Lipophilicity Studies on Thiosemicarbazide Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Relationship between the Retention Parameter logk and the Concentration of Organic Modifier φ
2.2. The Calibration Equation logP vs. logkw
n = 7; r = 0.9976; se = 0.08; F = 1047.8
2.3. Theoretical Calculation clogP
2.4. Correlation of Lipophilicity with Inhibitory Potency towards Bacterial Type IIA Topoisomerases
3. Materials and Methods
3.1. Chromatographic Analysis
3.2. Standard Solutes
3.3. Statistical Analysis
3.4. LogP Calculations
3.5. Quantum-Chemical Calculations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hansch, C.; Leo, A. Exploring QSAR: Fundamentals and Applications in Chemistry and Biology; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Testa, B.; Crivori, P.; Reist, M.; Carrupt, P.-A. The influence of lipophilicity on the pharmacokinetic behavior of drugs: Concepts and examples. Perspect. Drug Discov. Des. 2000, 17, 179–211. [Google Scholar] [CrossRef]
- Lewis, M.L.; Cucurull-Sanchez, L. Structural pairwise comparisons of HLM stability of phenyl derivatives: Introduction of the Pfizer metabolism index (PMI) and metabolism-lipophilicity efficiency (MLE). J. Comput. Aided Mol. Des. 2009, 23, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Shamovsky, I.; Connolly, S.; David, L.; Ivanova, S.; Norden, B.; Springthorpe, B.; Urbahns, K. Overcoming undesirable hERG potency of chemokine receptor antagonists using baseline lipophilicity relationships. J. Med. Chem. 2008, 51, 1162–1178. [Google Scholar] [CrossRef] [PubMed]
- Briciu, R.D.; Kot-Wasik, A.; Wasik, A.; Namieśnik, J.; Sârbu, C. The lipophilicity of artificial and natural sweeteners estimated by reversed-phase thin-layer chromatography and computed by various methods. J. Chromatogr. A 2010, 1217, 3702–3706. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Yalkowsky, S.H. Estimation of the aqueous solubility I: Application to organic nonelectrolytes. J. Pharm. Sci. 2001, 90, 234–252. [Google Scholar] [CrossRef]
- Yoshida, F.; Topliss, J.G. QSAR model for drug human oral bioavailability. J. Med. Chem. 2000, 43, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Kanaoka, M. Computational prediction of the plasma protein-binding percent of diverse pharmaceutical compounds. J. Pharm. Sci. 2004, 93, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
- Austin, R.P.; Barton, P.; Cockroft, S.L.; Wenlock, M.C.; Riley, R.J. The influence of nonspecific microsomal binding on apparent intrinsic clearance and its prediction from physicochemical properties. Drug Metab. Dispos. 2002, 30, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Austin, R.P.; Barton, P.; Mohmed, S.; Riley, R.J. The binding of drugs to hepatocytes and its relationship to physicochemical properties. Drug Metab. Dispos. 2005, 33, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; DeCrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef] [PubMed]
- Alelyunasa, Y.W.; Pelosi-Kilbya, L.; Turcotteb, P.; Karyb, M.-B.; Spreena, R.C. A high throughput dried DMSO LogD lipophilicity measurement based on 96-well shake-flask and atmospheric pressure photoionization mass spectrometry detection. J. Chromatogr. A 2010, 1217, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Hollósy, F.; Seprödi, J.; Örfi, L.; Erös, D.; Kéri, G.; Idei, M. Evaluation of lipophilicity and antitumour activity of parallel carboxamide libraries. J. Chromatogr. B 2002, 780, 355–363. [Google Scholar] [CrossRef]
- Testa, B.; Carrupt, P.-A.; Gaillard, P.; Tsai, R.-S. Lipophilicity in Drug Action and Toxicology; Pliska, V., Testa, B., van de Waterbeemd, H., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA (VCH): Weinheim, Germany, 1996; pp. 49–71. [Google Scholar]
