Osteoprotective Effect of Radix Scutellariae in Female Hindlimb-Suspended Sprague-Dawley Rats and the Osteogenic Differentiation Effect of Its Major Constituent
Abstract
:1. Introduction
2. Results
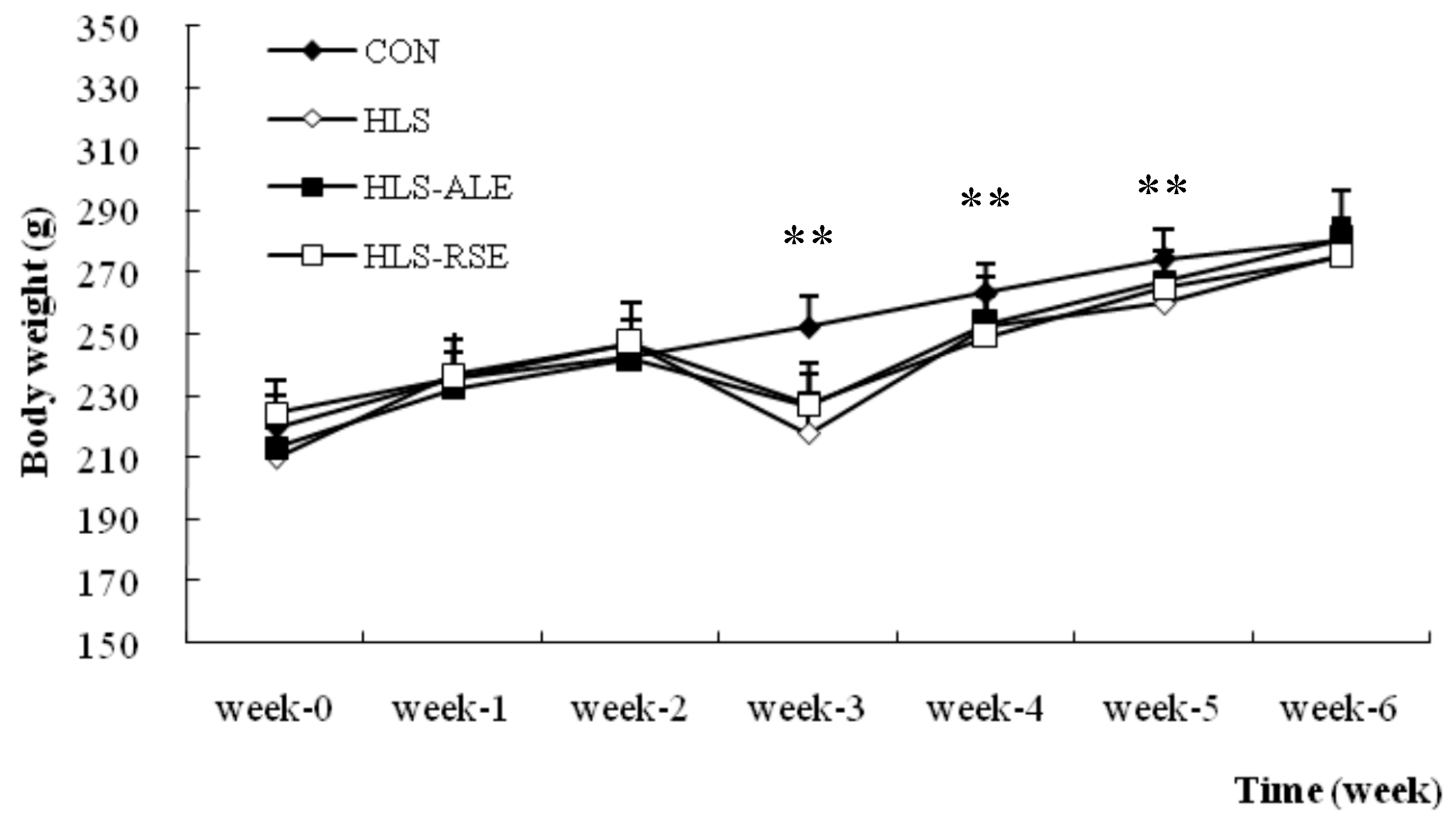
2.1. Body Weights
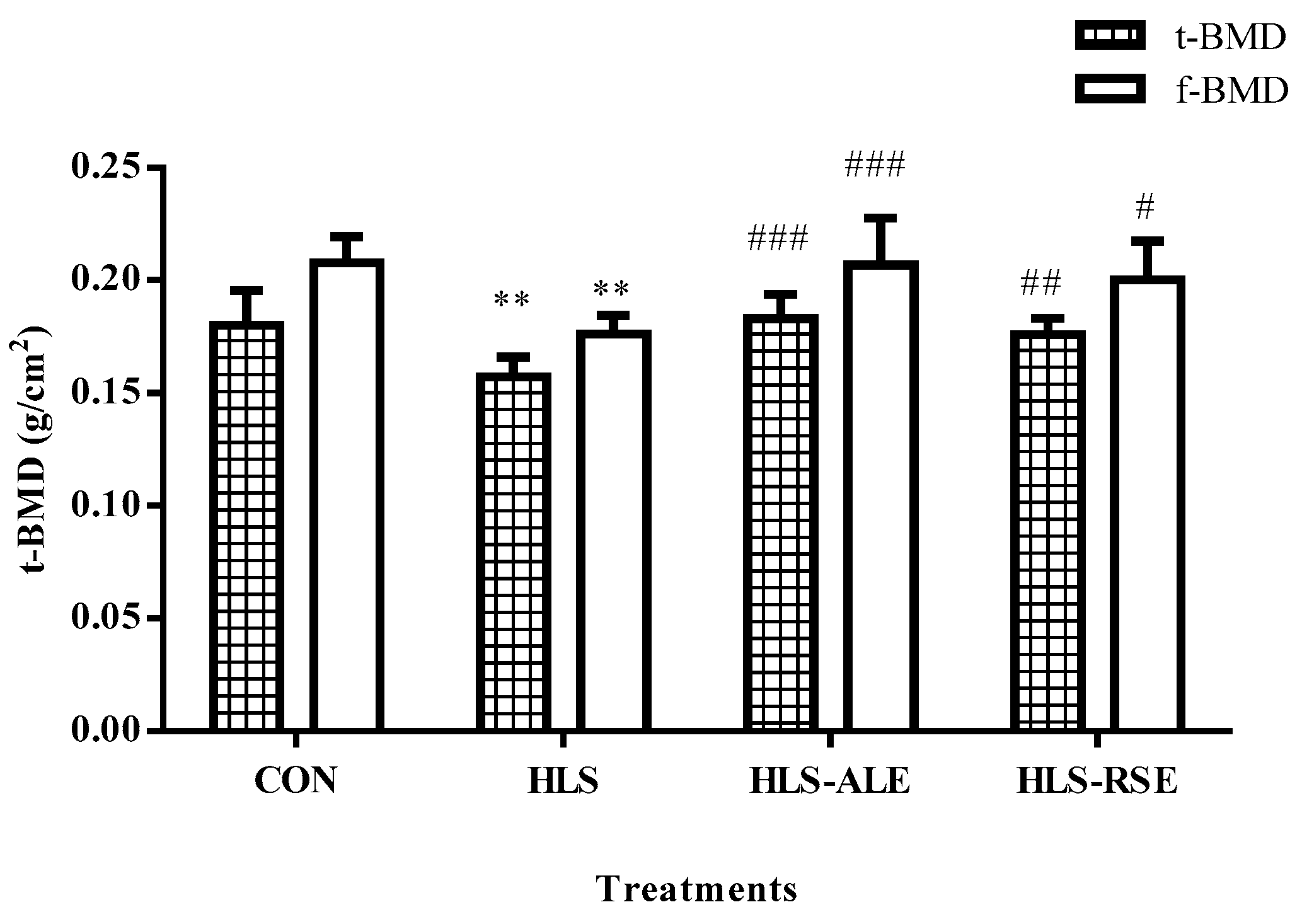
2.2. BMD Evaluation of Femur and Tibia
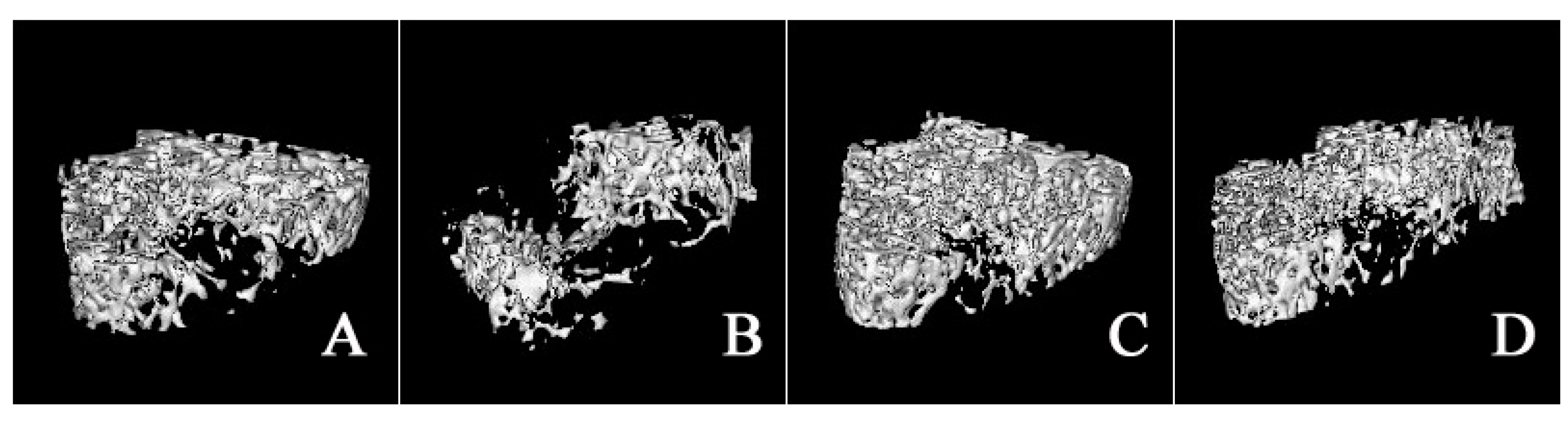
2.3. Micro-Architecture of Femoral Trabecula
2.4. Bone Biomechanical Test
2.5. Assay of Bone Turnover Markers in Serum
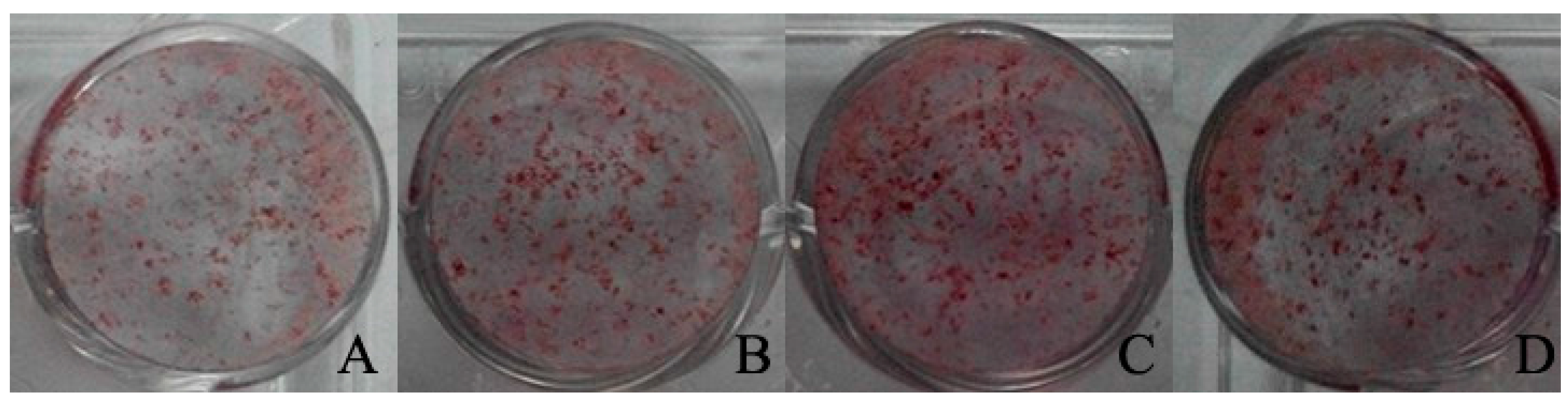
2.6. The Effect of Baicalin to Enhance Osteogenic Differentiation of rBMSC
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Treatments
4.3. Bone Mineral Density (BMD) Analysis
4.4. Micro-Computed Tomography (Micro-CT) Analysis
4.5. Biomechanical Test
4.6. Measurements of Serum Bone Turnover Markers
4.7. Cell Culture
4.8. Alkaline Phosphatase Assay
4.9. Mineralization Assay
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clarke, B.L.; Khosla, S. Physiology of bone loss. Radiol. Clin. N. Am. 2010, 48, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Gates, B.J.; Sonnett, T.E.; Duvall, C.A.; Dobbins, E.K. Review of osteoporosis pharmacotherapy for geriatric patients. Am. J. Geriatr. Pharmacother. 2009, 7, 293–323. [Google Scholar] [CrossRef] [PubMed]
- Blaber, E.; Marcal, H.; Burns, B.P. Bioastronautics: The influence of microgravity on astronaut health. Astrobiology 2010, 10, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hamamura, K.; Yokota, H. A brief review of bone adaptation to unloading. Genom. Proteom. Bioinform. 2008, 6, 4–7. [Google Scholar] [CrossRef]
