The Investigation of Electrochemistry Behaviors of Tyrosinase Based on Directly-Electrodeposited Grapheneon Choline-Gold Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Materials andApparatus
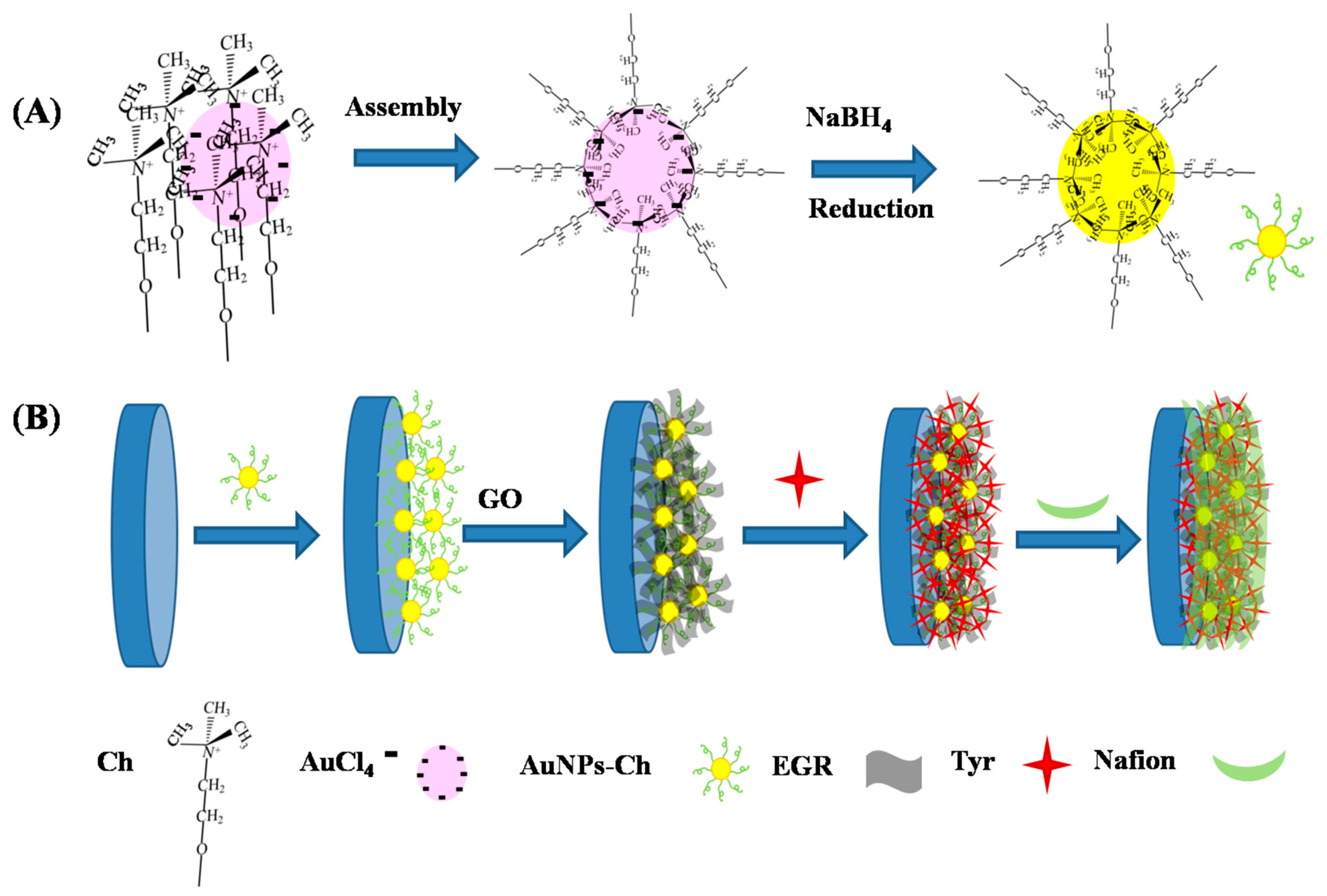
2.2. Preparation and Modification of Nafion/Tyr/EGR-AuNPs-Ch/GCE
3. Results and Discussion
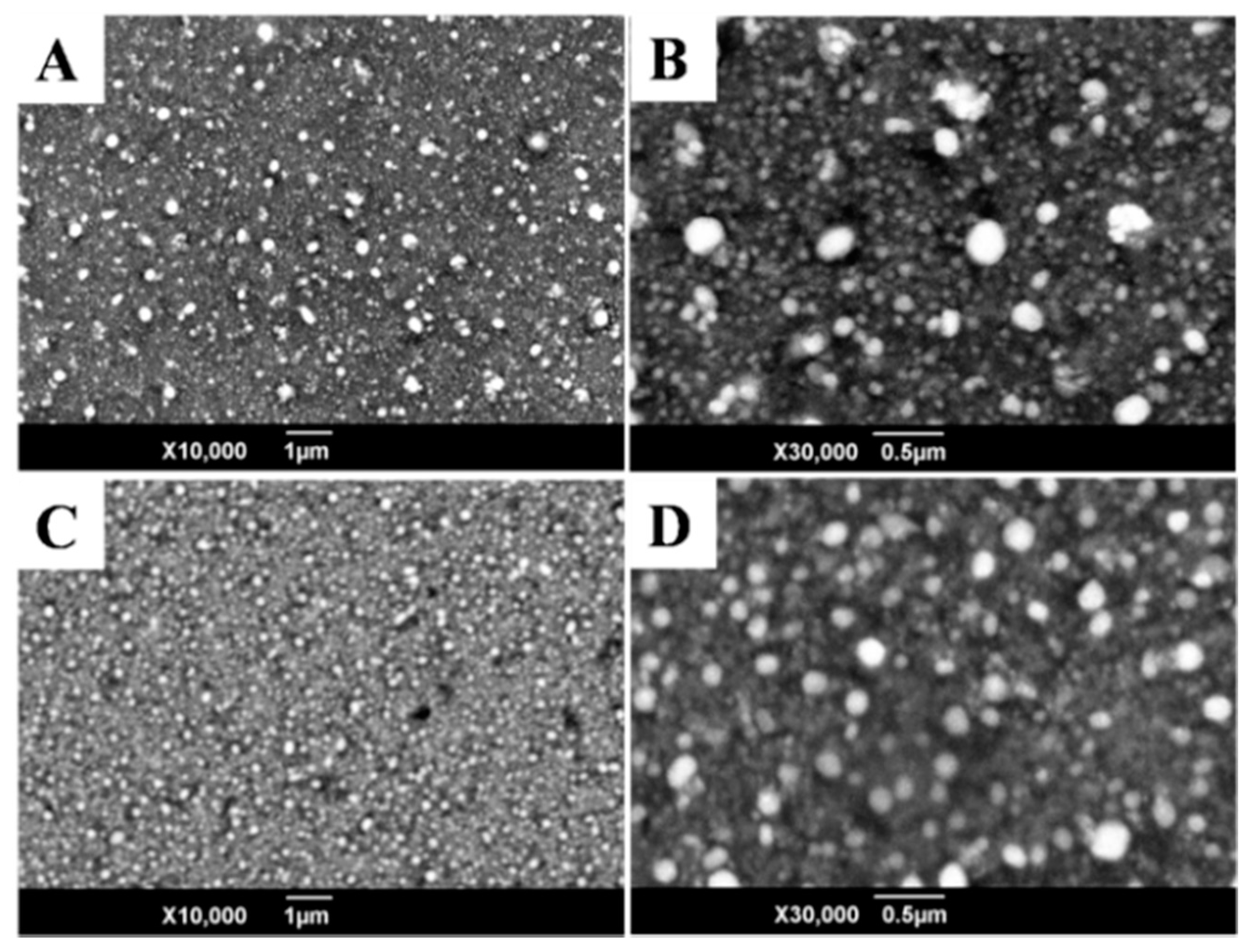
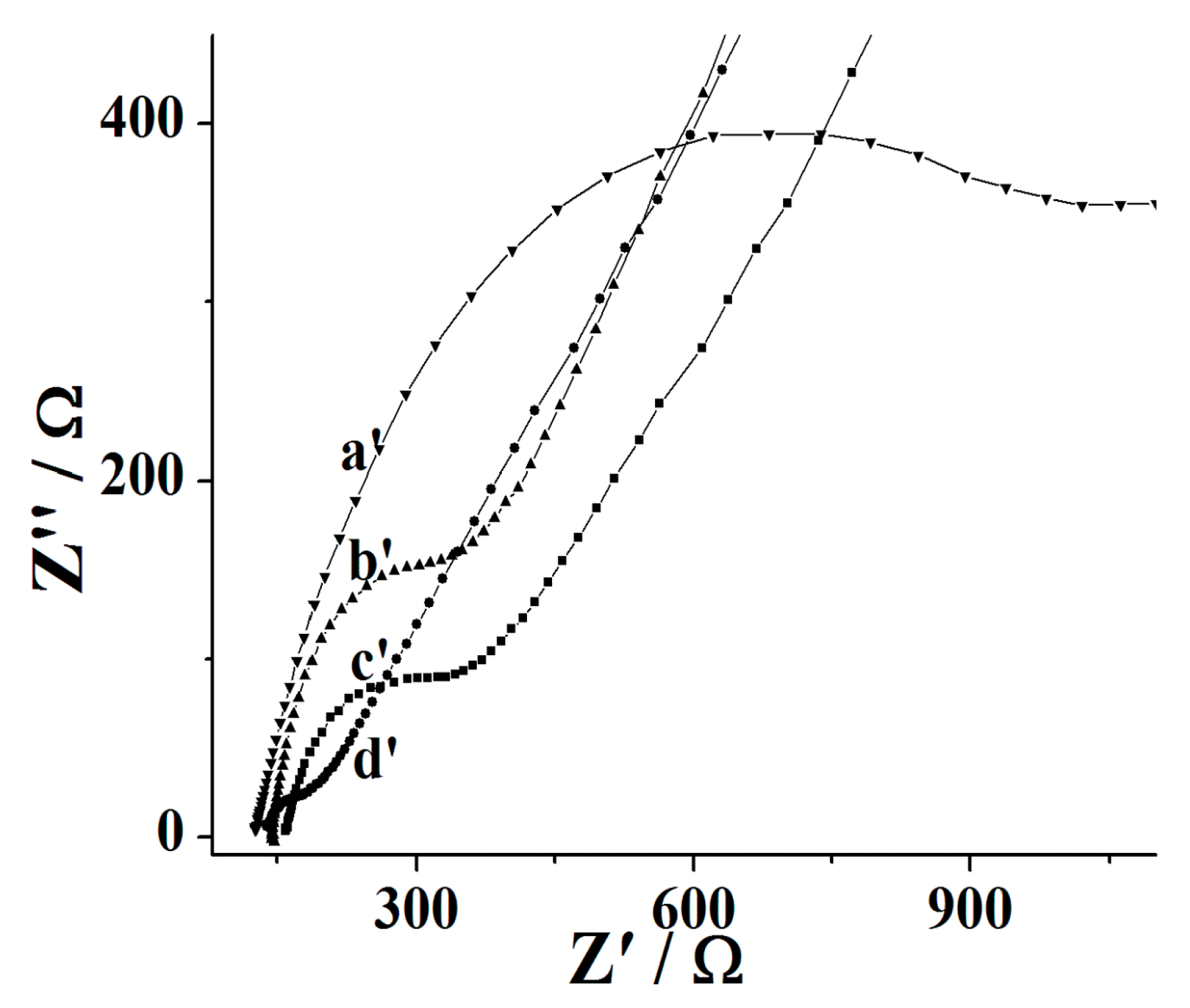
3.1. Fabrication and Characterization of the Nafion/Tyr/EGR-AuNPs-Ch/GCE
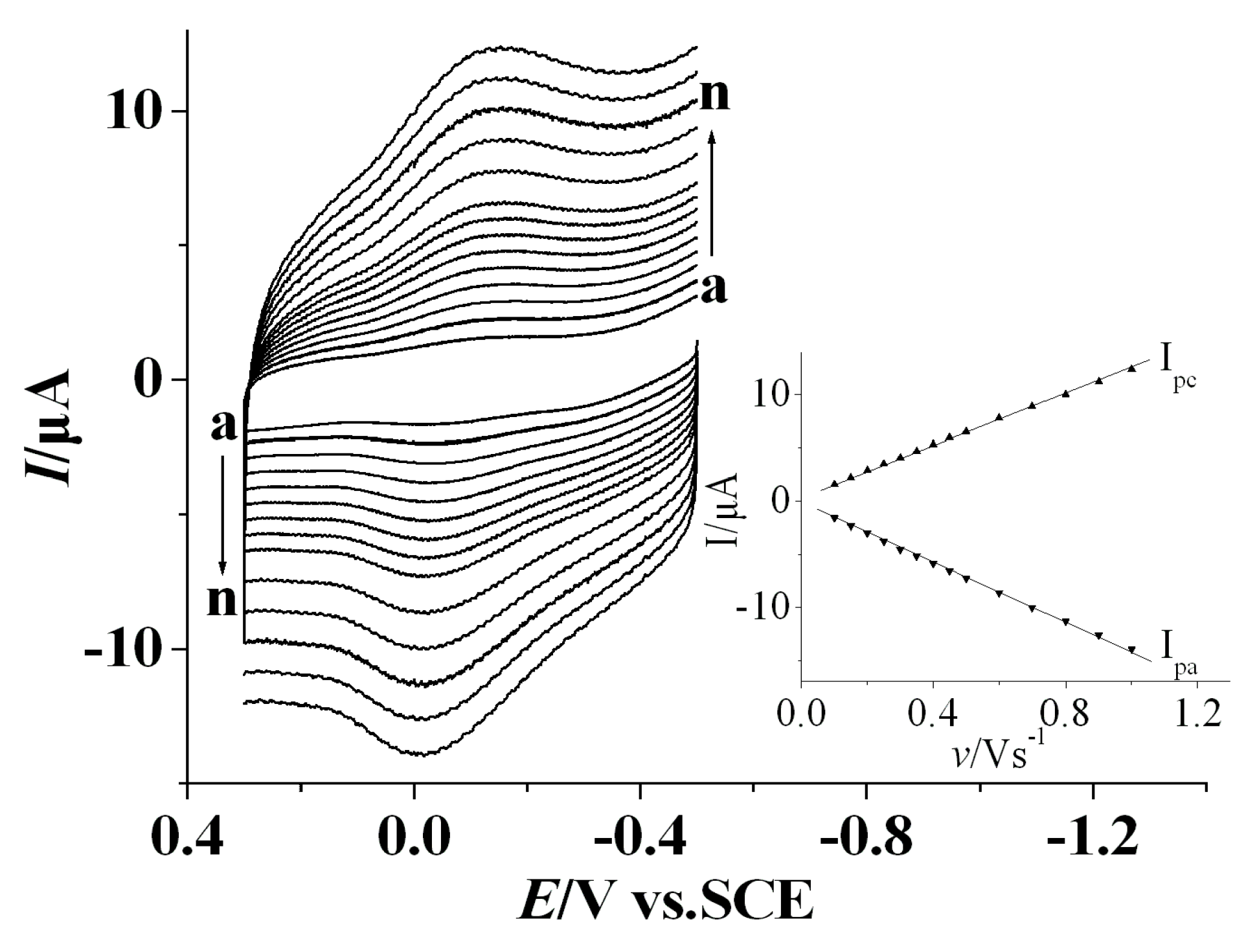
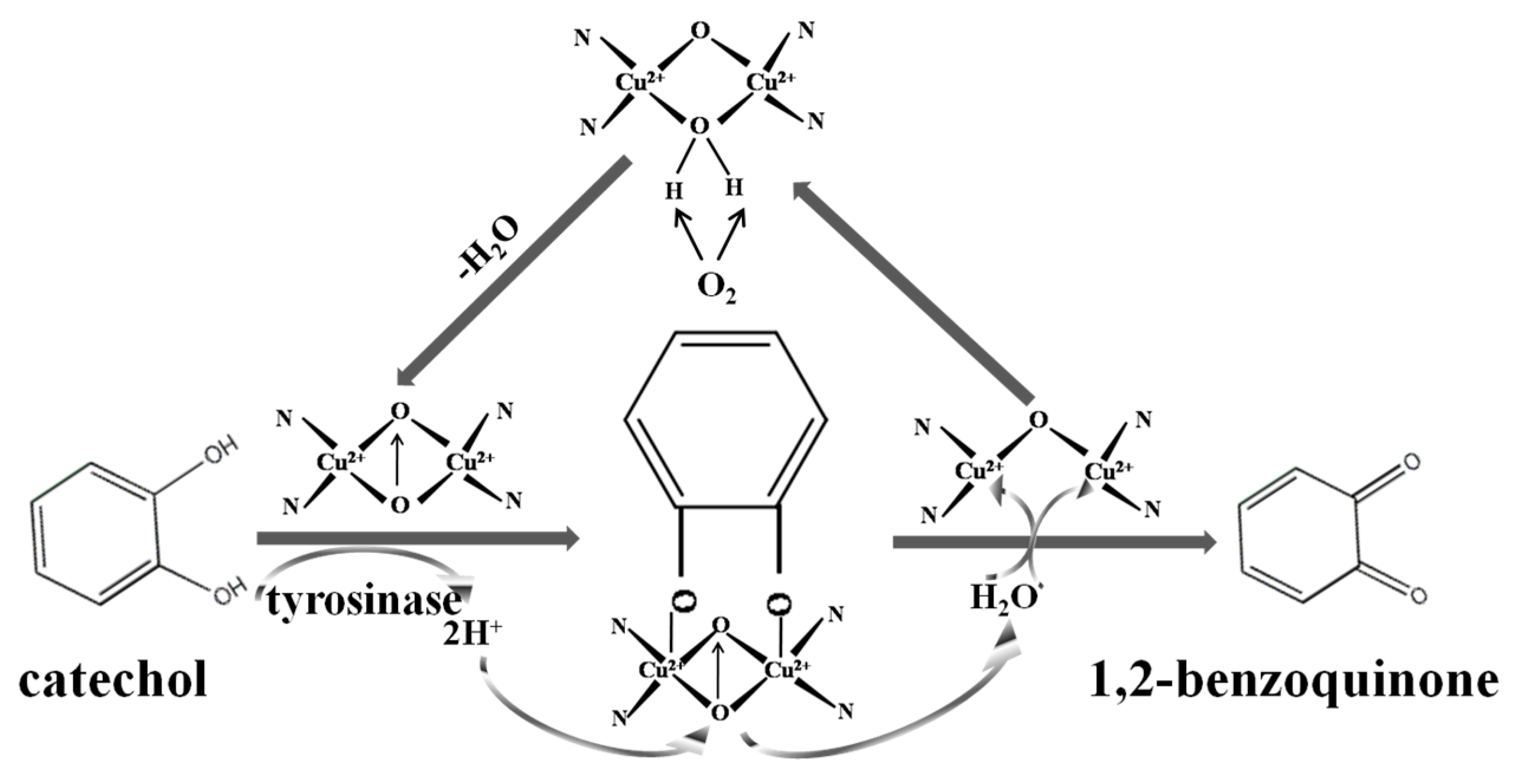
3.2. Direct Electrochemistry of Tyr on the Nafion/Tyr/EGR-AuNPs-Ch/GCE
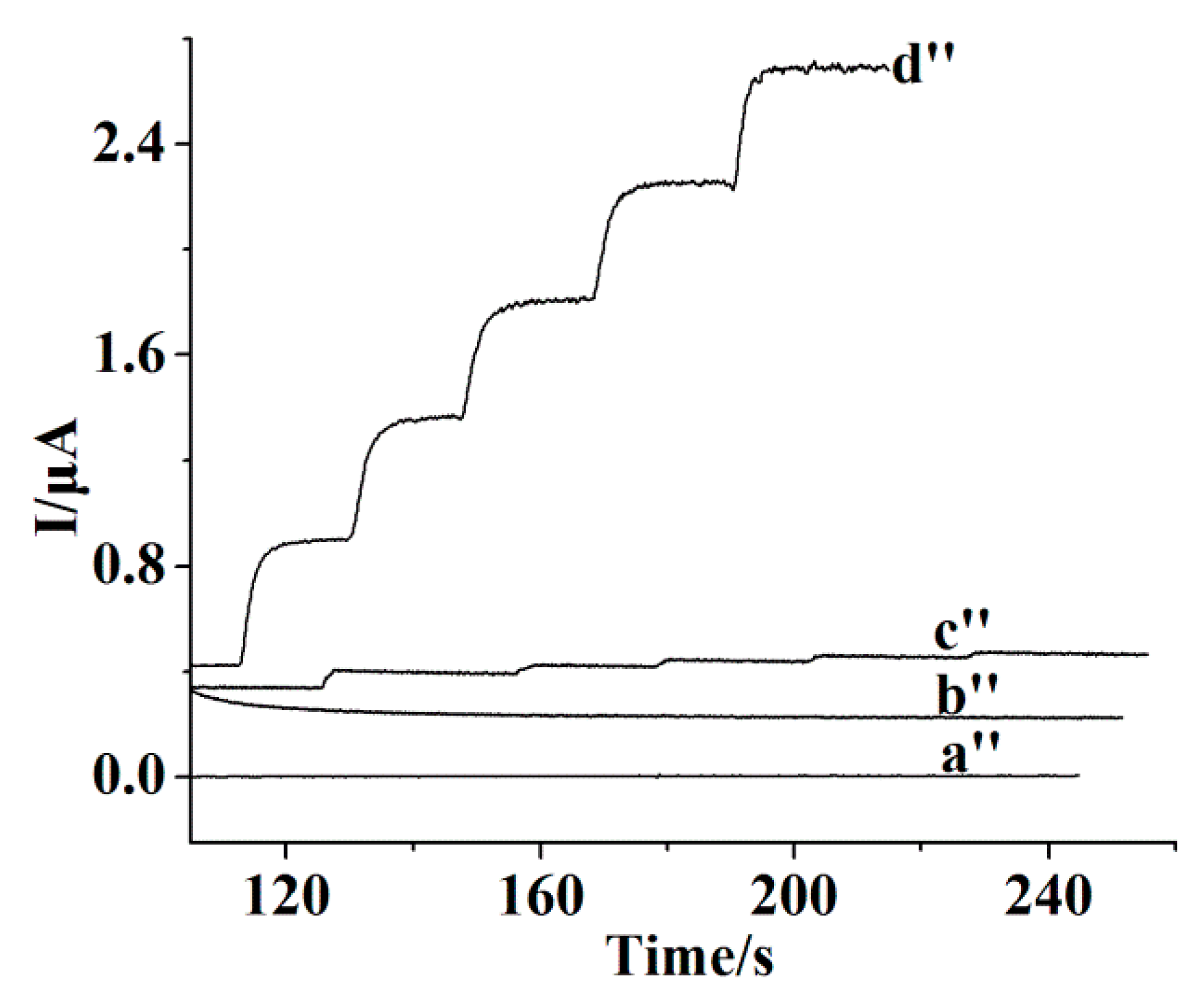
3.3. Amperometric Response Towards CA
3.4. Repeatability and Stability of CA Biosensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alessandra, A.; Matteo, S.; Stephan, D.; Saverio, M. Nanofibrous membrane based tyrosinase-biosensor for the detection of phenolic compounds. Anal. Chim. Acta 2010, 659, 133–136. [Google Scholar]
- Du, W.; Zhao, F.Q.; Zeng, B.Z. Novel multiwalled carbon nanotubes-polyaniline composite film coated platinum wire for headspace solid-phase microextraction and gaschromatographic determination of phenolic compounds. J. Chrom. A 2009, 1216, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Guix, M.; Pérez-López, B.; Sahin, M.; Roldán, M.; Ambrosi, A.; Merkoçi, A. Structural characterization by confocal laser scanning microscopy and electrochemical study of multi walled carbon nanotube tyrosinase matrix for phenol detection. Analyst 2010, 135, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Tan, Y.M.; Zhao, D.M.; Liu, G.D. Amperometric tyrosinase biosensor based on Fe3O4nanoparticles-chitosan nanocomposite. Biosens. Bioelectron. 