Augmented Anticancer Effects of Cantharidin with Liposomal Encapsulation: In Vitro and In Vivo Evaluation
Abstract
:1. Introduction
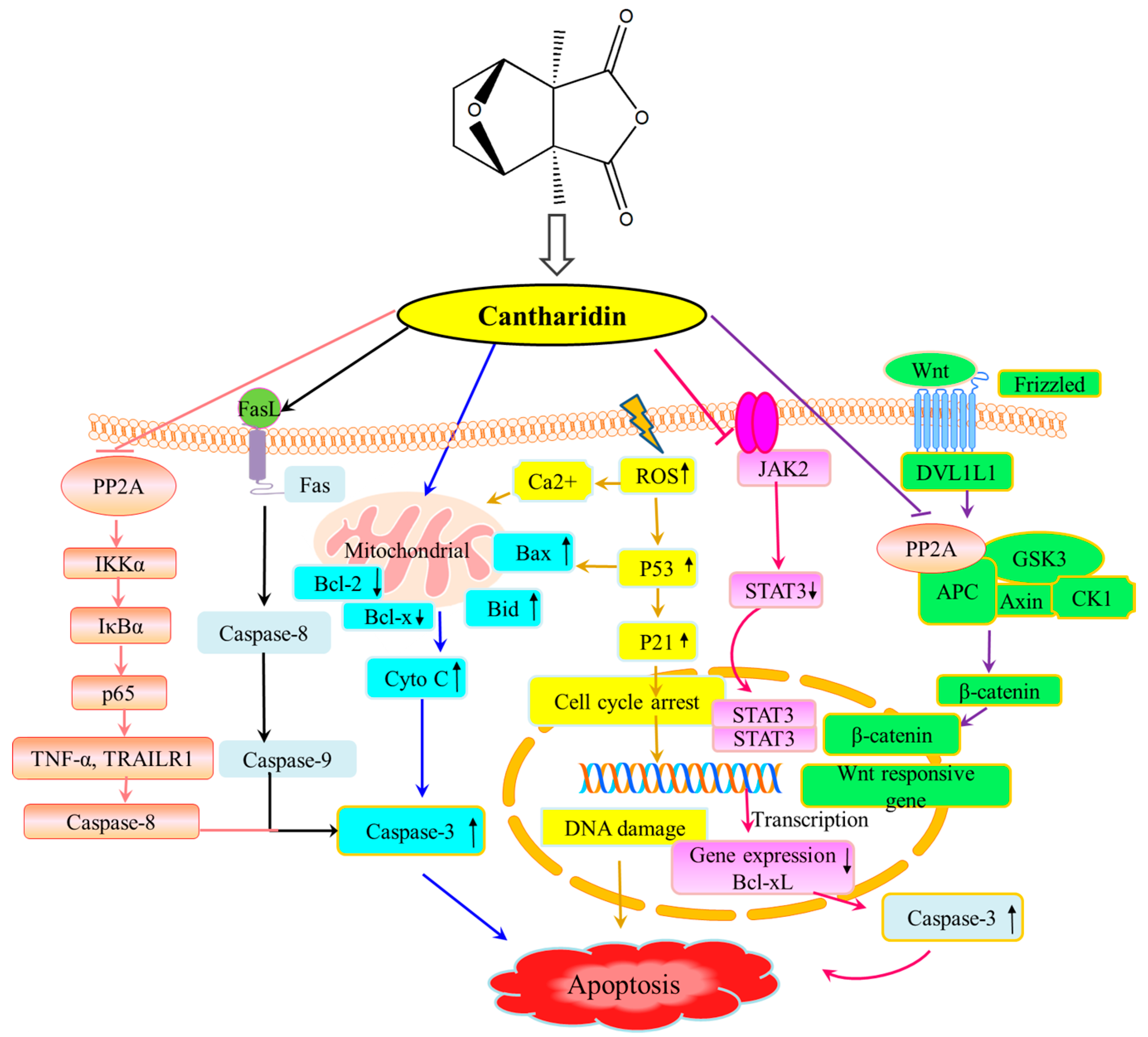
2. Results and discussion
2.1. Preparation of Liposomal Cantharidin and Its Characterization
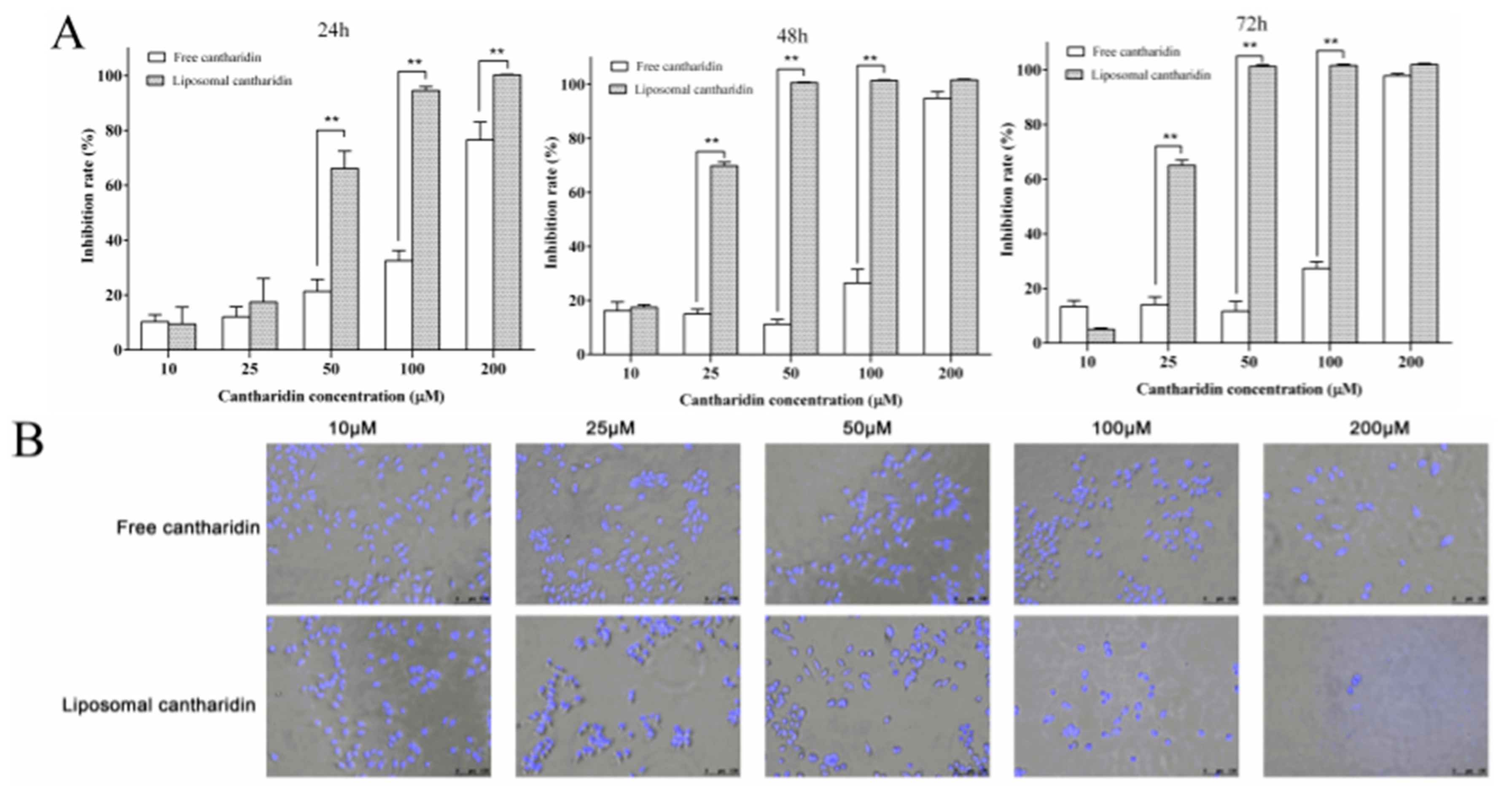
2.2. Anti-Proliferative Effects of Liposomal Cantharidin on HepG2 Cells
2.3. Cell and Nuclear Morphology Changes
2.4. Cell Cycle Analysis
2.5. Apoptosis Induced by Liposomal Cantharidin
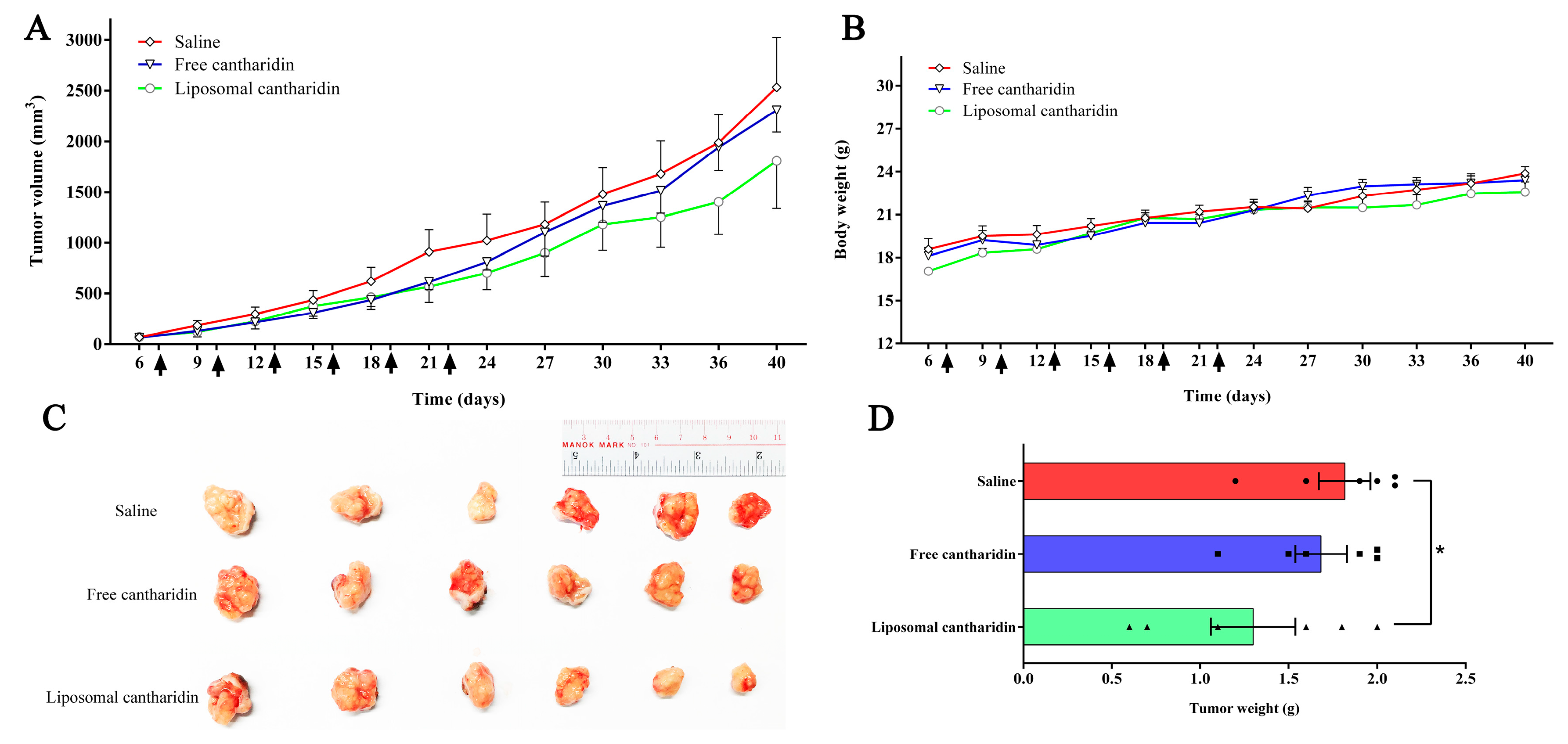
2.6. In vivo Therapeutic Efficacy
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Preparation of PEGylated Liposome Encapsulating Cantharidin
3.4. Drug Analysis
3.5. Characterization of Liposomal Cantharidin
3.6. Cell Culture
3.7. Cell Proliferation Assay
3.8. Detection of Apoptosis Morphologically with Hoechst 33342 Staining
3.9. Cell Cycle Analysis
3.10. Apoptosis Analysis by Flow Cytometry
3.11. In Vivo Therapeutic Efficacy
3.12. Statistical Analysis
4. Conclusion
Acknowledgments
Author contributions
Declaration of interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA: A Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Hernandez-Gea, V.; Llovet, J.M. Medical therapies for hepatocellular carcinoma: A critical view of the evidence. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Li, P.C.; Konkimalla, V.S.; Kaina, B. From traditional Chinese medicine to rational cancer therapy. Trends Mol. Med. 2007, 13, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S. Medical uses of mylabris in ancient China and recent studies. J. Ethnopharmacol. 1989, 26, 147–162. [Google Scholar] [CrossRef]