- Sibonga, J.D.; Evans, H.J.; Sung, H.G.; Spector, E.R.; Lang, T.F.; Oganov, V.S.; Bakulin, A.V.; Shackelford, L.C.; LeBlanc, A.D. Recovery of spaceflight-induced bone loss: Bone mineral density after long-duration missions as fitted with an exponential function. Bone 2007, 41, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, L.C.; LeBlanc, A.D.; Driscoll, T.B.; Evans, H.J.; Rianon, N.J.; Smith, S.M.; Spector, E.; Feeback, D.L.; Lai, D. Resistance exercise as a countermeasure to disuse-induced bone loss. J. Appl. Physiol. 2004, 97, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading of growing rats: A model for predicting skeletal changes during space flight. Bone 1998, 22, 83s–88s. [Google Scholar] [CrossRef]
- Knopp-Sihota, J.A.; Cummings, G.G.; Homik, J.; Voaklander, D. The association between serious upper gastrointestinal bleeding and incident bisphosphonate use: A population-based nested cohort study. BMC Geriatr. 2013, 13, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat. Rev. Cancer 2006, 6, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Li, C.R.; Li, Q.; Liu, R.J.; Niu, Y.B.; Pan, Y.L.; Zhai, Y.K.; Mei, Q.B. Medicinal herbs in the prevention and treatment of osteoporosis. Am. J. Chin. Med. 2014, 42, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [PubMed]
- Guo, A.J.; Choi, R.C.; Cheung, A.W.; Chen, V.P.; Xu, S.L.; Dong, T.T.; Chen, J.J.; Tsim, K.W. Baicalin, a flavone, induces the differentiation of cultured osteoblasts: An action via the Wnt/beta-catenin signaling pathway. J. Biol. Chem. 2011, 286, 27882–27893. [Google Scholar] [PubMed]
- Kim, M.H.; Ryu, S.Y.; Bae, M.A.; Choi, J.S.; Min, Y.K.; Kim, S.H. Baicalein inhibits osteoclast differentiation and induces mature osteoclast apoptosis. Food Chem. Toxicol. 2008, 46, 3375–3382. [Google Scholar] [PubMed]