2008, 23, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.M.; Zhang, L.; Zhang, X.B.; Huan, S.Y.; Shen, G.L.; Yu, R.Q. A novel tyrosinase biosensor based on hydroxyapatite-chitosan nanocomposite for the detection of phenolic compounds. Anal. Chim. Acta 2010, 665, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Burestedt, E.; Narvaez, A.; Ruzgas, T.; Gorton, L.; Emnéus, J.; Domínguez, E.; Marko-Varga, G. Rate limiting steps of tyrosinase-modified electrode for detection of catechol. Anal. Chem. 1996, 68, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lei, J.P.; Liu, Y.Y.; Zhao, J.W.; Ju, H.X. Highly sensitive amperometric biosensors for phenols based on polyaniline-ionic liquid-carbon nanofiber composite. Biosens. Bioelectron. 2009, 24, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Alwarappan, S.; Liu, C.; Kumar, A.; Li, C.Z. Enzyme-doped graphenenanosheets for enhanced glucose biosensing. J. Phys. Chem. C 2010, 114, 12920–12924. [Google Scholar] [CrossRef]
- Pérez-López, B.; Merkoçi, A. Magnetic nanoparticles modified with carbon nanotubes for electrocatalytic magnetoswitchable biosensing applications. Adv. Funct. Mater. 2011, 21, 255–260. [Google Scholar] [CrossRef]
- Reuillard, B.; Goff, A.L.; Agnès, C.; Zebda, A.; Holzinger, M.; Cosnier, S. Direct electron transfer between tyrosinase and multi-walled carbon nanotubes for bioelectrocatalytic oxygen reduction. Electrochem. Commun. 2012, 20, 19–22. [Google Scholar] [CrossRef]