- Tian, X.; Zeng, G.; Li, X.; Wu, Z.; Wang, L. Cantharidin inhibits cell proliferation and promotes apoptosis in tongue squamous cell carcinoma through suppression of miR-214 and regulation of p53 and Bcl-2/Bax. Oncol. Rep. 2015, 33, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.H.; Chu, Y.L.; Yang, J.S.; Lin, J.P.; Lai, K.C.; Kuo, H.M.; Hsia, T.C.; Chung, J.G. Cantharidin induces apoptosis in human bladder cancer TSGH 8301 cells through mitochondria-dependent signal pathways. Int. J. Oncol. 2010, 37, 1243–1250. [Google Scholar] [PubMed]
- Sagawa, M.; Nakazato, T.; Uchida, H.; Ikeda, Y.; Kizaki, M. Cantharidin induces apoptosis of human multiple myeloma cells via inhibition of the JAK/STAT pathway. Cancer Sci. 2008, 99, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Z.; Zong, Y.; Gong, F.; Zhu, Y.; Zhu, Y.; Lv, J.; Zhang, J.; Xie, L.; Sun, Y.; et al. PP2A inhibitors induce apoptosis in pancreatic cancer cell line PANC-1 through persistent phosphorylation of IKKalpha and sustained activation of the NF-kappaB pathway. Cancer Lett. 2011, 304, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Wu, M.Y.; Shen, M.; Zhi, Q.; Liu, Z.Y.; Gong, F.R.; Tao, M.; Li, W. Cantharidin and norcantharidin impair stemness of pancreatic cancer cells by repressing the beta-catenin pathway and strengthen the cytotoxicity of gemcitabine and erlotinib. Int. J. Oncol. 2015, 47, 1912–1922. [Google Scholar] [PubMed]
- Kadioglu, O.; Kermani, N.S.; Kelter, G.; Schumacher, U.; Fiebig, H.H.; Greten, H.J.; Efferth, T. Pharmacogenomics of cantharidin in tumor cells. Biochem. Pharmacol. 2014, 87, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Hsia, T.C.; Yu, C.C.; Hsu, S.C.; Tang, N.Y.; Lu, H.F.; Huang, Y.P.; Wu, S.H.; Lin, J.G.; Chung, J.G. Cantharidin induces apoptosis of H460 human lung cancer cells through mitochondria-dependent pathways. Int. J. Oncol. 2014, 45, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Chen, Z.T.; Zhou, X.L.; Xu, W.; Wang, G.; Tang, X.X.; Luo, L.S.; Tu, J.F.; Zhu, Y.M.; Hu, W.; et al. Cantharidin induces G(2)/M phase arrest and apoptosis in human gastric cancer SGC-7901 and BGC-823 cells. Oncol. Lett. 2014, 8, 2721–2726. