159) | Inhibits P-gp activity via binding with its active sites. | Shows P-gp inhibitory action on MDR-1 gene-transfected L1210 mouse lymphoma cells. | Not found. | [192] |
| Euphorbia peplus (Family: Euphorbiaceae) | Pepluanin A (160) | Inhibits P-gp activity via binding with its active sites. | Shows P-gp inhibitory action on MDR-1 gene-transfected L1210 mouse lymphoma cells; promotes Rh 123 and epirubicin accumulation. Pepluanin A prevents daunomycin efflux from K562/R7 human leukemic cells via P-gp inhibition. | Not found. | [188,192] |
| Euphorbia piscatoria (Family: Euphorbiaceae) | Jolkinol B (161) | Inhibits P-gp activity via binding with its active sites. | Shows P-gp inhibition on human MDR-1 gene transfected and parenteral L5178 mouse lymphoma cells. | Not found. | [190] |
| Euphorbia portlandica (Family: Euphorbiaceae) | Euphoportlandol A (162), Euphoportlandol B (163) | Inhibit P-gp activity via binding with its active sites. | Reversal of MDR was evaluated via Rh 123 exclusion in L5178 mouse lymphoma cells transfected with the pHa MDR1/A gene. | Not found. | [193] |
| Euphorbia spp. (Family: Euphorbiaceae) | Helioscopinolide A (164), Helioscopinolide B (165), Helioscopinolide E (166), Helioscopinolide F (167) | Inhibit P-gp activity via binding with its active sites. | Exhibit high anti-neoplastic activity against human MDR-1 gene-transfected mouse lymphoma cells. | Not found. | [194] |
| Euphorbia tuckeyana (Family: Euphorbiaceae) | Tuckeyanols A (168), Tuckeyanols B (169), Euphotuckeyanol (170) | Inhibit P-gp activity via binding with its active sites. | All show P-gp inhibition on human MDR-1 gene transfected and parenteral L5178 mouse lymphoma cells and potentiate epirubicin action. | Not found. | [195] |
| Gentiana verna (Family: Gentianaceae) | Loganine (171) | Not found. | Inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] |
| Glycyrrhiza glabra (Family: Fabaceae) | Glycyrrhizin (172), | Inhibit P-gp ATPase activity. | Inhibits P-gp in MDR1-MDCKII and Caco-2 cell. | 21.78 µM | [196] |
| Not found. | Potentially Inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] | ||
| Laurencia fliformis (Family: Rhodomelaceae) | Parguerene I (173), Parguerene II (174) | Inhibits both P-gp and MRP1. | Both show reversal of vinblastine, doxorubicin and paclitaxel resistance in SW620 AD-300, HEK293/ABCB1, CEM/VLB100 cells. | Not found. | [7] |
| Licania tomentosa, Chrysobalanus icaco (Family: Chrysobalanaceae) | Betulinic acid (175), pomolic acid (176) | Not found. | Inhibit the proliferation of a vincristine-resistant derivative of K562 cells and reduced MDR activity. | Not found. | [197] |
| Maytenus spp. (Family: Celastraceae) | Dihydro-β-agarofuran (177) | Similar to verapamil. | It is found to inhibit leucine uptake in LNCaP cells. It also shows higher P-gp reversal activity on human MDR1-transfected NIH-3T3 cells. Dihydro-β-agarofuran was observed as an inhibitor of a P-gp like transporter in multidrug-resistant Leishmania tropica. | Not found. | [198,199,200] |
| Olea europea (Family: Oleaceae) | Oleanolic acid (178) | Not found. | Potentially inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] |
| Origanum vulgare (Family: Lamiaceae) | Carvacrol (179) | Not found. | Potentially inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] |
| Pastinaca sativa (Family: Apiaceae) | Terpinolene (180) | Not found. | Potentially inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] |
| Phellodendron amurense (Family: Rutaceae) | Obacunone (181) | Shows P-gp mediated MDR inhibition activity. | Shows P-gp inhibition on MES-SA/DX5 (human MDR uterine sarcoma cells) and HCT15 cells (Human colorectal cancer cell line). | ED50 values are 0.028 and 0.0011 µg/mL, respectively. | [201] |
