Light-Assisted Advanced Oxidation Processes for the Elimination of Chemical and Microbiological Pollution of Wastewaters in Developed and Developing Countries
Abstract
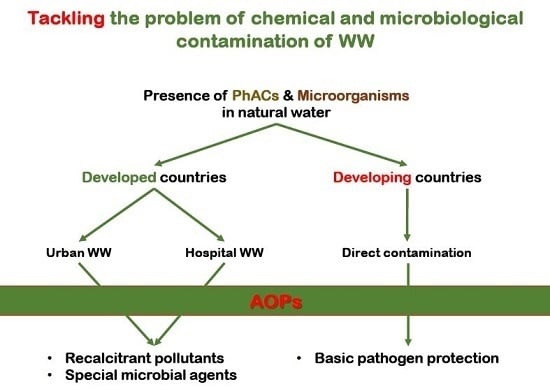
:1. Problems Related with Presence of Microorganisms and Micropollutants
2. Hospital Wastewater Effluents and Types and Pollutants
2.1. Hospital Wastewater and Micropollutants
- (i)
- Human sewage
- (ii)
- Kitchen and laundry
- (iii)
- Heating and cooling processes
- (iv)
- Laboratorial discharge (clinics and research centers)
- (v)
- Wards and outpatients contribution
2.2. Hospital Wastewaters and Microorganisms
3. Light-Assisted AOPs and Their Action in Chemical and Microbiological Pollutants’ Degradation
- Ozone-based: O3/H2O2, O3/UV, O3/UV/H2O2
- UV-based: UV, UV/H2O2
- Fenton-related: (Fe/H2O2), including photo-Fenton, electro-Fenton, etc.
- Heterogeneous photocatalysis, such as (TiO2/hv)
- γ-radiolysis
- Ultrasound-based: sonolysis, ultrasound-supported Fenton, etc.
3.1. UV-Based Processes (UV, UV/H2O2)