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.W.; Ko, S.W.; Tsai, H.Y.; Chung, J.G.; Chiang, J.H.; Chen, K.T.; Chen, Y.C.; Chen, H.Y.; Chen, Y.F.; Yang, J.S. Cantharidin induces G2/M phase arrest and apoptosis in human colorectal cancer colo 205 cells through inhibition of CDK1 activity and caspase-dependent signaling pathways. Int. J. Oncol. 2011, 38, 1067–1073. [Google Scholar] [PubMed]
- Li, W.; Xie, L.; Chen, Z.; Zhu, Y.; Sun, Y.; Miao, Y.; Xu, Z.; Han, X. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Sci. 2010, 101, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, Y.Z.; Song, L.; Wang, C.H.; Qi, T.G.; Shao, G.R. Effect of cantharidins in chemotherapy for hepatoma: a retrospective cohort study. Am. J. Chin. Med. 2014, 42, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.H.; Bao, Y.L.; Wu, Y.; Yu, C.L.; Meng, X.Y.; Li, Y.X. Cantharidin reverses multidrug resistance of human hepatoma HepG2/ADM cells via down-regulation of P-glycoprotein expression. Cancer Lett. 2008, 272, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Ying, K.; Xiao, Z.Y.; Zhou, B.; Huang, Q.S.; Wu, H.M.; Yin, M.; Xie, Y.; Mao, Y.M.; Rui, Y.C. Analysis of gene expression profiles in human HL-60 cell exposed to cantharidin using cDNA microarray. Int. J. Cancer 2004, 108, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ng, H.L.; Lu, A.; Lin, C.; Zhou, L.; Lin, G.; Zhang, Y.; Yang, Z.; Zhang, H. Drug delivery system targeting advanced hepatocellular carcinoma: Current and future. Nanomedicine: NBM 2016, 12, 853–869. [Google Scholar] [CrossRef] [PubMed]