- Tetko, I.V.; Poda, G.I.; Ostermann, C.; Mannhold, R. Accurate in silico logP predictions: One can’t embrace the unembraceable. QSAR Comb. Sci. 2009, 28, 845–849. [Google Scholar] [CrossRef]
- Lombardo, F.; Shalaeva, M.Y.; Tupper, K.A.; Gao, F.; Abraham, M.H. ElogPoct: A tool for lipophilicity determination in drug discovery. J. Med. Chem. 2000, 43, 2922–2928. [Google Scholar] [CrossRef] [PubMed]
- Paneth, A.; Stączek, P.; Plech, T.; Strzelczyk, A.; Dzitko, K.; Wujec, M.; Kuśmierz, E.; Kosikowska, U.; Grzegorczyk, A.; Paneth, P. Biological evaluation and molecular modelling study of thiosemicarbazide derivatives as bacterial type IIA topoisomerases inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Siwek, A.; Stą̨czek, P.; Wujec, M.; Stefańska, J.; Kosikowska, U.; Malm, A.; Jankowski, S.; Paneth, P. Biological and docking studies of topoisomerase IV inhibition by thiosemicarbazides. J. Mol. Model. 2011, 17, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Siwek, A.; Stą̨czek, P.; Stefańska, J. Synthesis and structure-activity relationship studies of 4-arylthiosemicarbazides as topoisomerase IV inhibitors with Gram-positive antibacterial activity. Search for molecular basis of antibacterial activity of thiosemicarbazides. Eur. J. Med. Chem. 2011, 46, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, H.H. A reexamination of the Hammet equation. Chem. Rev. 1953, 53, 191–261. [Google Scholar] [CrossRef]
- Leśniewska, M.A.; Gdaniec, Z.; Musialska, I. Calculation procedures and HPLC method for analysis of the lipophilicity of acyclovir esters. Drug Dev. Ind. Pharm. 2014, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rabtti, E.H.M.A.; Natic, M.M.; Milojkovic-Opsenica, D.M.; Trifkovic, J.D.; Vuckovic, I.M.; Vajs, V.E.; Tešic, Ž.L. RP TLC based lipophilicity assessment of some natural and synthetic coumarins. J. Braz. Chem. Soc. 2012, 23, 522–530. [Google Scholar] [CrossRef]
- Hawrył, A.M.; Popiołek, Ł.P.; Hawrył, M.A.; Świeboda, R.S.; Niejedli, M.A. Chromatographic and calculation methods for analysis of the lipophilicity of newly synthesized thiosemicarbazides and their cyclic analogues 1,2,4-triazol-3-thiones. J. Braz. Chem. Soc. 2015, 26, 1617–1624. [Google Scholar] [CrossRef]
- Pachuta-Stec, A.; Hawrył, A.M.; Wróbel, A.; Hawrył, M.A.; Pitucha, M. Chromatographic evaluation of the lipophilic properties of some 1,2,4-triazole with potential antitumour activity. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1199–1206. [Google Scholar] [CrossRef]
- Meyer, H. Zur theorie der alkoholnarkose, I. Mit welch eigenshaft der anasthetika bedingt ihre narkotische Wirkung? Arch. Exp. Path. Pharmakol. (Naunyn Schmiedebergs) 1899, 42, 109–137. [Google Scholar] [CrossRef]
- Tanitame, A.; Oyamada, Y.; Ofuji, K.; Suzuki, K.; Ito, H.; Kawasaki, M.; Wachi, M.; Yamagishi, J.-I. Potent DNA gyrase inhibitors; novel 5-vinylpyrazole analogues with Gram-positive antibacterial activity. Bioorg. Med. Chem. Lett. 2004, 14, 2863–2866. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-S.; Lin, S.-T. Prediction of pH Effect on the octanol−water partition coefficient of ionizable pharmaceuticals. Ind. Eng. Chem. Res. 2016, 55, 9284–9294. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Test No. 117: Partition Coefficient (n-Octanol/Water), HPLC method. In OECD Guidelines for the Testing of Chemicals; Section 1; OECD Publishing: Paris, French, 2004. [Google Scholar]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory—Design and description. J. Comput. Aid. Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Virtual Computational Chemistry Labolatory. Available online: http://www.vcclab.org (accessed on 27 May 2017).