- Tembe, S.; Inamdar, S.; Haram, S.; Karve, M.; D’Souza, S.F. Electrochemical biosensor for catechol using agarose-guar gum entrapped tyrosinase. J. Biotechnol. 2007, 128, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.J.; Son, K.J.; Kim, B.; Koh, W.G. Phenol biosensor based on hydrogel microarrays entrapping tyrosinase and quantum dots. Analyst 2010, 135, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Xiong, H.Y.; Zhang, X.H.; Wang, S.F. A novel tyrosinase biosensor based on chitosan-carbon-coated nickel nanocomposite film. Bioelectrochemistry 2012, 84, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Y.; Kan, J.Q.; Li, S.Q. Amperometric biosensor for catechol using electrochemical template process. Sens. Actuators B Chem. 2011, 152, 285–288. [Google Scholar] [CrossRef]
- Zhao, J.W.; Wu, D.H.; Zhi, J.F. A novel tyrosinase biosensor based on the nanocrystalline diamond electrode for detection of phenolic compounds. Bioelectrochemistry 2009, 75, 44–49. [Google Scholar] [CrossRef] [PubMed]
- ElKaoutit, M.; Naranjo-Rodriguez, I.; Temsamani, K.R.; Domínguez, M.; Hidalgo-Hidalgo de Cisneros, J.L. A comparison of three amperometric phenoloxidase-Sonogel-Carbon based biosensor for determination of polyphenols in beers. Talanta 2008, 75, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.S.; Zhou, Y.L.; Xu, J.; Ai, S.Y.; Cui, L.; Zhu, L.S. Amperometric biosensor based on tyrosinase film and its applicationto determine bisphenol A. Anal. Chim. Acta 2010, 659, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Faridnouri, H.; Ghourchian, H.; Hashemnia, S. Direct electron transfer enhancement of covalently bound tyrosinase to glassy carbon via Woodward’s reagent K. Bioelectrochemistry 2011, 82, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Moghaddam, A.B.; Dinarvand, R.; Rezaei-Zarchi, S. Direct electron transfer of polyphenol oxidase on carbon nanotube surfaces: Application in biosensing. Int. J. Electrochem. Sci. 2009, 4, 895–905. [Google Scholar]