- van der Veldt, A.A.; Hendrikse, N.H.; Smit, E.F.; Mooijer, M.P.; Rijnders, A.Y.; Gerritsen, W.R.; van der Hoeven, J.J.; Windhorst, A.D.; Lammertsma, A.A.; Lubberink, M. Biodistribution and radiation dosimetry of 11C-labelled docetaxel in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Polanska, H.; Rodrigo, M.A.M.; Guran, R.; Kulich, P.; Kopel, P.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Kizek, R.; et al. Prostate tumor attenuation in the nu/nu murine model due to anti-sarcosine antibodies in folate-targeted liposomes. Sci. Rep. 2016, 6, 33379. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Gumulec, J.; Fojtu, M.; Raudenska, M.; Sztalmachova, M.; Skotakova, A.; Vlachova, J.; Skalickova, S.; Nejdl, L.; Kopel, P.; Knopfova, L.; et al. Modulation of Induced Cytotoxicity of Doxorubicin by Using Apoferritin and Liposomal Cages. Int. J. Mol. Sci. 2014, 15, 22960–22977. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar] [PubMed]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of large unilamellar vesicles by a rapid extrusion procedure: characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta 1985, 812, 55–65. [Google Scholar] [CrossRef]
- Rello, S.; Stockert, J.C.; Moreno, V.; Gamez, A.; Pacheco, M.; Juarranz, A.; Canete, M.; Villanueva, A. Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis 2005, 10, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Reubold, T.F.; Eschenburg, S. A molecular view on signal transduction by the apoptosome. Cell. Signal. 2012, 24, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.W.; Tao, W.; Zhang, H.L.; Liu, G.; Wang, T.; Zhang, L.H.; Zeng, X.W.; Mei, L. Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system fore the treatment of liver cancer. Acta Biomater. 2016, 30, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Ruan, L.M.; Mao, W.W.; Wang, J.Q.; Shen, Y.Q.; Sui, M.H. Preparation of RGD-modified long circulating liposome loading matrine, and its in vitro anti-cancer effects. Int. J. Med. Sci. 2010, 7, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.C.; Zhang, H.; Qin, L.; Chen, H.; Fang, C.; Lu, A.; Yang, Z. Carbonic anhydrase IX-directed immunoliposomes for targeted drug delivery to human lung cancer cells in vitro. Drug Des. Devel. Ther. 2014, 8, 993–1001. [Google Scholar] [PubMed]