- Li, C.R.; Zhang, G.W.; Niu, Y.B.; Pan, Y.L.; Zhai, Y.K.; Mei, Q.B. Antiosteoporosis effect of Radix Scutellariae extract on density and microstructure of long bones in tail-suspended Sprague-Dawley rats. Evid. Based Complement. Altern. Med. 2014. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Allen, M.R.; Hogan, H.A.; Delp, M.D. Site- and compartment-specificchanges in bone with hindlimb unloading in mature adult rats. Bone 2002, 31, 149–157. [Google Scholar] [PubMed]
- Giangregorio, L.; Blimkie, C.J. Skeletal adaptations to alterations in weight-bearing activity: A comparison of models of disuse osteoporosis. Sports Med. 2002, 32, 459–476. [Google Scholar] [PubMed]
- Wimalawansa, S.M.; Wimalawansa, S.J. Simulatedweightlessness-inducedattenuation of testosteroneproduction may be responsible for boneloss. Endocrine 1999, 10, 253–260. [Google Scholar] [PubMed]
- Cremers, S.C.; Papapoulos, S. Pharmacology of bisphosphonates. Bone 2011, 49, 42–49. [Google Scholar] [PubMed]
- Drake, M.T.; Cremers, S.C. Bisphosphonate therapeutics in bone disease: The hard and soft data on osteoclast inhibition. Mol. Interv. 2010, 10, 141–152. [Google Scholar] [PubMed]
- Qi, W.; Yan, Y.B.; Lei, W.; Wu, Z.X.; Zhang, Y.; Liu, D.; Shi, L.; Cao, P.C.; Liu, N. Prevention of disuse osteoporosis in rats by Cordyceps sinensis extract. Osteoporos. Int. 2012, 23, 2347–2357. [Google Scholar] [PubMed]
- Laurent, M.R.; Jardi, F.; Dubois, V.; Schollaert, D.; Khalil, R.; Gielen, E.; Carmeliet, G.; Claessens, F.; Vanderschueren, D. Androgens have antiresorptive effects on trabecular disuse osteopenia independent from muscle atrophy. Bone 2016, 93, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L. Mechanisms of osteoporosis therapy: A bone strength perspective. Clin. Cornerstone 2003, 2 (Suppl. S1), 13S–21S. [Google Scholar] [CrossRef]