| Phellodendron amurense (Family: Rutaceae) | Limonin (182) | Shows P-gp mediated MDR inhibition activity. | Shows P-gp inhibition on MES-SA/DX5 (human MDR uterine sarcoma cells) and HCT15 cells (Human colorectal cancer cell line) | ED50 values are 0.021 and 0.392 µg/mL, respectively. | [100,201] |
| Pinus nigra (Family: Pinaceae) | Isopimaric acid (183) | Inhibits microbial TetK or NorA efflux pumps. | Potentiate antibiotic activity in Staphylococcus aureus. | Not found. | [202] |
| β-myrcene (184) | Not found. | Potentially inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] | |
| Podocarpus totara (Family: Podocarpaceae) | Totarol (185) | Inhibits NorA efflux pump. | Inhibits Staphylococcus aureus NorA efflux pump. | Not found. | [203] |
| Sinocalycanthus chinensis (Family: Calycanthaceae) | Sinocalycanchinensin E (186) | Not found. | Shows reversal of MDR activity in MDR KB cells and enhanced colchicines induced cytotoxicity. | Not found. | [204] |
| Siphonochalina siphonella (Family: Callyspongidae) | Sipholenol A (187), Sipholenol L (188) | Show P-gp mediated MDR inhibition activity. | Enhance cytotoxicity of P-gp substrate in KB C2 cells and human cervix carcinoma subclone derived from KB-3 1cells via P-gp inhibition. | Not found. | [205,206] |
| Tamarindus indica (Family: Fabaceae) | Lupeol (189) | Not found. | Potentially inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] |
| Thymus vulgaris (Family: Lamiaceae) | Thymol (190) | Not found. | Potentially inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] |
| Zanthoxylum piperitum (Family: Rutaceae) | Citronellal (191), Citronellol (192) | Not found. | Potentially inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] |
| Saponins, Sapogenins and Sterols | |||||
| Astragalus membranaceus (Family: Fabaceae) | Astragaloside II (193) | Downregulates the expression of the P-gp and MDR1 genes. | Astragaloside II in low concentration shows strong potency to increase 5-fluorouracil cytotoxicity toward 5-fluorouracil-resistant human hepatic cancer cells Bel-7402/FU. It also downregulates the P-gp and MDR1 genes. | Not found. | [207] |
| Citrus jambhiri, Citrus pyriformis (Family: Rutaceae) | Stigmasterol (194), β-sitosterol-O-glucoside (195) | Not found. | Inhibit P-gp in Caco-2 cells and enhance accumulation of Rh 123. | Not found. | [148] |
| Cucurbita andreana, Hemsleya endecaphylla, Ecballium elaterium, Citrullus colocynthis etc. (Family: Cucurbitaceae) | Cucurbitacin I (196) | Not found. | Potentially inhibits P-gp mediated efflux of [3H] digoxin in LLCGA5-COL150 cells. | Not found. | [6] |
| Dioscorea opposite (Family: Dioscoreaceae) | Gracillin (197) | Inhibits P-gp via direct interaction with active binding sites. | Shows inhibition of P-gp mediated daunorubicin efflux in K567/R7 cells (human leukemic). | Not found. | [208] |
| Isis hippuris (Family: Isididae) | Gorgosterol (198), Hippuristanol (199) | Not found. | Show P-gp inhibitory activity on KB-C2 cells. | Not found. | [7] |
| Labisia pumila (Family: Primulaceae) | Primulanin (200) | Not found. | Inhibits P-gp mediated drug efflux, tested in hMDR1-MDCKII cells using 3H-digoxin. | IC50 value is 6.4 ± 2.3 µM. | [209] |
| Marsdenia tenacissima (Family: Apocynaceae) | Tenacissimoside A (201) | Modulates P-gp mediated MDR through direct interaction with P-gp substrate site. | Reverses MDR in P-gp overexpressing MDR cancer cells (HepG2/Dox). The sensitivity of HepG2/Dox cells to antitumor drugs doxorubicin, vinblastine, puromycin and paclitaxel was increased by 18-, 10-, 11- and 6-fold by 20 µg/mL (or 25 µM) in presence of tenacissimoside A. | Not found. | [210] |