- Di Paolo, D.; Pastorino, F.; Zuccari, G.; Caffa, I.; Loi, M.; Marimpietri, D.; Brignole, C.; Perri, P.; Cilli, M.; Nico, B.; et al. Enhanced anti-tumor and anti-angiogenic efficacy of a novel liposomal fenretinide on human neuroblastoma. J. Control. Release 2013, 170, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Hanelt, M.; Gareis, M.; Kollarczik, B. Cytotoxicity of mycotoxins evaluated by the MTT-cell culture assay. Mycopathologia 1994, 128, 167–174. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Liposome Type | Particle Size (nm) | PDI | Encapsulation Efficacy (%) | |
|---|---|---|---|---|
| Liposomal cantharidin | Before extrusion | 547.6 ± 2.3 | 0.329 ± 0.006 | |
| After extrusion | 129.9 ± 2.5 | 0.087 ± 0.004 | 88.9 ± 0.1 | |
| Blank liposome after extrusion | 132.1 ± 0.8 | 0.071 ± 0.022 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Lin, C.-C.; Chan, W.-K.-N.; Liu, K.-L.; Yang, Z.-J.; Zhang, H.-Q. Augmented Anticancer Effects of Cantharidin with Liposomal Encapsulation: In Vitro and In Vivo Evaluation. Molecules 2017, 22, 1052. https://doi.org/10.3390/molecules22071052
Zhang X, Lin C-C, Chan W-K-N, Liu K-L, Yang Z-J, Zhang H-Q. Augmented Anticancer Effects of Cantharidin with Liposomal Encapsulation: In Vitro and In Vivo Evaluation. Molecules. 2017; 22(7):1052. https://doi.org/10.3390/molecules22071052
Chicago/Turabian StyleZhang, Xue, Cong-Cong Lin, Wai-Kei-Nickie Chan, Kang-Lun Liu, Zhi-Jun Yang, and Hong-Qi Zhang. 2017. "Augmented Anticancer Effects of Cantharidin with Liposomal Encapsulation: In Vitro and In Vivo Evaluation" Molecules 22, no. 7: 1052. https://doi.org/10.3390/molecules22071052




