Evaluation of the Cardiotoxicity of Evodiamine In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. In Vitro Cardiotoxicity in Primary Neonatal Rat Cardiomyocytes
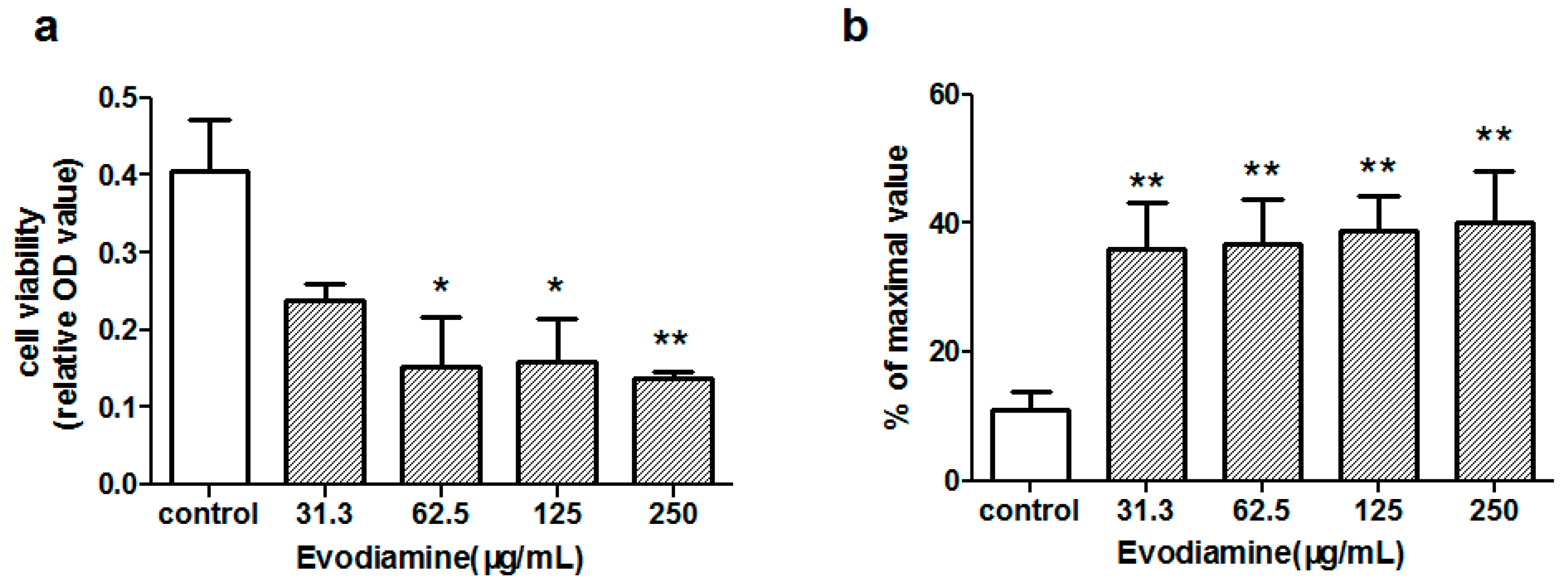
2.1.1. Evodiamine-Induced Changes in Cardiomyocyte Viability
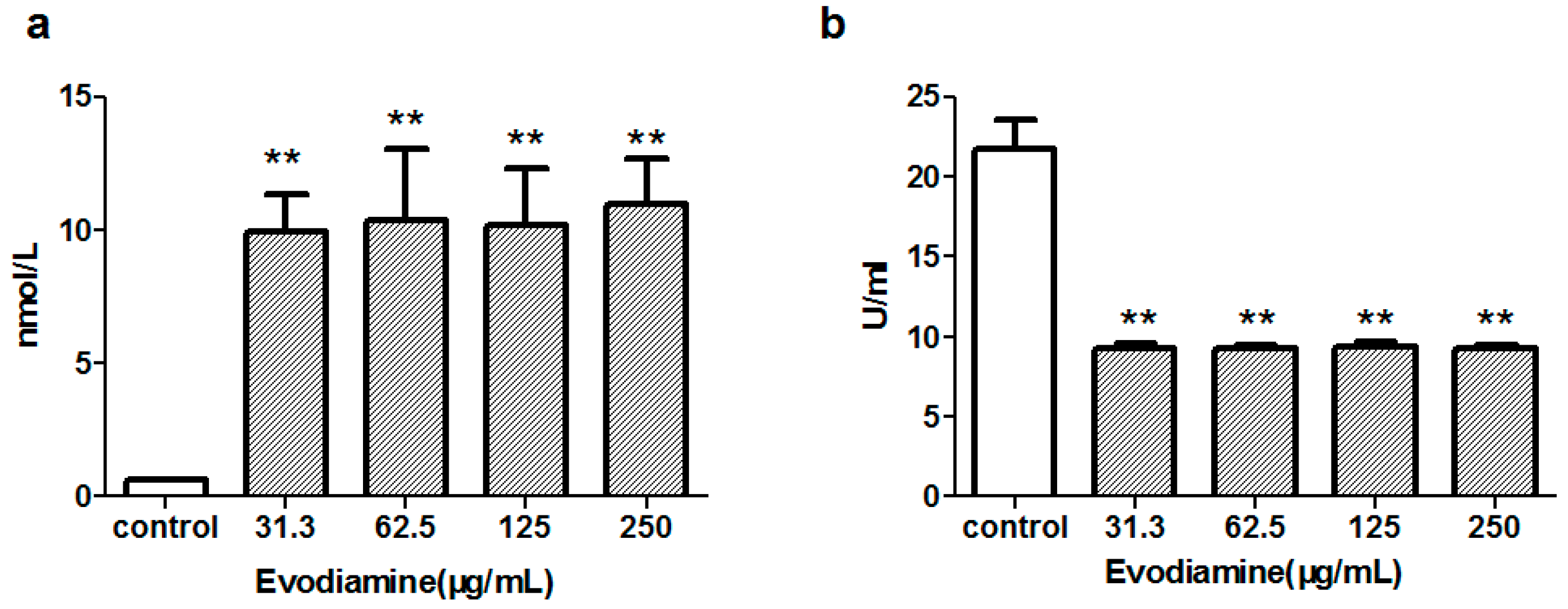
2.1.2. Evodiamine-Induced Changes in Maleic Dialdehyde (MDA) Levels and Superoxide Dismutase (SOD) Activity
2.2. In Vivo Cardiotoxicity in Zebrafish
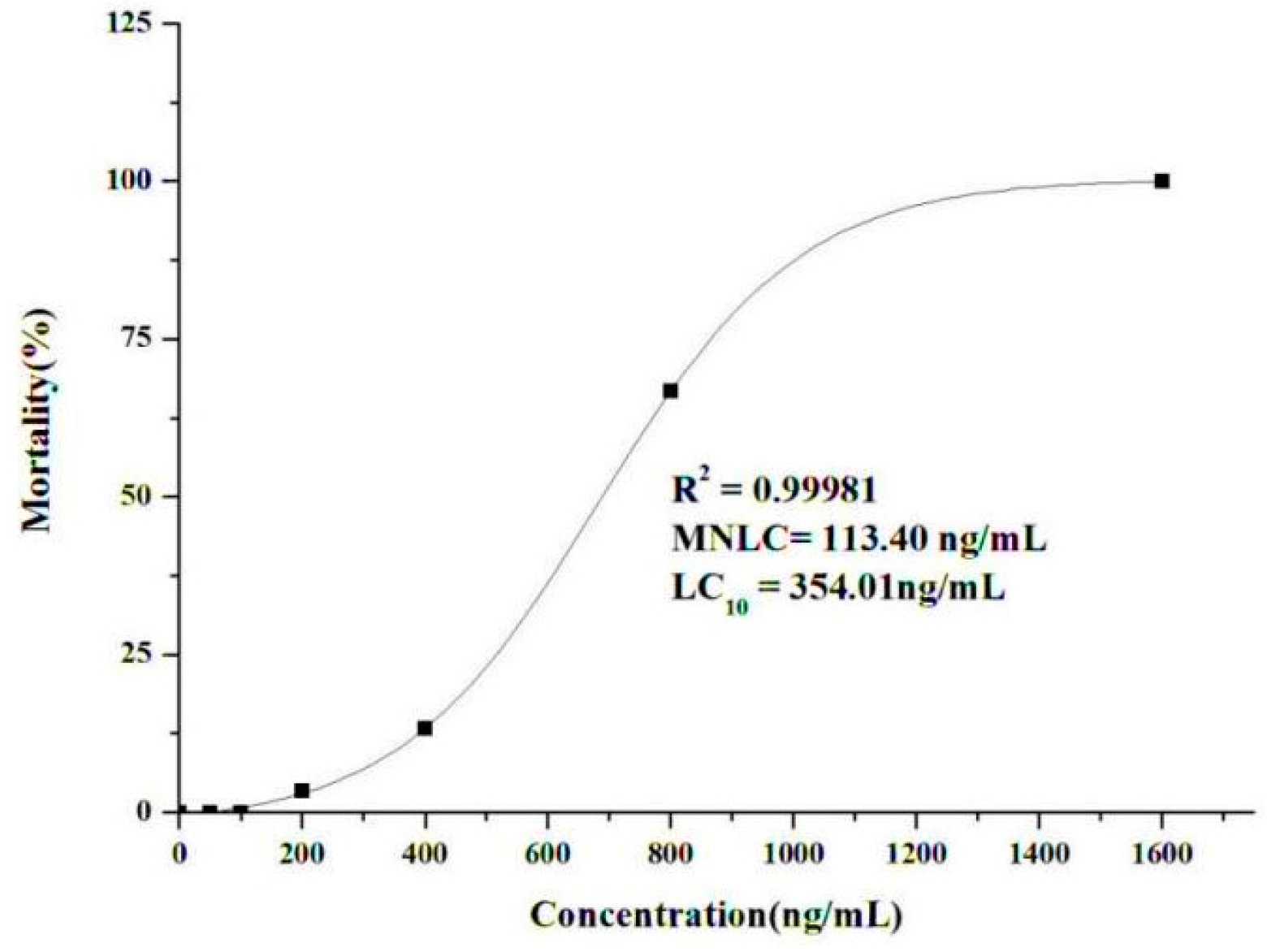
2.2.1. Determination of the Maximum Non-Lethal Concentration (MNLC) and 10% Lethal Concentration (LC10)
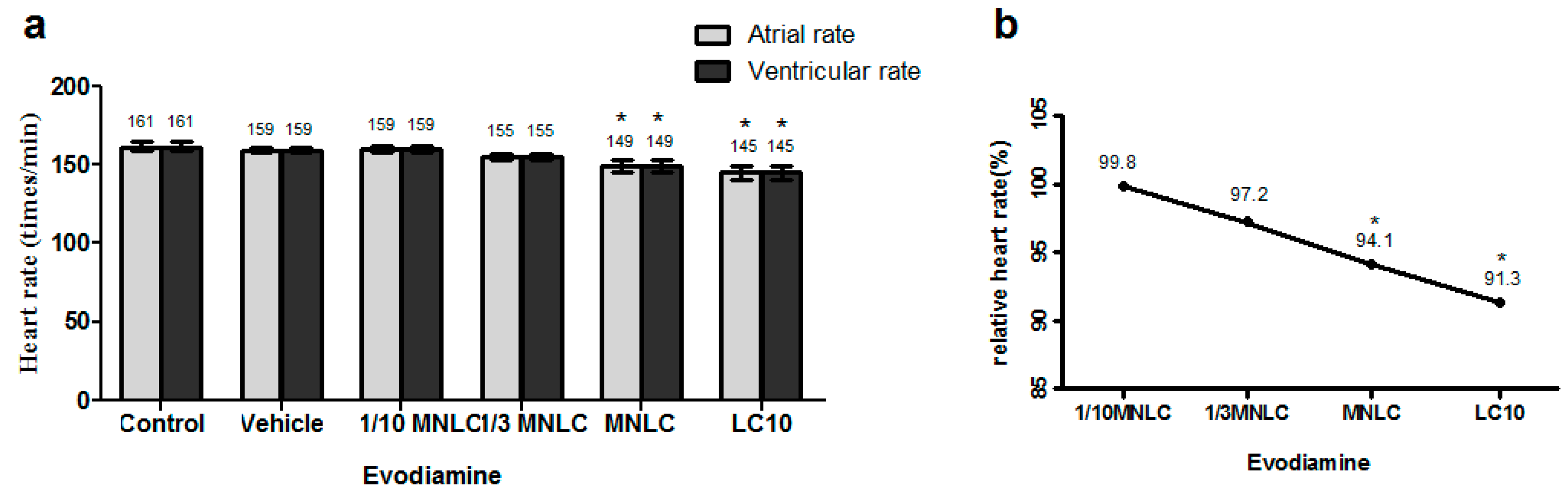
2.2.2. Evodiamine-Induced Effects on Heart Rate and Rhythm
2.2.3. Morphological Assessment of Cardiotoxicity
3. Discussion
4. Materials and Methods
4.1. Chemicals, Drugs and Reagents
4.2. Animals
4.3. In Vitro Cardiotoxicity in Primary Neonatal Rat Cardiomyocytes
4.3.1. Experimental Protocol
4.3.2. Isolation and Culture of Primary Neonatal Rat Cardiomyocytes
4.3.3. Cell Viability Assay
4.3.4. Determination of LDH Release, MDA and SOD Activity
4.4. In Vivo Cardiotoxicity in Zebrafish
4.4.1. Experimental Protocol
4.4.2. Determination of the MNLC and LC10
4.4.3. Cardiovascular Toxicity Assessment
4.4.4. Morphological Assessment