3.2. Fenton-Related Reactions (Fenton, Photo-Fenton, Solar Light)
4. Problem Identification and Contextualization: Micropollutants and Microorganisms in Developed and Developing Countries
5. Treatment Strategy and Research Results
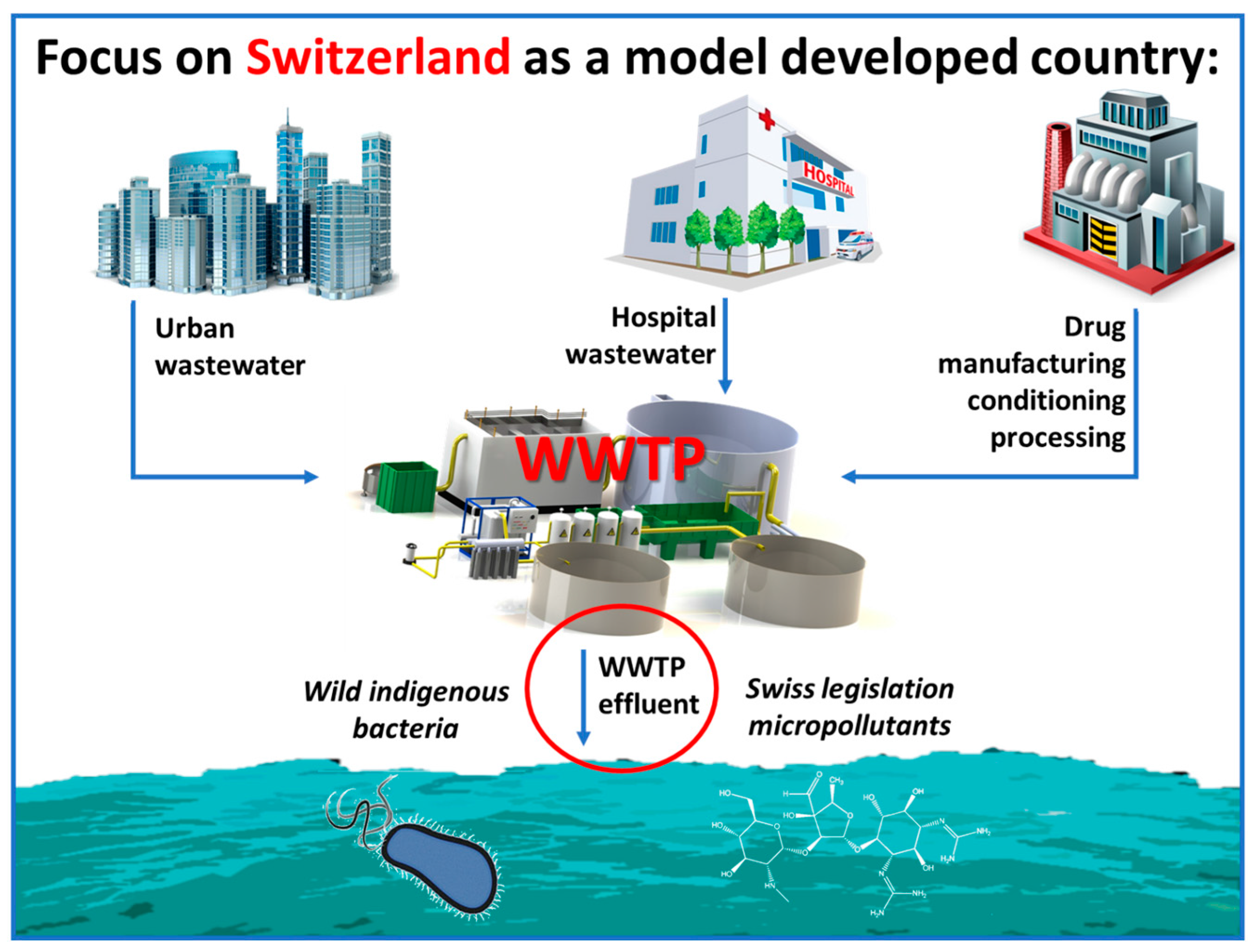
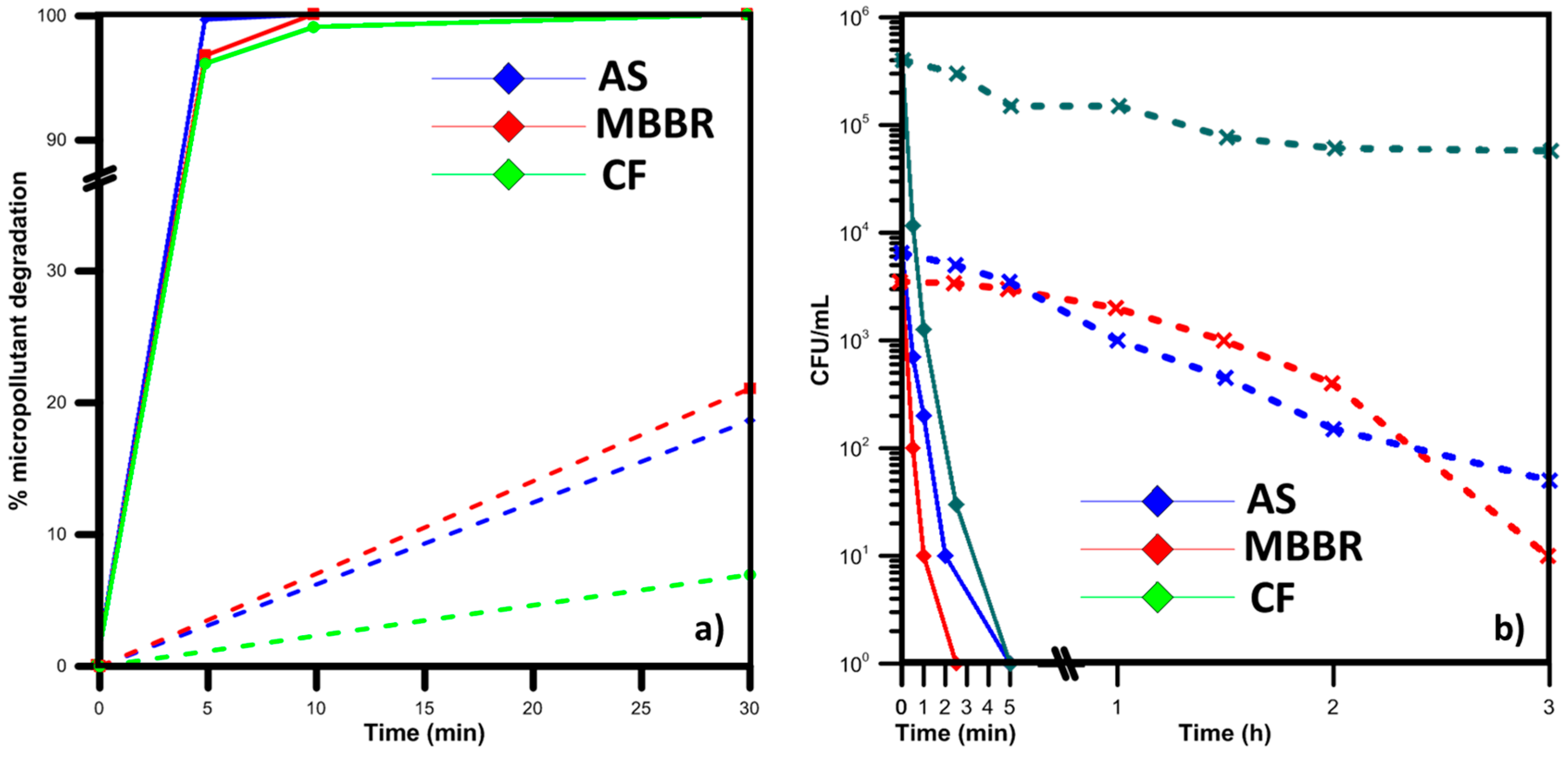
5.1. Developed Countries and Municipal WWTPs: Treatment of MPs and MOs by Light-Assisted AOPs
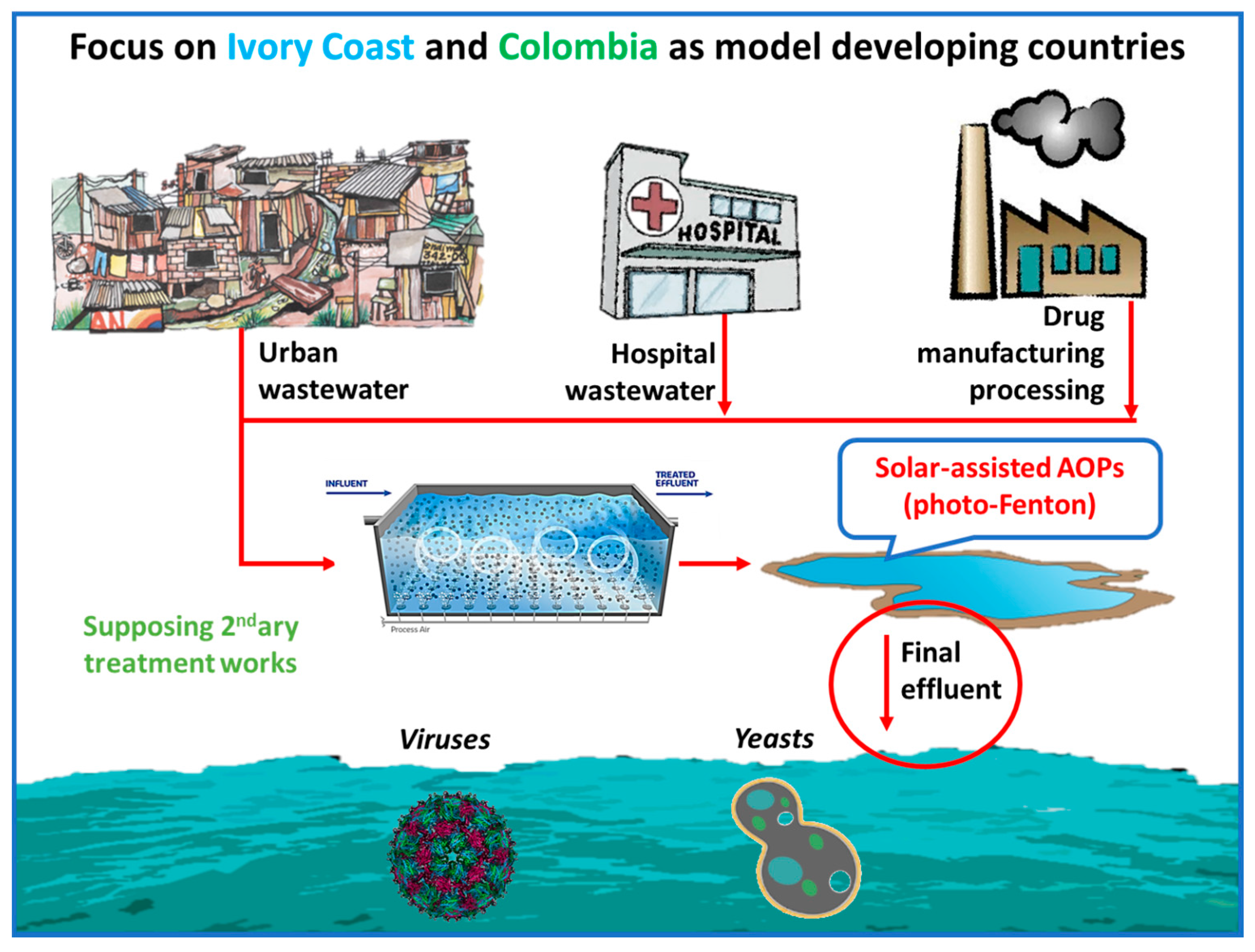
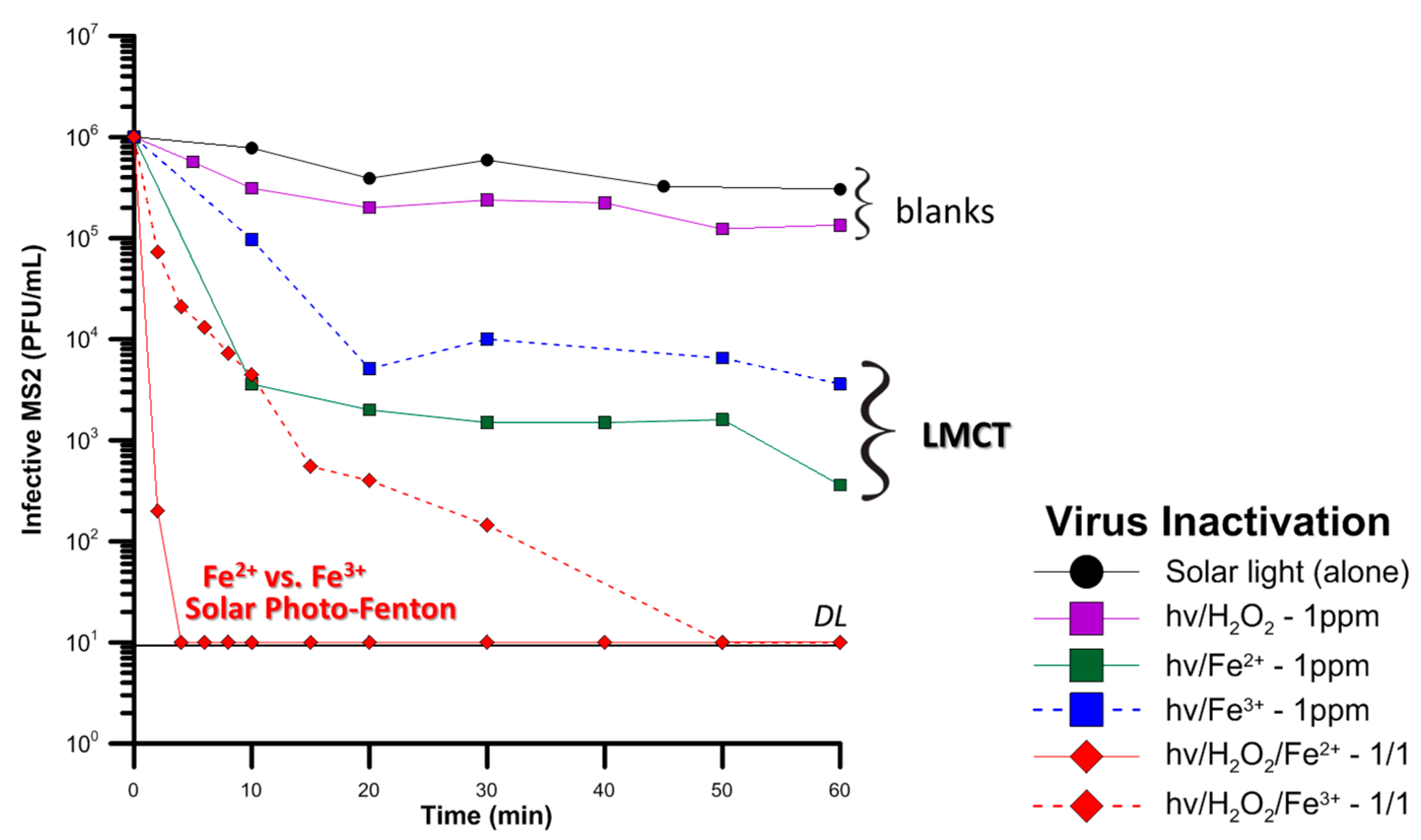
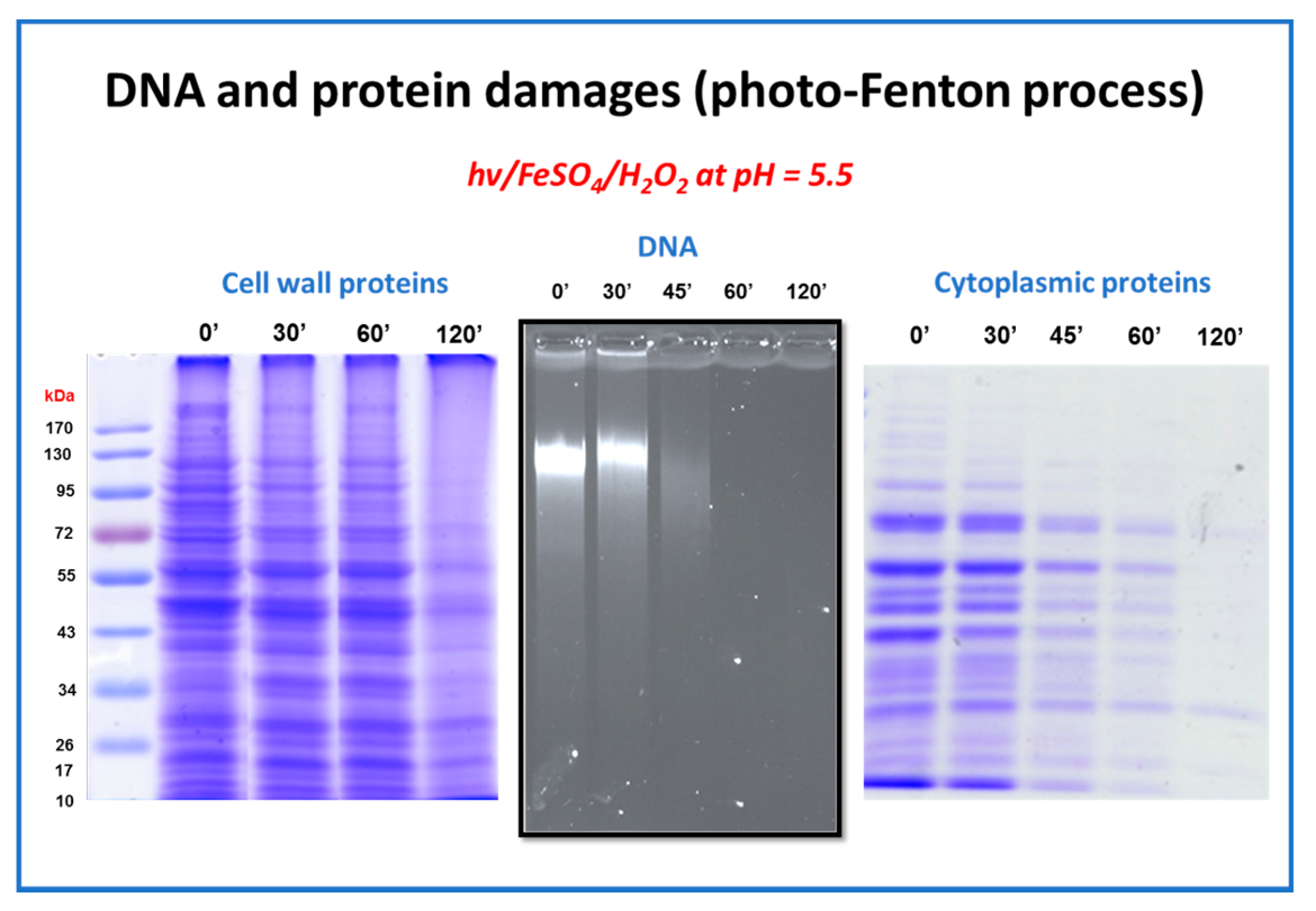
5.2. Developing Countries and Microorganisms Disinfection by Photo-Fenton
- (i)
- Solar light alone is unable to inflict high removal of MS2, hence underlines the need for application of an oxidative process.
- (ii)
- Adding 1 mg/L H2O2, which is a moderate amount for inactivation of microorganisms, but could simulate the in-situ generation of H2O2 by irradiation of Dissolved Organic Matter (DOM) [84], again has almost no effect. The mild oxidative potential of H2O2 is unable to inactivate more than 1 log of MS2.