- Rocha, G.B.; Freire, R.O.; Simas, A.M.; Stewart, J.J. RM1: A reparameterization of AM1 for H, C, N, O, P, S, F, Cl, Br and I. J. Comput. Chem. 2006, 27, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.M.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the density functional ladder: Nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys. Rev. Lett. 2003, 91, 146401–146404. [Google Scholar] [CrossRef] [PubMed]
- Baronel, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti conelation energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistentmolecular orbital methods: 9. Extended gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods. 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; Defrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. 23. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Lynch, B.J.; Fast, P.L.; Harris, M.; Truhlar, D.G. Adiabatic connection for kinetics. J. Phys. Chem. A 2000, 104, 4811–4815. [Google Scholar] [CrossRef]
- Fast, P.L.; Sanchez, M.L.; Truhlar, D.G. Multi-coefficient Gaussian-3 method for calculating potential energy surfaces. Chem. Phys. Lett. 1999, 306, 407–410. [Google Scholar] [CrossRef]
- Môller, C.; Plesset, M.S. Note on an approximation treatment for many-electron systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Gonzalez-Lafont, A.; Truong, T.N.; Truhlar, D.G. Direct dynamics calculations with neglect of diatomic differential overlap molecular orbital theory with specific reaction parameters. J. Phys. Chem. 1991, 95, 4618–4627. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence,triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
Sample Availability: Not Available. |


| Compound | logkw | −S | r | n | F | SD of Estimation | |
|---|---|---|---|---|---|---|---|
| 1 | 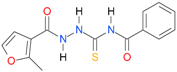 | 2.9992 | 4.3720 | 0.9986 | 7 | 1796.1 | 0.027 |
| 2 | 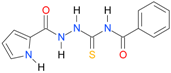 | 2.5105 | 4.1416 | 0.9983 | 7 | 1487.0 | 0.028 |
| 3 |  | 4.2230 | 6.1166 | 0.9983 | 7 | 1440.4 | 0.043 |
| 4 | 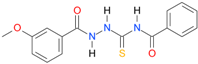 | 3.3130 | 4.8205 | 0.9984 | 7 | 1598.1 | 0.032 |
| 5 | 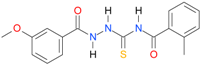 | 3.4487 | 4.8562 | 0.9987 | 7 | 1864.2 | 0.030 |
| 6 |  | 3.7864 | 5.1137 | 0.9976 | 7 | 1049.6 | 0.042 |
| 7 | 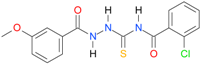 | 3.8283 | 5.6579 | 0.9934 | 7 | 372.8 | 0.078 |
| 8 | 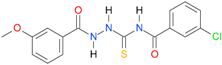 | 3.7422 | 5.0742 | 0.9972 | 7 | 901.4 | 0.045 |
| 9 | 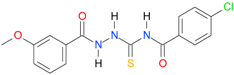 | 3.9422 | 5.3417 | 0.9974 | 7 | 975.2 | 0.045 |
| 10 |  | 3.2498 | 5.7291 | 0.9838 | 7 | 150.3 | 0.124 |
| 11 | 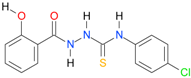 | 3.3324 | 4.8778 | 0.9983 | 7 | 2138.6 | 0.028 |