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Madan, A.K.; Bajaj, S.; Dureja, H. Classification Models for Safe Drug Molecules. In Computational Toxicology; Reisfeld, B., Mayeno, A.N., Eds.; Humana Press: New York, NY, USA, 2013; Volume 930, pp. 99–124. [Google Scholar]
- Toropov, A.A.; Toropova, A.P.; Raska, I., Jr.; Leszczynska, D.; Leszczynski, J. Comprehension of drug toxicity: Software and databases. Comput. Biol. Med. 2014, 45, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wang, X.Z.; Zhang, L.; Kang, L.; Yang, C.; Zhu, Y.L.; Ye, Z.G.; Qian, X.P. QSAR Study on Rat Cardiotoxicity of Chemical Component of Chinese Herbs. World Sci. Technol. Mod. TCM Mater. Med. 2015, 17, 1833–1837. [Google Scholar]
- National Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China, Part 1; The Medicine Science and Technology Press of China: Beijing, China, 2010; pp. 160–161. [Google Scholar]
- Tang, X.; Huang, Z.; Chen, Y.; Liu, Y.; Liu, Y.; Zhao, J.; Yi, J. Simultaneous determination of six bioactive compounds in Evodiae Fructus by high-performance liquid chromatography with diode array detection. J. Chromatogr. Sci. 2014, 52, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, C. Evodiamine: A Novel Anti-cancer Alkaloid from Evodia rutaecarpa. Molecules 2009, 14, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.F.; Chou, C.J.; Shum, A.Y.; Chen, C.F. The vasorelaxant effect of evodiamine in rat isolated mesenteric arteries: Mode of action. Eur. J. Pharmacol. 1992, 215, 277–283. [Google Scholar] [CrossRef]