- Janegitz, B.C.; Medeiros, R.A.; Rocha-Filho, R.C.; Fatibello-Filho, O. Direct electrochemistry of tyrosinase and biosensing for phenol based on gold nanoparticles electrodeposited on a boron doped dimondelectrode. Diam. Relat. Mater. 2012, 25, 128–133. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Ganjali, M.R.; Saboury, A.A.; Moosavi-Movahedi, A.A.; Norouzi, P. Electrodeposition of nickel oxide nanoparticles on glassy carbon surfaces: Application to the direct electron transfer of tyrosinase. J. Appl. Electrochem. 2008, 38, 1233–1239. [Google Scholar] [CrossRef]
- Hou, S.F.; Kasner, M.L.; Su, S.J.; Patel, K.; Cuellari, R. Highly sensitive and selective dopamine biosensor fabricated with silanized grapheme. J. Phys. Chem. C 2010, 114, 14915–14921. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, S.S.; Zhang, H.Y.; Jiang, J.Q.; Liu, X.Y. A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal. Chim. Acta 2012, 709, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhai, Y.M.; Dong, S.J. Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Wang, Y.; Li, Y.M.; Feng, H.B.; Lu, J.; Li, J.H. Preparation, structure and electrochemical properties of graphene modified electrode. Adv. Funct. Mater. 2009, 19, 2782–2789. [Google Scholar] [CrossRef]