| 12 | 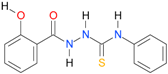 | 2.4813 | 4.1962 | 0.9992 | 7 | 3260.5 | 0.019 |
| 13 | 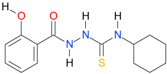 | 3.2386 | 4.7076 | 0.9987 | 7 | 1871.6 | 0.029 |
| 14 | 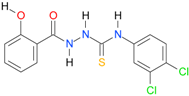 | 4.1453 | 5.6538 | 0.9980 | 7 | 1251.7 | 0.042 |
| 15 |  | 3.9451 | 5.5432 | 0.9964 | 7 | 690.2 | 0.056 |
| 16 | 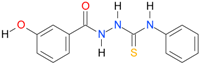 | 2.0687 | 4.1968 | 0.9993 | 7 | 3508.6 | 0.019 |
| 17 |  | 2.7583 | 4.3297 | 0.9993 | 7 | 3507.0 | 0.019 |
| Compound | AlogPs | AclogP | milogP | AlogP | MlogP | XlogP2 | XlogP3 | logkw | logPHPLC |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.90 | 1.70 | 1.28 | 1.80 | 1.33 | 1.62 | 2.59 | 2.9992 | 3.0469 |
| 2 | 1.66 | 1.06 | 1.27 | 1.94 | 1.06 | 1.59 | 2.25 | 2.5105 | 2.5254 |
| 3 | 2.86 | 2.59 | 2.23 | 2.95 | 3.20 | 3.43 | 3.95 | 4.2300 | 4.3606 |
| 4 | 2.36 | 2.17 | 2.15 | 2.53 | 2.42 | 2.70 | 3.10 | 3.3130 | 3.3819 |
| 5 | 2.41 | 2.49 | 2.55 | 3.02 | 2.66 | 3.14 | 3.46 | 3.4487 | 3.5267 |
| 6 | 2.41 | 2.49 | 2.57 | 3.02 | 2.66 | 3.14 | 3.46 | 3.7864 | 3.8871 |
| 7 | 3.00 | 2.79 | 2.78 | 3.20 | 2.93 | 3.32 | 3.73 | 3.8283 | 3.9318 |
| 8 | 2.93 | 2.79 | 2.80 | 3.20 | 2.93 | 3.32 | 3.73 | 3.7422 | 3.8399 |
| 9 | 2.88 | 2.79 | 2.83 | 3.20 | 2.93 | 3.32 | 3.73 | 3.9422 | 4.0534 |
| 10 | 2.62 | 1.59 | 1.53 | 2.81 | 2.57 | 2.43 | 3.06 | 3.2498 | 3.3144 |
| 11 | 2.82 | 2.66 | 3.31 | 3.08 | 3.39 | 3.41 | 3.52 | 3.3324 | 3.4026 |
| 12 | 1.95 | 2.05 | 2.63 | 2.41 | 2.86 | 2.78 | 3.21 | 2.4813 | 2.4942 |
| 13 | 1.72 | 2.05 | 3.48 | 2.69 | 2.44 | 3.05 | 3.14 | 3.2386 | 3.3025 |
| 14 | 3.32 | 3.27 | 3.91 | 3.74 | 3.64 | 4.03 | 4.15 | 4.1453 | 4.2702 |
| 15 | 3.02 | 2.97 | 3.69 | 3.56 | 3.64 | 3.63 | 3.88 | 3.9451 | 4.0565 |
| 16 | 1.99 | 2.05 | 1.64 | 2.41 | 2.35 | 2.36 | 2.34 | 2.0687 | 2.0538 |
| 17 | 1.74 | 2.05 | 2.48 | 2.69 | 1.93 | 2.62 | 2.59 | 2.7583 | 2.7898 |
| AlogPs | AclogP | milogP | AlogP | MlogP | XlogP2 | XlogP3 | logkw | logPHPLC | |
|---|---|---|---|---|---|---|---|---|---|
| AlogPs | 1.0000 | 0.7241 | 0.7833 | 0.9335 | 0.9132 | 0.8906 | 0.8518 | 0.7676 | 0.7676 |
| AclogP | 1.0000 | 0.9500 | 0.8541 | 0.8641 | 0.9348 | 0.9403 | 0.9052 | 0.9052 | |
| milogP | 1.0000 | 0.8993 | 0.8226 | 0.9282 | 0.8755 | 0.8013 | 0.8013 | ||
| AlogP | 1.0000 | 0.9425 | 0.9529 | 0.9177 | 0.8361 | 0.8361 | |||
| MlogP | 1.0000 | 0.9632 | 0.9667 | 0.9251 | 0.9251 | ||||
| XlogP2 | 1.0000 | 0.9776 | 0.9265 | 0.9265 | |||||
| XlogP3 | 1.0000 | 0.9716 | 0.9716 | ||||||
| logkw | 1.0000 | 1.0000 | |||||||
| logPHPLC | 1.0000 | 1.0000 |
| AlogPs | AclogP | milogP | AlogP | MlogP | XlogP2 | XlogP3 | logkw | logPHPLC | |
|---|---|---|---|---|---|---|---|---|---|