- Hung, P.H.; Lin, L.C.; Wang, G.J.; Chen, C.F.; Wang, P.S. Inhibitory effect of evodiamine on aldosterone release by Zona glomerulosa cells in male rats. Chin. J. Physiol. 2001, 44, 53–57. [Google Scholar] [PubMed]
- Heo, S.K.; Yun, H.J.; Yi, H.S.; Noh, E.K.; Park, S.D. Evodiamine and rutaecarpine inhibit migration by light via suppression of NADPH oxidase activation. J. Cell. Biochem. 2009, 107, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Fang, C.; Wu, X.; Fan, J.; Dong, S. Perfluorooctane Sulfonate Impairs the Cardiac Development of a Marine Medaka (Oryzias melastigma). Aquat. Toxicol. 2011, 105, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Traditional Chinese Medicine Induced Liver Injury. J. Clin. Transl. Hepatol. 2014, 2, 80–94. [Google Scholar] [PubMed]
- Yang, X.W. Toxicological Assessment on Safety of Water and 70% ethanolic extracts of nearly ripe fruit of Evodia rutaecarpa. Zhongguo Zhong Yao Za Zhi 2008, 33, 1317–1321. [Google Scholar] [PubMed]
- Zhou, Q.; Zhang, Q.; Jin, R.M. Time-effect and Dose-effect of Evodia rutaecarpa on Hepatotoxicity in Mice. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 232–235. [Google Scholar]
- Huang, W.; Sun, R. Study on Chronic Toxicity of Water Extraction Components from Evodia fructus in Rats. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 269–273. [Google Scholar]