- (iii)
- Continuing with the components of the photo-Fenton process, iron, in salts form, Fe2+ or Fe3+ was tested. Natural waters in Africa have often been found to contain high amounts of iron, especially in the form of oxides [85] but also dissolved. Although iron has no oxidative actions, its complexation with the viral capsid enables, upon irradiation, a LMCT reaction, with the reduction of Fe3+ to Fe2+ and oxidation of the ligand. This oxidation damages the external capsid proteins, thus reducing the infectivity of the virus.
- (iv)
- The voluntary addition of very low amounts of H2O2 in the bulk indicate that the photo-Fenton process is potentially a very efficient treatment technique in the elimination of viral pathogens; the inactivation is either very sharp, or at least faster than the Fe or solar alone. Fe2+ is readily oxidizable, generating HO• species, reaching viral inactivation, and Fe3+ after an initial reduction step, participates in the photo-Fenton catalytic cycle.
- (v)
- Finally, the presence of organic matter in all the experiments did not appear to significantly hinder the inactivation of viruses. In fact, in presence of organic matter, the quantity of dissolved iron was followed, and the precipitation due to the neutral operating pH was avoided. Hence, a sustainable catalytic cycle can be maintained, aided by the iron-DOM complexes in WW [86].
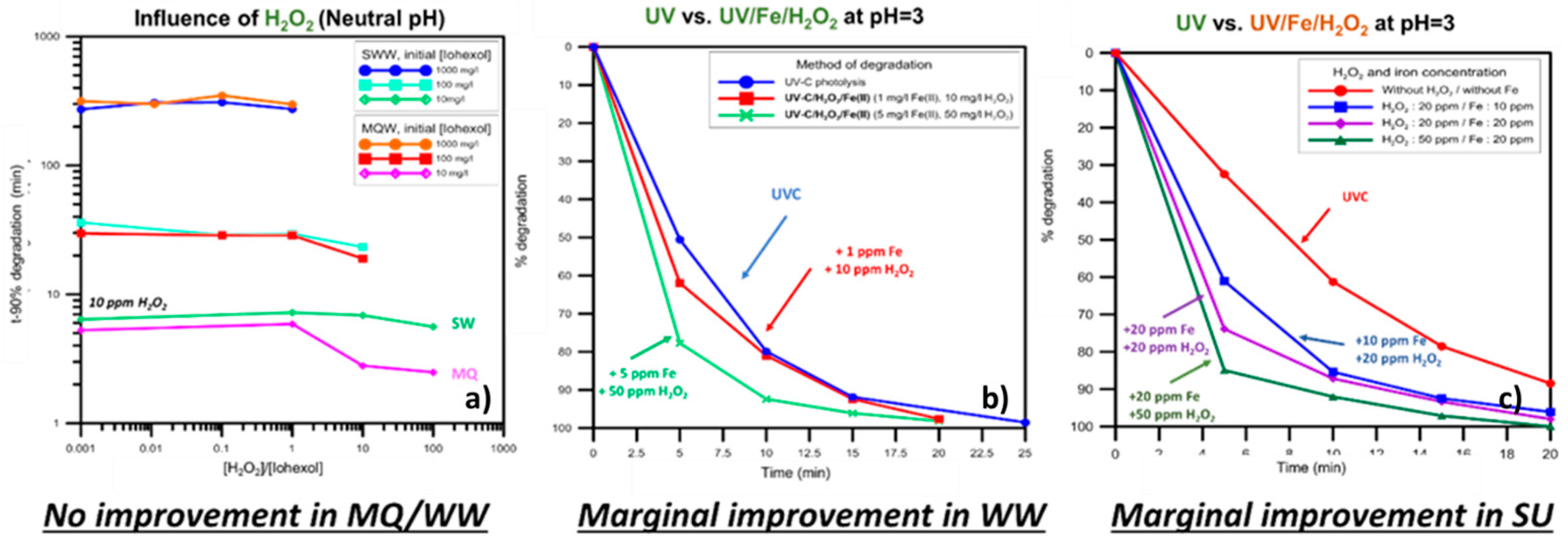
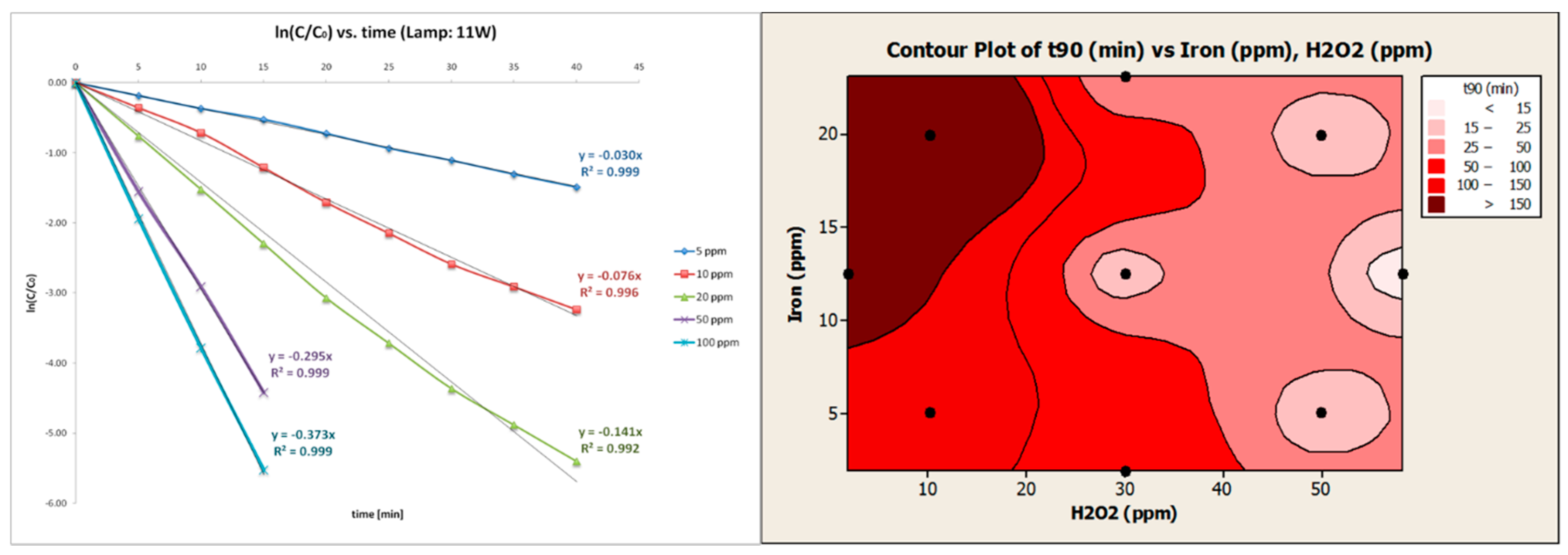
5.3. Hospital-Derived and Highly Concentrated Flows in Developed and Developing Countries: Iodinated Contrast Media and Drugs Treatment by Light-Assisted AOPs
6. Conclusions
- (1)
- UV-based AOPs are efficient for MP removal and MO inactivation. Although changing dynamically, the Swiss reality on hospital wastewater treatment dictates their discharge in the municipal collection network, and therefore implies their co-treatment with municipal wastes. The UV-based AOPs (UV and UV/H2O2) were found to be effective micropollutant removal strategies in ng/L level and bacterial inactivating processes, after biological secondary pre-treatment, as found in municipal wastewaters. When used in simulated hospital wastewaters and urine treatment, as alternative micropollutant elimination strategies, their efficiency was measured and established against a list of contaminants, with parallel elimination of the contained organic matter. The degradation was fast, and the reactants addition and necessary light doses were moderate.
- (2)
- The solar photo-Fenton process and its constituents can be very effective in the proper context. Despite the lower apparent efficiency of this process when compared with its UV-based counterparts, photo-Fenton was found to effectively and non-selectively remove micropollutants and effluent organic matter. Furthermore, their application resulted in high bacterial removal, regrowth suppression, and yeasts and viruses inactivation from water and wastewater effluents. Most importantly, through systematic studies the mechanism and the key points of the process against the aforementioned targets were characterized. Special emphasis was given to the organic matter present in WW, as it is found to hinder the inactivation process but other benefits, such as iron complexation, also occur.
- (3)
- The selected model hospital/industrial contaminants (Iohexol, Venlafaxine) helped elucidate the pitfalls and opportunities in HWW treatment by AOPs. The AOPs were found to work particularly well against the concentrated, (simulated) industrial wastewater, hospital flows and urine. Therefore, their application in hospitals and related industrial activities is promising. In addition, the structural deformation of the selected pollutants provided helpful insights on the operational and chemical constraints on applying the various AOPs; for instance the use of iron (when H2O2 is present) is strongly recommended for faster and more intense degradation of the contaminants in HWW. Finally, apart from the degradation point of view, the AOPs studied increased the biodegradability of the selected compounds treated solutions, which could allow their use as pre-treatment methods in HWWTPs.