- Hefferan, T.E.; Evans, G.L.; Lotinun, S.; Zhang, M.; Morey-Holton, E.; Turner, R.T. Effect of gender on bone turnover in adult rats during simulated weightlessness. J. Appl. Physiol. 2003, 95, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Habold, C.; Momken, I.; Ouadi, A.; Bekaert, V.; Brasse, D. Effect of prior treatment with resveratrol on density and structure of rat long bones under tail-suspension. J. Bone Miner. Metab. 2011, 29, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Durbin, S.M.; Jackson, J.R.; Ryan, M.J.; Gigliotti, J.C.; Alway, S.E.; Tou, J.C. Resveratrol supplementation influences bone properties in the tibia of hindlimb-suspended mature Fisher 344× Brown Norway male rats. Appl. Physiol. Nutr. Metab. 2012, 37, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Kim, M.H.; Gwak, N.G.; Im, Y.S.; Lee, L.Y.; Sohn, Y.; Choi, H.; Yang, W.M. Antiallergic effects of Scutellaria baicalensis on inflammation in vivo and in vitro. J. Ethnopharmacol. 2012, 141, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Li, C.R.; Fong, S.Y.; Mei, Q.B.; Lin, G.; Zuo, Z. Influence of mefenamic acid on the intestinal absorption and metabolism of three bioactive flavones in Radix Scutellariae and potential pharmacological impact. Pharm. Biol. 2014, 52, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.D.; Zhang, C.; Chen, G.J.; Tang, Z.H.; Liu, Q.W.; Chen, J.R.; Tong, X.M.; Wang, J.F. Effects of BMP-2 and FGF2 on the osteogenesis of bone marrow-derived mesenchymal stem cells in hindlimb-unloaded rats. Cell Biochem. Biophys. 2014, 70, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.J.; Yang, J.F.; Guo, C.J.; Shi, D.Y.; Shen, D.; Zheng, Q.; Chen, R.; Xu, Y.L.; Xi, Y.M.; Wang, J.F. Effects of hindlimb unloading on ex vivo growth and osteogenic/adipogenic potentials of bone marrow-derived mesenchymal stem cells in rats. Stem Cells Dev. 2008, 17, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Li, C.R.; Zhou, L.M.; Lin, G.; Zuo, Z. Contents of major bioactive flavones in proprietary traditional Chinese