- Cai, Q.; Wei, J.; Zhao, W.; Shi, S.; Zhang, Y.; Wei, R.; Zhang, Y.; Li, W.; Wang, Q. Toxicity of Evodiae fructus on Rat Liver Mitochondria: The Role of Oxidative Stress and Mitochondrial Permeability Transition. Molecules 2014, 19, 21168–21182. [Google Scholar] [CrossRef] [PubMed]
- Zon, L.; Peterson, R.T. In vivo Drug Discovery in the Zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, S.; Marrs, J.A. Zebrafish as a Vertebrate Model System to Evaluate Effects of Environmental Toxicants on Cardiac Development and Function. Int. J. Mol. Sci. 2016, 17, 2123–2138. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.G.; Milan, D.J.; Grande, E.J.; Rottbauer, W.; MacRae, C.A.; Fishman, M.C. High-throughput Assay for Small Molecules that Modulate Zebrafish Embryonic Heart Rate. Nat. Chem. Biol. 2005, 1, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.F.; Wang, B.X.; Li, T.J.; Tashiro, S.; Minami, M.; Xing, D.J.; Ikejima, T. Evodiamine, a Constituent of Evodiae Fructus, Induces Anti-proliferating Effects in Tumor Cells. Cancer Sci. 2003, 94, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.J.; Tashiro, S.; Onodera, S.; Ikejima, T. Intracellular Regulation of Evodiamine-induced A375-S2 Cell Death. Biol. Pharm. Bull. 2003, 26, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.J.; Tashiro, S.; Onodera, S.; Ikejima, T. Evodiamine Induces Tumor Cell Death through Two Different Pathways: Apoptosis and Necrosis. Acta Pharmacol. Sin. 2004, 25, 83–89. [Google Scholar] [PubMed]