- Chen, D.; Tang, L.H.; Li, J.H. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Lai, Y.J.; Jiang, D.W.; Zeng, Y.B.; Xian, Y.Z.; Xiao, F.; Zhang, N.D.; Hou, J.; Jin, L.T. Ultrasensitive electrochemical immunoassay based on graphene oxide-Ag composites for rapid determination of clenbuterol. Analyst 2012, 137, 4349–4355. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.B.; He, Y.P.; Sheng, Q.L.; Zhang, H.F. DNA as a linker for biocatalytic deposition of Au nanoparticles on graphene and its application in glucose detection. J. Mater. Chem. 2011, 21, 12873–12879. [Google Scholar] [CrossRef]
- He, Y.P.; Sheng, Q.L.; Zheng, J.B.; Wang, M.Z.; Liu, B. Magnetite-graphene for the direct electrochemistry of hemoglobin and its biosensing application. Electrochim. Acta 2011, 56, 2471–2476. [Google Scholar] [CrossRef]
- Liu, C.B.; Wang, K.; Luo, S.L.; Tang, Y.H.; Chen, L.Y. Direct electrodeposition of graphene enabling the one-step synthesis of grapheme-metal nanocomposite films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. A green approach to the synthesis of graphenenanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, B.; Palanisamy, S.; Chen, S.M. A simple electrochemical approach to fabricate a glucose biosensor based on grapheme-glucose oxidase biocomposite. Biosens. Bioelectron. 2013, 39, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.L.; He, Y.P.; Zhang, D.W.; Zheng, J.B. Ultrasonic-electrodeposition of flower-like graphenenanosheets in ionic liquid and its sensing for ascorbic acid. J. Chin. Chem. Soc. 2013, 60, 199–203. [Google Scholar] [CrossRef]