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. Environmental considerations on solar disinfection of wastewater and the subsequent bacterial (re)growth. Photochem. Photobiol. Sci. 2015, 14, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Merino Gamo, A.I.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. Monitoring the post-irradiation E. coli survival patterns in environmental water matrices: Implications in handling solar disinfected wastewater. Chem. Eng. J. 2014, 253, 366–376. [Google Scholar] [CrossRef]
- Liberti, L.; Notarnicola, M.; Petruzzelli, D. Advanced treatment for municipal wastewater reuse in agriculture. UV disinfection: Parasite removal and by-product formation. Desalination 2003, 152, 315–324. [Google Scholar] [CrossRef]
- McGuigan, K.G.; Conroy, R.M.; Mosler, H.J.; du Preez, M.; Ubomba-Jaswa, E.; Fernandez-Ibanez, P. Solar water disinfection (SODIS): A review from bench-top to roof-top. J. Hazard. Mater. 2012, 235–236, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Wichelns, D.; Raschid-Sally, L.; McCornick, P.G.; Drechsel, P.; Bahri, A.; Minhas, P. The challenges of wastewater irrigation in developing countries. Agric. Water Manag. 2010, 97, 561–568. [Google Scholar] [CrossRef]
- Hoebe, C.J.; Vennema, H.; de Roda Husman, A.M.; van Duynhoven, Y.T. Norovirus outbreak among primary schoolchildren who had played in a recreational water fountain. J. Infect. Dis. 2004, 189, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Focazio, M.J.; Kolpin, D.W.; Barnes, K.K.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Barber, L.B.; Thurman, M.E. A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States—(II) Untreated drinking water sources. Sci. Total Environ. 2008, 402, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The Challenge of Micropollutants in Aquatic Systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, V.; Chèvre, N. Assessing the risks posed by mixtures of chemicals in freshwater environments: Case study of Lake Geneva, Switzerland. Wiley Interdiscip. Rev. Water 2014, 1, 229–247. [Google Scholar] [CrossRef]
- Backhaus, T.; Altenburger, R.; Arrhenius, Å.; Blanck, H.; Faust, M.; Finizio, A.; Gramatica, P.; Grote, M.; Junghans, M.; Meyer, W.; et al. The BEAM-project: Prediction and assessment of mixture toxicities in the aquatic environment. Cont. Shelf Res. 2003, 23, 1757–1769. [Google Scholar] [CrossRef]
- Deneer, J.W. Toxicity of mixtures of pesticides in aquatic systems. Pest Manag. Sci. 2000, 56, 516–520. [Google Scholar] [CrossRef]
- Junghans, M.; Backhaus, T.; Faust, M.; Scholze, M.; Grimme, L.H. Application and validation of approaches for the predictive hazard assessment of realistic pesticide mixtures. Aquat. Toxicol. 2006, 76, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Rodney, S.I.; Teed, R.S.; Moore, D.R.J. Estimating the Toxicity of Pesticide Mixtures to Aquatic Organisms: A Review. Hum. Ecol. Risk Assess. Int. J. 2013, 19, 1557–1575. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Al Aukidy, M.; Galletti, A.; Petrovic, M.; Barcelo, D. Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci. Total Environ. 2012, 430, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, E.; Keck, G.; Blanchard, J.-M.; Vermande, P.; Perrodin, Y. Toxicological effects of disinfections using sodium hypochlorite on aquatic organisms and its contribution to AOX formation in hospital wastewater. Environ. Int. 2004, 30, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Boillot, C.; Bazin, C.; Tissot-Guerraz, F.; Droguet, J.; Perraud, M.; Cetre, J.C.; Trepo, D.; Perrodin, Y. Daily physicochemical, microbiological and ecotoxicological fluctuations of a hospital effluent according to technical and care activities. Sci. Total Environ. 2008, 403, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, E.; Perrodin, Y.; Keck, G.; Blanchard, J.M.; Vermande, P. Ecotoxicological risk assessment of hospital wastewater: A proposed framework for raw effluents discharging into urban sewer network. J. Hazard. Mater. 2005, 117, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hawkshead, J.J., III. Hospital wastewater containing pharmaceutically active compounds and drug-resistant organisms: A source of environmental toxicity and increased antibiotic resistance. J. Residuals Sci. Technol. 2008, 5, 51–60. [Google Scholar]
- Altin, A.; Altin, S.; Degirmenci, M. Characteristics and treatability of hospital(medical) wastewaters. Fresenius Environ. Bull. 2003, 12, 1098–1108. [Google Scholar]