- Zhang, Y.; Zhang, Q.H.; Wu, L.J.; Tashiro, S.; Onodera, S.; Ikejima, T. A Typical Apoptosis in L929 Cells Induced by Evodiamine Isolated from Evodia rutaecarpa. J. Asian Nat. Prod. Res. 2004, 6, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, L.J.; Tashino, S.; Onodera, S.; Ikejima, T. Critical Roles of Reactive Oxygen Species in Mitochondrial Permeability Transition in Mediating Evodiamine-induced Human MelanomaA375-S2 cell Apoptosis. Free Radic. Res. 2007, 41, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, L.J.; Tashiro, S.; Onodera, S.; Ikejima, T. Nitric Oxide Activated by p38 and NF-kappaB Facilitates Apoptosis and Cell Cycle Arrest under Oxidative Stress in Evodiamine-treated Human Melanoma A375-S2 Cells. Free Radic. Res. 2008, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, L.J.; Tashino, S.; Onodera, S.; Ikejima, T. Reactive Oxygen Species and Nitric Oxide Regulate Mitochondria-dependent Apoptosis and Autophagy in Evodiamine-treated Human Cervix Carcinoma HeLa Cells. Free Radic. Res. 2008, 42, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, L.J.; Tashino, S.; Onodera, S.; Ikejima, T. Protein Tyrosine Kinase Pathway-derived ROS/NO Productions