- He, Y.P.; Zheng, J.B.; Dong, S.Y. Ultrasonic-electrodeposition of hierarchical flower-like cobalt on petalage-like graphene hybrid microstructures for hydrazine sensing. Analyst 2012, 137, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- He, Y.P.; Zheng, J.B. One-pot ultrasonic-electrodeposition of copper-graphene nanoflowers in Ethaline for glucose sensing. Anal. Methods 2013, 5, 767–772. [Google Scholar] [CrossRef]
- Shan, C.S.; Yang, H.F.; Han, D.X.; Zhang, Q.X.; Ivaska, A.; Niu, L. Water-soluble graphene covalently functionalized by biocompatible poly-l-lysine. Langmuir 2009, 25, 12030–12033. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.P.; Yuan, R.; Chai, Y.Q.; Yuan, Y.L.; Bai, L.J.; Wang, Y. Aptamer-based highly sensitive electrochemical detection of thrombin via the amplification of grapheme. Analyst 2012, 137, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zou, Z.X.; Shin, Y.S.; Wang, J.; Wu, H.; Engelhard, M.H.; Liu, J.; Aksay, I.A.; Lin, Y.H. Sensitive immunosensor for cancer biomarker based on dual signal amplification strategy of graphene sheets and multienzyme functionalized carbon nanospheres. Anal. Chem. 2010, 82, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.N.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; John, S.A. Determination of nanomolar uric and ascorbic acids using enlarged gold nanoparticles modified electrode. Anal. Biochem. 2009, 386, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Jena, B.K.; Percival, S.J.; Zhang, B. Highly sensitive detection of exocytotic dopamine release using a gold-nanoparticle-network microelectrode. Anal. Chem. 2011, 83, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Li, D.W.; Li, Y.T.; Li, Y.; Long, Y.T. Disposable biosensor based on graphene oxide conjugated with tyrosinase assembled gold nanoparticles. Biosens. Bioelectron. 