- Pauwels, B.; Verstraete, W. The treatment of hospital wastewater: An appraisal. J. Water Health 2006, 4, 405–416. [Google Scholar] [PubMed]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Elimination of pharmaceuticals in sewage treatment plants in Finland. Water Res. 2007, 41, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece. J. Hazard. Mater. 2010, 179, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—A review. Chemosphere 2001, 45, 957–969. [Google Scholar] [CrossRef]
- Mahnik, S.; Lenz, K.; Weissenbacher, N.; Mader, R.; Fuerhacker, M. Fate of 5-fluorouracil, doxorubicin, epirubicin, and daunorubicin in hospital wastewater and their elimination by activated sludge and treatment in a membrane-bio-reactor system. Chemosphere 2007, 66, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.; Lema, J.M.; Omil, F. Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresour. Technol. 2009, 100, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J. Hydrol. 2010, 389, 416–428. [Google Scholar] [CrossRef]
- Jones, O.H.; Voulvoulis, N.; Lester, J. Human pharmaceuticals in wastewater treatment processes. Crit. Rev. Environ. Sci. Technol. 2005, 35, 401–427. [Google Scholar] [CrossRef]
- Ikehata, K.; Jodeiri Naghashkar, N.; Gamal El-Din, M. Degradation of Aqueous Pharmaceuticals by Ozonation and Advanced Oxidation Processes: A Review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Lienert, J.; Burki, T.; Escher, B. Reducing micropollutants with source control: Substance flow analysis of 212 pharmaceuticals in faeces and urine. Water Sci. Technol. 2007, 56, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Carraro, E.; Bonetta, S.; Bertino, C.; Lorenzi, E.; Bonetta, S.; Gilli, G. Hospital effluents management: Chemical, physical, microbiological risks and legislation in different countries. J. Environ. Manag. 2016, 168, 185–199. [Google Scholar] [CrossRef] [PubMed]
- El-Ogri, F.; Ouazzani, N.; Boraâm, F.; Mandi, L. A survey of wastewaters generated by a hospital in Marrakech city and their characterization. Desalination Water Treat. 2016, 57, 17061–17074. [Google Scholar]
- Kilunga, P.I.; Kayembe, J.M.; Laffite, A.; Thevenon, F.; Devarajan, N.; Mulaji, C.K.; Mubedi, J.I.; Yav, Z.G.; Otamonga, J.-P.; Mpiana, P.T. The impact of hospital and urban wastewaters on the bacteriological contamination of the water resources in Kinshasa, Democratic Republic of Congo. J. Environ.Sci. Health Part A 2016, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gernjak, W.; Fuerhacker, M.; Fernández-Ibañez, P.; Blanco, J.; Malato, S. Solar photo-Fenton treatment—Process parameters and process control. Appl. Catal. B Environ. 2006, 64, 121–130. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Kalogerakis, N. Treatment of olive mill effluents: Part I. Organic matter degradation by chemical and biological processes—An overview. Environ. Int. 2005, 31, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Oller, I.; Malato, S.; Sanchez-Perez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.; Silva, A.M. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Frontistis, Z.; Fatta-Kassinos, D. Removal of pharmaceuticals from environmentally relevant matrices by advanced oxidation processes (AOPs). Compr. Anal. Chem. 2013, 62, 345–407. [Google Scholar]
- Oppenländer, T. Photochemical Purification of Water and Air: Advanced Oxidation Processes (AOPs)—Principles, Reaction Mechanisms, Reactor Concepts; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Legrini, O.; Oliveros, E.; Braun, A. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Kim, I.; Yamashita, N.; Tanaka, H. Performance of UV and UV/H2O2 processes for the removal of pharmaceuticals detected in secondary effluent of a sewage treatment plant in Japan. J. Hazard. Mater. 2009, 166, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Yamashita, N.; Tanaka, H. Photodegradation of pharmaceuticals and personal care products during UV and UV/H2O2 treatments. Chemosphere 2009, 77, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Alpert, S.M.; Knappe, D.R.; Ducoste, J.J. Modeling the UV/hydrogen peroxide advanced oxidation process using computational fluid dynamics. Water Res. 2010, 44, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Zapata, A.; Oller, I.; Rizzo, L.; Hilgert, S.; Maldonado, M.; Sánchez-Pérez, J.; Malato, S. Evaluation of operating parameters involved in solar photo-Fenton treatment of wastewater: Interdependence of initial pollutant concentration, temperature and iron concentration. Appl. Catal. B Environ. 2010, 97, 292–298. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Stasinakis, A. Use of selected advanced oxidation processes (AOPs) for wastewater treatment—A mini review. Glob. NEST J. 2008, 10, 376–385. [Google Scholar]