Contribute to G2/M Cell Cycle Arrest in Evodiamine-treated Human Cervix Carcinoma Hela Cells. Free Radic. Res. 2010, 44, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Pan, S.L.; Guh, J.H.; Chang, Y.L.; Pai, H.C.; Lin, C.H.; Teng, C.M. Antitumor Mechanism of Evodiamine, a Constituent from Chinese Herb Evodiae fructus, in Human Multipledrug Resistant Breast Cancer NCI/ADR-RES cells in vitro and in vivo. Carcinogenesis 2005, 6, 968–975. [Google Scholar]
- Menna, P.; Salvatorelli, E.; Minotti, G. Cardiotoxicity of Antitumor drugs. Chem. Res. Toxicol. 2008, 21, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Angsutararux, P.; Luanpitpong, S.; Issaragrisil, S. Chemotherapy-Induced Cardiotoxicity: Overview of the Roles of Oxidative Stress. Oxid. Med. Cell. Longev. 2015, 29, 13. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; McBride, R.B.; Eisenberger, A.; Wei, Y.T.; Grann, V.R.; Jacobson, J.S. Doxorubicin, Cardiac Risk Factors, and Cardiac Toxicity in Elderly Patients with Diffuse B-cell non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2008, 26, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Simunek, T.; Sterba, M.; Popelova, O.; Adamcova, M.; Hrdina, R.; Gersl, V. Anthracycline-induced Cardiotoxicity: Overview of Studies Examining the Roles of Oxidative Stress and Free Cellular Iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef]