medicine products and reference herb of Radix Scutellariae. J. Pharm. Biomed. Anal. 2009, 50, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Li, C.R.; Zhang, L.; Lin, G.; Zuo, Z. Identification and quantification of baicalein, wogonin, oroxylin A and their major glucuronide conjugated metabolites in rat plasma after oral administration of Radix Scutellariae product. J. Pharm. Biomed. Anal. 2011, 54, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Mosekilde, L.; Thomsen, J.S.; Mackey, M.S.; Phipps, R.J. Treatment with resedronate or alendronate prevents hind-limb immobilization-induced loss of bone density and strength in adult female rats. Bone 2000, 27, 639–645. [Google Scholar] [CrossRef]
- Ngueguim, F.T.; Khan, M.P.; Donfack, J.H.; Tewari, D.; Dimo, T.; Tamtchouing, P.; Maurya, R.; Chattopadhyay, N. Ethanol extract of Peperomia pellucida (Piperaceae) promotes fracture healing by an anabolic effect on osteoblasts. J. Ethnopharmacol. 2013, 148, 62–68. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds including Radix Scutellariae extract, baicalin, baicalin, wogonin, wogonoside, oroxylin A and oroxylin A-7-O-glucuronide are available from the authors. |


| Parameters | CON | HLS | HLS-ALE | HLS-RSE |
|---|---|---|---|---|
| Maximum stress (MPa) | 111.24 ± 13.05 | 89.55 ± 5.28 * | 109.84 ± 16.46 # | 116.37 ± 8.85 ## |
| Young’s modulus (MPa) | 3576.52 ± 237.04 | 2724.98 ± 128.70 * | 3415.29 ± 556.66 # | 3671.41 ± 314.43 # |
| Maximum load (N) | 77.02 ± 9.04 | 61.77 ± 3.70 ** | 80.05 ± 9.38 ### | 80.57 ± 6.13 ### |
| Stiffness (N/mm) | 198.96 ± 18.64 | 120.72 ± 11.50 *** | 210.01 ± 26.34 ### | 196.26 ± 13.39 ### |
| Parameters | CON | HLS | HLS-ALE | HLS-RSE |
|---|---|---|---|---|
| Ca (mM) | 2.46 ± 0.10 | 2.41 ± 0.16 | 2.34 ± 0.123 | 2.37 ± 0.06 |
| P (mM) | 1.82 ± 0.08 | 2.08 ± 0.05 | 1.80 ± 0.21 | 2.04 ± 0.11 |