| Momordica balsamina (Family: Cucurbitaceae) | Karavilagenin C (202), Balsaminol (203), balsaminagenin B (204), balsaminoside A (205) | Karavilagenin C inhibits Rv1258c efflux pump. Karavilagenin, balsaminol and balsaminagenin inhibit AcrAB-TolC efflux pump. Show synergistic interactions with P-gp substrate and inhibit P-gp function | Karavilagenin C enhances antimicrobial activity of ethidium bromide on Enterococcus faecalis. Karavilagenin, balsaminol and balsaminagenin potentiate actimicrobial activity in S. aureus and E. coli. Balsaminagenin B, balsaminoside A and karavilagenin C show MDR reversing activity on human MDR1 gene transfected mouse lymphoma cells and enhance cytotoxicity of doxorubicin. | Not found. | [211,212] |
| Panax ginseng (Family: Araliaceae) | Protopanaxatriol (206), 20(S)-ginsenoside F1 (207) | Protopanaxatriol directly inhibits P-gp mediated substrate transport. Ginsenoside F1 inhibits P-gp ATPase activity. | Directly inhibit P-gp in daunorubicin- and doxorubicin-resistant acute myelogenous leukemia sublines (AML-2/D100 and AML-2/DX100) and enhance daunorubicin concentration inside cell. 20(S)-ginsenoside F1 shows P-gp inhibitory activity on MDR1-MDCKII and Caco-2 cells. | Not found. | [196,213] |
| Paris polyphylla (Family: Melianthaceae) | Pennogenine (208) | Inhibits P-gp via direct interaction with active binding sites. | Inhibits P-gp-mediated daunorubicin efflux in K562/R7 cells. | Not found. | [208] |
| Tacca chantrieri (Family: Dioscoreace) | Teccalonolides A (209), Teccalonolides E (210), Teccalonolides B (211), Teccalonolides N (212) | Not found. | All of the teccalonolides are active against P-gp expressed and MRP7 transfacted MDR cancer cells. Taccalonolides A and E are highly active in vivo against a doxorubicin- and paclitaxel- resistant P-gp-expressing tumor (Mam17/ADR) and also bind with tubuline. | Not found. | [214] |
| Spongia spp. (Family: Spongiidae) | Agosterol A (213) | Inhibits ATP-dependent drug efflux by P-gp and MRP1. | Reverses the resistance to colchicine in KB-C2 cells and also reverses the resistance to vincristine in KBCV60 cells via P-gp and MRP inhibition. | Not found. | [7,215] |
| Trillium tschonoskii (Family: Trilliaceae) | Paris saponin VII (214) | Inhibits P-gp ATPase activity. | It reverses MDR in adriamycin-resistant MCF-7/ADR cells and intracellular Rh 123 accumulation is increased via P-gp inhibition. | Not found. | [195] |
| Vegetables oils, legumes, nut, seeds | β-sitosterol (215), Z-guggulsterone (216) | Not found. | β-sitosterol shows P-gp inhibitory activity in multidrug resistant NCI/ADR-RES cell line. Z-guggulsterone enhances accumulation of daunorubicin or Rh 123 in P-gp-overexpressing human carcinoma KB-C2 cells and human MRP1 gene-transfected KB/MRP cells via P-gp inhibition. | Not found. | [216] |
| Vitex scabra (Family: Verbenaceae) | Pinnatasterone (217) | Inhibits P-gp via direct interaction with active binding sites. | Shows inhibition of P-gp-mediated daunorubicin efflux in K562/R7 (human leukemic) cells. | Not found. | [195] |
| Coumarins | |||||
| Angelica gigas (Family: Apiaceae) | Decursinol (218) | Inhibits P-gp, MRP-2 and BCRP via acting as substrate. | Acts on Caco-2 cell monolayer and inhibites efflux transporter like BCRP/MDP 2. | Not found. | [217] |
| Calophyllum brasillense (Family: Clusiaceae) | GUT 70 (219) | Inhibits P-gp expression. | Acts by inhibiting the P-gp activity at human leukemia cells. | 2–5 µM | [218] |
| Citrus hybrids (Family: Rutaceae) | Bergamottin (220), 6′,7′-dihydroxy bergamottin (221), 6′,7′-epoxy bergamottin (222) | Inhibit P-gp expression. | Inhibit P-gp (ABCB1) mediated transport of talinolol in Caco-2 cells. | Not found. | [143,144] |
| Citrus paradisi (Family: Rutaceae) | Bergaptol (223) | Specific inhibitor of P-gp and/or MRP2 function. | Inhibited [3H]-Vinblastine efflux from LLC-GA5-COL300 cells (a transformant cell line derived by transfecting LLC-PK1 with human MDR1 cDNA isolated from normal adrenal gland). Also inhibites P-gp function in human breast cancer cells. | Not found. | [219,220] |