2011, 26, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lin, X.Q. Modified electrode based on immobilizing horseradish peroxidase on nano-gold with choline covalently modified glassy carbon electrode as a base. Chin. J. Anal. Chem. 2008, 36, 604–608. [Google Scholar] [CrossRef]
- Li, Y.X.; Lin, X.Q.; Jiang, C.M. Fabrication of a nanobiocomposite film containing heme proteins and carbon nanotubes on a choline modified glassy carbon electrode: Direct electrochemistry and electrochemical catalysis. Electroanalysis 2006, 18, 2085–2091. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.X.; Huang, X.; Wang, L. Fabrication of layer-by-layer modified multilayer films containing choline and gold nanoparticles and its sensing application for electrochemical determination of dopamine and uric acid. Talanta 2007, 73, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Hu, S.S. Electrochemical sensors based on graphene materials. Microchim. Acta 2011, 175, 1–19. [Google Scholar] [CrossRef]
- Shleev, S.; Tkac, J.; Christenson, A.; Ruzgas, T.; Yaropolov, A.I.; Whittaker, J.W.; Gorton, L. Amperometric biosensors based on recombinant laccases for phenols determination. Biosens. Bioelectron. 2005, 20, 2517–2554. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Ye, B.X.; Zhou, X.Y. Direct electrochemical redox of tyrosinase at silver electrodes. Talanta 1997, 44, 831–836. [Google Scholar] [CrossRef]
- Xu, D.Y.; Yang, Y.; Yang, Z. Activity and stability of cross-linked tyrosinase aggregates in aqueous and nonaqueous media. J. Biotechnol. 2011, 152, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kamin, R.A.; Wilson, G.S. Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal. Chem. 1980, 52, 1198–1205. [Google Scholar] [CrossRef]