| BAP (μg/L) | 55.86 ± 1.55 | 55.03 ± 2.56 | 57.41 ± 1.25 | 56.29 ± 5.53 |
| TRACP (pg/L) | 2055.53 ± 172.20 | 2560.55 ± 114.58 | 2178.42 ± 287.16 | 2221.74 ± 203.29 |
| *** | # | # |
| Group | Area (mm2/Well) | Number (Mineralized Nodule/Well) | Intensity |
|---|---|---|---|
| CON | 30.34 ± 6.53 | 702.78 ± 49.50 | 21955.34 ± 2397.71 |
| 1 μmol·L−1 | 54.55 ± 6.23 ** | 973.64 ± 74.70 ** | 71181.98 ± 5769.01 *** |
| 10 μmol·L−1 | 68.14 ± 4.93 *** | 1218.64 ± 147.13 ** | 90613.62 ± 6423.42 *** |
| 50 μmol·L−1 | 64.03 ± 5.41 ** | 1157.68 ± 85.70 *** | 74511.73 ± 5773.22 *** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Li, C.; Niu, Y.; Yu, Q.; Chen, Y.; Liu, E. Osteoprotective Effect of Radix Scutellariae in Female Hindlimb-Suspended Sprague-Dawley Rats and the Osteogenic Differentiation Effect of Its Major Constituent. Molecules 2017, 22, 1044. https://doi.org/10.3390/molecules22071044
Zhang G, Li C, Niu Y, Yu Q, Chen Y, Liu E. Osteoprotective Effect of Radix Scutellariae in Female Hindlimb-Suspended Sprague-Dawley Rats and the Osteogenic Differentiation Effect of Its Major Constituent. Molecules. 2017; 22(7):1044. https://doi.org/10.3390/molecules22071044
Chicago/Turabian StyleZhang, Guangwei, Chenrui Li, Yinbo Niu, Qi Yu, Yulong Chen, and Enqi Liu. 2017. "Osteoprotective Effect of Radix Scutellariae in Female Hindlimb-Suspended Sprague-Dawley Rats and the Osteogenic Differentiation Effect of Its Major Constituent" Molecules 22, no. 7: 1044. https://doi.org/10.3390/molecules22071044
APA StyleZhang, G., Li, C., Niu, Y., Yu, Q., Chen, Y., & Liu, E. (2017). Osteoprotective Effect of Radix Scutellariae in Female Hindlimb-Suspended Sprague-Dawley Rats and the Osteogenic Differentiation Effect of Its Major Constituent. Molecules, 22(7), 1044. https://doi.org/10.3390/molecules22071044