| Ferula persica (Family: Umbelliferae) | Farnesiferol A (224), Farnesiferol B (225), Farnesiferol C (226), Lehmferin (227) | Inhibit P-gp active substrate binding sites. | Farnesiferol A is observed as a potential P-gp inhibitor tested via Rh 123 efflux assay in doxorubicin resistant breast cancer cell line (MCF7/Adr). Farnesiferol B, farnesiferol C and lehmferin inhibit P-gp efflux pump and enhanced performance of doxorubicin on breast cancer cell line (MCF7/ADR). | IC50 values for doxorubicin + farnesiferol B is 10.68 μM, doxorubicin + farnesiferol C is 6.72 μM and doxorubicin + lehmferin is 5.08 μM | [221,222] |
| Ferula schtschurowskiana (Family: Umbelliferae) | Conferone (228) | Inhibits P-gp via competitive binding with P-gp active sites. | Inhibits efflux of vinblastine in MDCK-MDR1 cells. | Not found. | [223] |
| Ferula szowitsiana (Family: Umbelliferae) | Galbanic acid (229) | Inhibits P-gp via competitive binding with P-gp active sites. Also inhibits NorA or NorB efflux pump | Galbanic acid is observed as a potential P-gp inhibitor tested via Rh 123 efflux assay in doxorubicin resistant breast cancer cells (MCF7/Adr). Enhances the performance of Ethidium bromide in Staphylococcus aureus. | Not found. | [222,224] |
| Peucedanum praeruptorium (Family: Oenanthe) | Praeruptorin A (230) | Inhibits P-gp fuction via depleting ATP and/or suppressing P-gp gene expression. | Inhibits P-gp mediated drug resistance for doxorubicin, paclitaxel, puromycin and vincristine in MDR human oral epidermoid carcinoma cells (KB-V1). | Not found. | [225] |
| Tordylium opulum (Family: Apiaceae) | Cnidiadin (231) | Acts as chemo-sensitiser for P-gp and inactivates it via blocking its efflux function. | Enhances vinblastine or vincristine performance in two cell lines overexpressing P-gp namely, MDCK-MDR1 and KB/VCR cells. | Not found. | [226] |
| Peptides | |||||
| Discodermia dissoluta (Family: Theonellidae) | Discodermolide (232) | Not found. | Reverses the resistance of paclitaxal in ovarian carcinoma cells (A2780AD). | 580 nM. | [227] |
| Reverses paclitaxal resistance in colon carcinoma cells (SW620AD-300). | 70 nM. | ||||
| Haliclona caerulea (Family: Chalinidae) | Kendarimide (233) | Reverses P-gp mediated MDR. | Reverses the resistance to colchicin in human carcinoma cells (KB-C2). | Not found. | [228] |
| Hapalosiphon welwitschii (Family: Hapalosiphonaceae) | Hapalosin (234) | Reverses P-gp mediated efflux via direct inhibition of efflux mechanism. | Reverses MDR in P-gp overexpressing, vinblastine-resistant human ovarian adenocarcinoma cell line with higher effect than the known P-gp inhibitor verapamil. | Not found. | [229] |
| Nocardiopsis spp. (Family: Nocardiopsaceae) | Nocardioazine A (235) | Inhibits membrane bound P-gp efflux protein. | Reverses the resistance to doxorubicin in SW620AD-300 cells. | Not found. | [7,230] |
| Resins | |||||
| Garcinia hamburyi (Family: Clusiaceae) | Gambogic acid (236) | Dose dependently inhibits ABCB1 activity. It directly inhibits ABCB1 and protein degradation of ABCB1 via the proteasome pathway. | In the MCF-7/Adm cells, gambogic acid enhances the cytotoxicities of docetaxel and adriamycin. | Not found. | [231] |
| Ipomoea violacea (Family: Convolvulaceae) | Orizabin (237) | Inhibits NorA efflux pump. | Reverses norfloxacin resistance in Staphylococcus aureus. | Not found. | [232] |
| Miscellaneous | |||||
| Alpinia galangal (Family: Zingiberaceae) | Acetoxy cavicolacetate (238) | Inhibits NorA efflux pump. | Potentiates the activity of ethidium bromide in Staphylococcus aureus. | Not found. | [233] |