- Ky, B.; Vejpongsa, P.; Yeh, E.T.; Force, T.; Moslehi, J.J. Emerging Paradigms in Cardiomyopathies Associated with Cancer Therapies. Circ. Res. 2013, 113, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced Cardiomyopathy: From Molecular Mechanisms to Therapeutic Strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Monica, L.; Giancarlo, G.; Elpidio, M.G.; Franca, A.; Antonia, F.; Stefania, P.; Vincenzo, T.; Di Marina, D. Animal Models in Studies of Cardiotoxicity Side Effects from Antiblastic Drugs in Patients and Occupational Exposed Workers. Biomed. Res. Int. 2014, 2014, 240642. [Google Scholar]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced Mitochondrial Dysfunction and Cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.E.; Abi Gerges, N.; Bridgland-Taylor, M.H.; Easter, A.; Hammond, T.G.; Valentin, J.P. An Introduction to QT Interval Prolongation and Non-clinical Approaches to Assessing and Reducing Risk. Br. J. Pharmacol. 2010, 159, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Yongfeng, Y.; Xiangyun, B.; Cunjin, L.; Kuanquan, W.; Henggui, Z. The Virtual Heart as a Platform for Screening Drug Cardiotoxicity. J. Pharmacol. 2015, 172, 5531–5547. [Google Scholar]
- Zhu, Y.L.; Ye, Z.G. Computational Toxicology and its Application in Toxicity Study of Traditional Chinese medicine. Chin. J. New Drugs 2011, 20, 2424–2429. [Google Scholar]