| AlogPs | 1.0000 | 0.9809 | 0.6650 | 0.9168 | 0.9309 | 0.8909 | 0.8564 | 0.8014 | 0.8014 |
| AclogP | 1.0000 | 0.7475 | 0.9725 | 0.8927 | 0.9373 | 0.8881 | 0.8839 | 0.8839 | |
| milogP | 1.0000 | 0.8199 | 0.7237 | 0.9251 | 0.9135 | 0.9520 | 0.9521 | ||
| AlogP | 1.0000 | 0.8142 | 0.9450 | 0.8769 | 0.9510 | 0.9510 | |||
| MlogP | 1.0000 | 0.8805 | 0.9201 | 0.7670 | 0.7670 | ||||
| XlogP2 | 1.0000 | 0.9712 | 0.9650 | 0.9650 | |||||
| XlogP3 | 1.0000 | 0.9136 | 0.9136 | ||||||
| logkw | 1.0000 | 1.0000 | |||||||
| logPHPLC | 1.0000 | 1.0000 |
| Compound | XlogP3 | XlogP2 | Inhibitory Potency IC50 [μM] | ||
|---|---|---|---|---|---|
| DNA Gyrase | Topo IV | ||||
| 1 [16] | 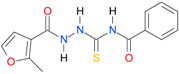 | 2.59 | n.d. * | 14.59 | n.a. ** |
| 2 [16] | 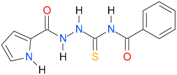 | 2.25 | n.d. | 93.30 | 41.04 |
| 18 [17] |  | 3.59 | n.d. | n.d. | 14 |
| 19 [16] | 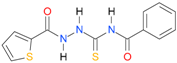 | 3.14 | n.d. | 83.63 | n.a. |
| 20 [17] | 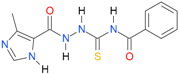 | 2.00 | n.d. | n.a. | 90.00 |
| 21 [18] | 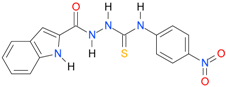 | n.d. | 2.82 | n.a. | 14.00 |
| 22 [16] | 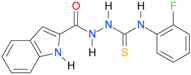 | n.d. | 3.09 | n.a. | 63.47 |
| 23 [16] | 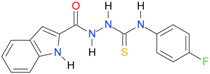 | n.d. | 3.09 | 127.68 | 267.04 |
| 24 [18] | 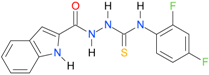 | n.d. | 3.25 | n.a. | 295.00 |
| 25 [16] | 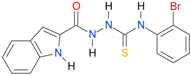 | n.d. | 3.73 | n.a. | n.a. |
| 26 [18] | 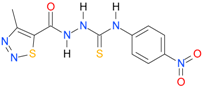 | n.d. | 2.25 | n.a. | n.a. |
| 27 [16] |  | n.d. | 1.46 | 64.21 | n.a. |
| 28 [18] |  | n.d. | 3.16 | n.a. | 403.00 |
| 29 [18] | 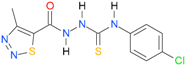 | n.d. | 2.98 | n.a. | n.a. |
| 30 [18] |  | n.d. | 2.52 | n.a. | n.a. |
| 31 [18] | 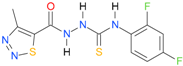 | n.d. | 2.68 | n.a. | n.a. |
| 32 [18] | 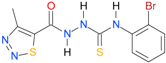 | n.d. | 3.16 | n.a. | n.a. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paneth, A.; Hawrył, A.; Plech, T.; Hawrył, M.; Świeboda, R.; Janowska, D.; Wujec, M.; Paneth, P. Lipophilicity Studies on Thiosemicarbazide Derivatives. Molecules 2017, 22, 952. https://doi.org/10.3390/molecules22060952
Paneth A, Hawrył A, Plech T, Hawrył M, Świeboda R, Janowska D, Wujec M, Paneth P. Lipophilicity Studies on Thiosemicarbazide Derivatives. Molecules. 2017; 22(6):952. https://doi.org/10.3390/molecules22060952
Chicago/Turabian StylePaneth, Agata, Anna Hawrył, Tomasz Plech, Mirosław Hawrył, Ryszard Świeboda, Dominika Janowska, Monika Wujec, and Piotr Paneth. 2017. "Lipophilicity Studies on Thiosemicarbazide Derivatives" Molecules 22, no. 6: 952. https://doi.org/10.3390/molecules22060952