| Arctium lappa (Family:Astereceae) | Arctigenin (239), Matairesinol (240), Arctiin (241), Isolappaol A (242), Lappaol C (243), Lappaol F (244) | Show synergistic activity with the cytotoxic drugs. | Potentiate doxorubicin mediated cytotoxicity in CaCo2 and CEM/ADR5000 cell lines. | Not found. | [234] |
| Ascidian of genus Didemnun (Family: Didemnidae) | Ningalin B (245) | Not found. | Inhibits P-gp function in P-gp overexpressing human colorectal carcinoma (HCT116/VM46) cells and increases sensitivity to vinblastine and doxorubicin. | Not found. | [235] |
| Berberis aetnensis (Family: Berberidaceae) | Porphyrin (246), pheophorbide A (247) | Pheophorbide A inhibits MexAB-OprM efflux pump. Porphyrin and pheophorbide also inhibit NorA efflux. | Pheophorbide A enhances the activity of Ciprofloxacin in Pseudomonas aeruginosa. Porphyrin and pheophorbide enhance the activities of ciprofloxacin and norfloxacin. | Not found. | [236] |
| Bugula neritina (Family: Bugulidae) | Bryostatin 1 (248) | Not found. | Reverses resistance of colchinin in KB-1 and human epitheliod cervix carcinoma cells. | Not found. | [237] |
| Cannabis sativa (Family: Cannabaceae) | Cannabinol (249), Cannabidiol (250) | Inhibit P-gp ATPase activity. Alter P-gp and BCRP mRNA expression. | Both stimulate the activity of ATPase activity of P-gp. On other hand, cannabidiol inhibited the verapamil stimulated ATPase activity of P-gp. Rh 123 and cyclosporine A accumulation is increased by cannabidiol in MCF-7/P-gp and choriocarcinoma cells. | Not found. | [238,239] |
| Cinnamonum camphora (Family: Lauraceae) | Anethole (251) | Not found. | Inhibites P-gp efflux in hepatocellular carcinoma cells. | Not found. | [234,240] |
| Eugenia caryophyllus (Family: Myrtaceae) | Eugenol (252) | Not found. | Inhibites P-gp efflux in hepatocellular carcinoma cells. | Not found. | [241] |
| Geranium caespitosum (Family: Geraniaceae) | Polyacylated neohesperidosides (253) | Inhibits NorA Efflux pump. | Enhances the activities of ciprofloxacin, norfloxacin, rhein, berberine in Staphylococcus aureus. | Not found. | [242] |
| Nicotiana tabacum (Family: solanaceae), Dalea versicolor (Family: Fabaceae) | Chalcone (254) | Inhibits NorA efflux pump. | Reverses resistance of berberine, erythromycin and tetracycline in Bacillus cereus and Staphylococcus aureus. | Not found. | [243] |
| Prunella vulgaris (Family: Lamiaceae) | Crude extract | Not found. | Inhibits Ebola virus glycoprotein (GP)-mediated virus entry and infection in different cell lines like HUVEC and macrophage. | Not found. | [244] |
| Schisandra chinensis (Family: Schisandraceae) | Gomisin (255), Pregomisin (256) | Act as uncompetitive inhibitor for P-gp-ATPase activity.Alter P-gp substrate interactions, noncompetitively | Show MDR phenomenon on human hepG2 hepatoma cells. | Not found. | [245] |
| Zingiber officinalis (Family: Zingiberaceae) | Phenylbutanoids (257) | Inhibits P-gp mediated MDR expression. | Shows potent P-gp inhibitory effect on breast cancer cell line (MCF-7/ADR) and enhances daunomycin uptake. | Not found. | [246] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; Feo, V.D.; Zia-Ul-Haq, M. Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition. Molecules 2017, 22, 871. https://doi.org/10.3390/molecules22060871
Dewanjee S, Dua TK, Bhattacharjee N, Das A, Gangopadhyay M, Khanra R, Joardar S, Riaz M, Feo VD, Zia-Ul-Haq M. Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition. Molecules. 2017; 22(6):871. https://doi.org/10.3390/molecules22060871
Chicago/Turabian StyleDewanjee, Saikat, Tarun K. Dua, Niloy Bhattacharjee, Anup Das, Moumita Gangopadhyay, Ritu Khanra, Swarnalata Joardar, Muhammad Riaz, Vincenzo De Feo, and Muhammad Zia-Ul-Haq. 2017. "Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition" Molecules 22, no. 6: 871. https://doi.org/10.3390/molecules22060871