- Shu, F.R.; Wilson, G.S. Rotating ring-disk enzyme electrode for surface catalysis study. Anal. Chem. 1976, 48, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- López, M.S.; Pez-Cabarcos, E.; López-Ruiz, B. Influence of the host matrix of the enzyme in the performance of amperometric biosensors. Sens. Actuators B Chem. 2012, 171–172, 387–397. [Google Scholar]
- Pandey, P.; Singh, S.P.; Arya, S.K.; Gupta, V.; Datta, M.; Singh, S.; Malhotra, B.D. Application of thiolated gold nanoparticles for the enhancement of glucose oxidase activity. Langmuir 2007, 23, 3333–3337. [Google Scholar] [CrossRef] [PubMed]
- Kiralp, S.; Toppare, L. Polyphenol content in selected Turkish wines, an alternative method of detection of phenolics. Process Biochem. 2006, 41, 236–239. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Yang, X.; Han, Q.; Zheng, J. The Investigation of Electrochemistry Behaviors of Tyrosinase Based on Directly-Electrodeposited Grapheneon Choline-Gold Nanoparticles. Molecules 2017, 22, 1047. https://doi.org/10.3390/molecules22071047
He Y, Yang X, Han Q, Zheng J. The Investigation of Electrochemistry Behaviors of Tyrosinase Based on Directly-Electrodeposited Grapheneon Choline-Gold Nanoparticles. Molecules. 2017; 22(7):1047. https://doi.org/10.3390/molecules22071047
Chicago/Turabian StyleHe, Yaping, Xiaohui Yang, Quan Han, and Jianbin Zheng. 2017. "The Investigation of Electrochemistry Behaviors of Tyrosinase Based on Directly-Electrodeposited Grapheneon Choline-Gold Nanoparticles" Molecules 22, no. 7: 1047. https://doi.org/10.3390/molecules22071047