Sample Availability: Samples of the compound evodiamine are available from the authors. |
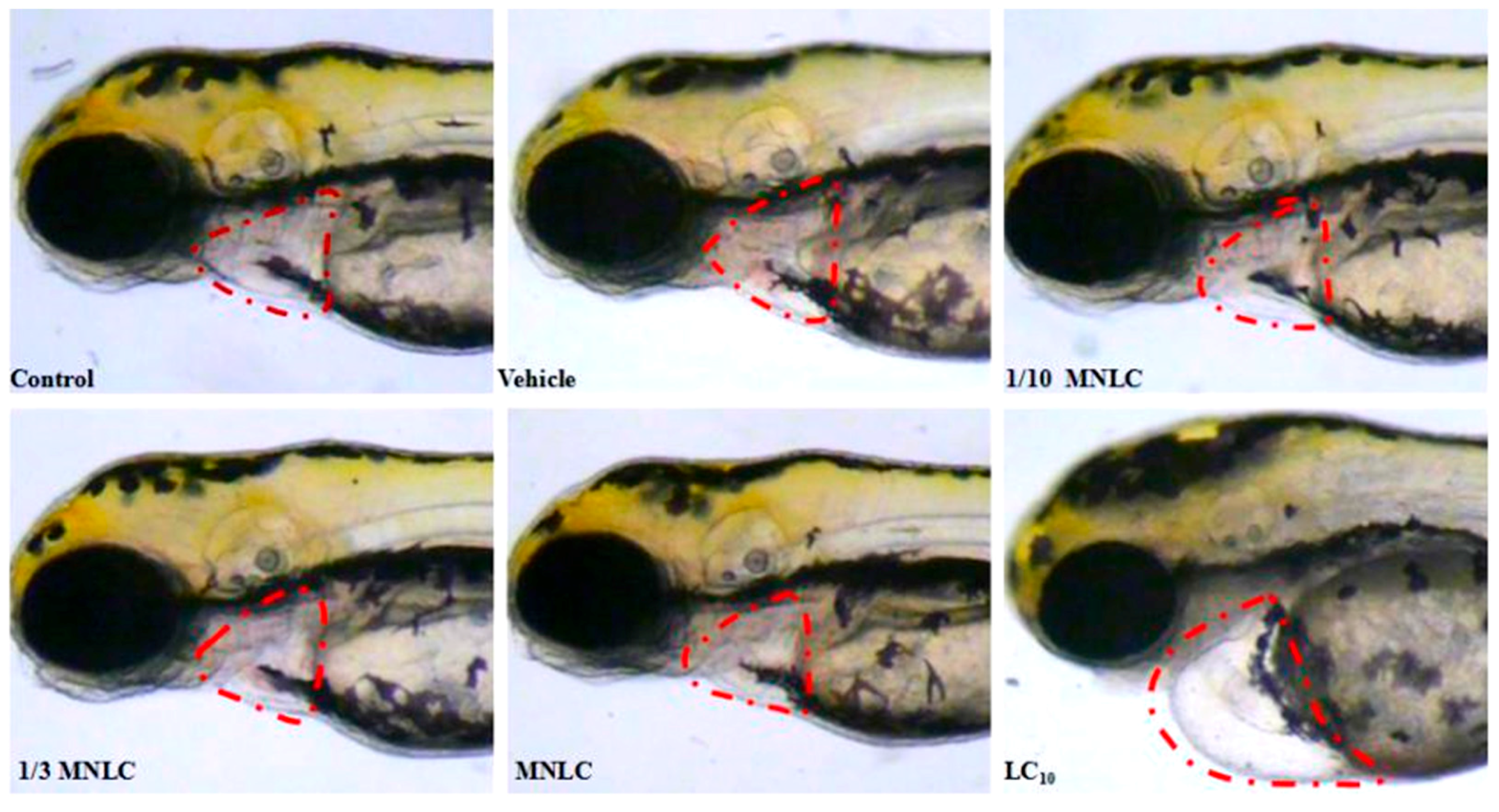
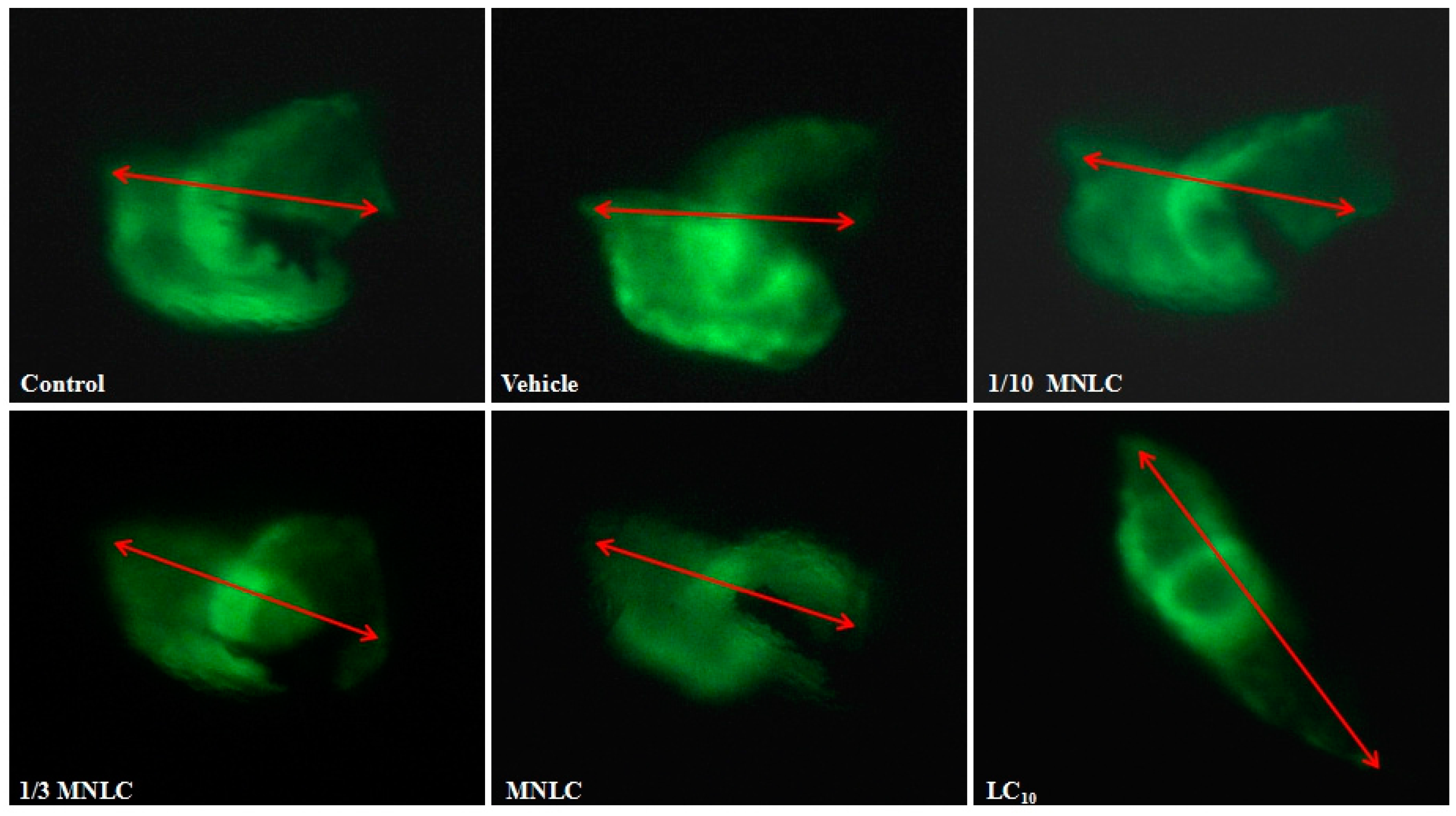
| Group | Evodiamine (ng/mL) | SV–BA Distance (Pixels) | Relative SV–BA Distance (%) |
|---|---|---|---|
| Control group | / | 231 ± 6.6 | 101 |
| Vehicle group | / | 229 ± 3.5 | / |
| 1/10 MNLC | 11 | 235 ± 2.4 | 102 |
| 1/3 MNLC | 38 | 250 ± 5.1 * | 109 * |
| MNLC | 113 | 254 ± 7.0 * | 111 * |
| LC10 | 354 | 326 ± 6.0 ** | 142 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Ma, L.; Li, S.; Cui, K.; Lei, L.; Ye, Z. Evaluation of the Cardiotoxicity of Evodiamine In Vitro and In Vivo. Molecules 2017, 22, 943. https://doi.org/10.3390/molecules22060943
Yang W, Ma L, Li S, Cui K, Lei L, Ye Z. Evaluation of the Cardiotoxicity of Evodiamine In Vitro and In Vivo. Molecules. 2017; 22(6):943. https://doi.org/10.3390/molecules22060943
Chicago/Turabian StyleYang, Weifeng, Lina Ma, Sidi Li, Kaiyu Cui, Lei Lei, and Zuguang Ye. 2017. "Evaluation of the Cardiotoxicity of Evodiamine In Vitro and In Vivo" Molecules 22, no. 6: 943. https://doi.org/10.3390/molecules22060943
APA StyleYang, W., Ma, L., Li, S., Cui, K., Lei, L., & Ye, Z. (2017). Evaluation of the Cardiotoxicity of Evodiamine In Vitro and In Vivo. Molecules, 22(6), 943. https://doi.org/10.3390/molecules22060943