- De Laat, J.; Gallard, H. Catalytic decomposition of hydrogen peroxide by Fe (III) in homogeneous aqueous solution: Mechanism and kinetic modeling. Environ. Sci. Technol. 1999, 33, 2726–2732. [Google Scholar] [CrossRef]
- Poyatos, J.; Muñio, M.; Almecija, M.; Torres, J.; Hontoria, E.; Osorio, F. Advanced oxidation processes for wastewater treatment: State of the art. Water Air Soil Pollut. 2010, 205, 187–204. [Google Scholar] [CrossRef]
- Giannakis, S.; Polo López, M.I.; Spuhler, D.; Sánchez Pérez, J.A.; Fernández Ibáñez, P.; Pulgarin, C. Solar disinfection is an augmentable, in situ-generated photo-Fenton reaction—Part 1: A review of the mechanisms and the fundamental aspects of the process. Appl. Catal. B Environ. 2016, 199, 199–223. [Google Scholar] [CrossRef]
- Giannakis, S.; Polo López, M.I.; Spuhler, D.; Sánchez Pérez, J.A.; Fernández Ibáñez, P.; Pulgarin, C. Solar disinfection is an augmentable, in situ-generated photo-Fenton reaction—Part 2: A review of the applications for drinking water and wastewater disinfection. Appl. Catal. B Environ. 2016, 198, 431–446. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Petrović, M.; Barceló, D. Wastewater treatment plants as a pathway for aquatic contamination by pharmaceuticals in the Ebro river basin (Northeast Spain). Environ. Toxicol. Chem. 2007, 26, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.-H.; Lin, A.Y.-C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, H.; Adams, C.D.; Gamagedara, S.; Stayton, I.; Timmons, T.; Ma, Y. Investigation of pharmaceuticals in Missouri natural and drinking water using high performance liquid chromatography-tandem mass spectrometry. Water Res. 2011, 45, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Vulliet, E.; Cren-Olivé, C.; Grenier-Loustalot, M.-F. Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Environ. Chem. Lett. 2011, 9, 103–114. [Google Scholar] [CrossRef]
- Kümmerer, K. Emerging Contaminants versus Micro-pollutants. CLEAN Soil Air Water 2011, 39, 889–890. [Google Scholar] [CrossRef]
- Giannakis, S.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. Solar disinfection modeling and post-irradiation response of Escherichia coli in wastewater. Chem. Eng. J. 2015, 281, 588–598. [Google Scholar] [CrossRef]
- Giannakis, S.; Merino Gamo, A.I.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. Impact of different light intermittence regimes on bacteria during simulated solar treatment of secondary effluent: Implications of the inserted dark periods. Sol. Energy 2013, 98, 572–581. [Google Scholar] [CrossRef]
- Giannakis, S.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. The antagonistic and synergistic effects of temperature during solar disinfection of synthetic secondary effluent. J. Photochem. Photobiol. A Chem. 2014, 280, 14–26. [Google Scholar] [CrossRef]
- Giannakis, S.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. Elucidating bacterial regrowth: Effect of disinfection conditions in dark storage of solar treated secondary effluent. J. Photochem. Photobiol. A Chem. 2014, 290, 43–53. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B. Photocatalytic treatment of high concentration carbamazepine in synthetic hospital wastewater. J. Hazard. Mater. 2012, 199, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ganzenko, O.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Electrochemical advanced oxidation and biological processes for wastewater treatment: A review of the combined approaches. Environ. Sci. Pollut. Res. 2014, 21, 8493–8524. [Google Scholar] [CrossRef] [PubMed]
- Kajitvichyanukul, P.; Suntronvipart, N. Evaluation of biodegradability and oxidation degree of hospital wastewater using photo-Fenton process as the pretreatment method. J. Hazard. Mater. 2006, 138, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Köhler, C.; Venditti, S.; Igos, E.; Klepiszewski, K.; Benetto, E.; Cornelissen, A. Elimination of pharmaceutical residues in biologically pre-treated hospital wastewater using advanced UV irradiation technology: A comparative assessment. J. Hazard. Mater. 2012, 239, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kovalova, L.; Siegrist, H.; von Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; McArdell, C.S. Elimination of Micropollutants during Post-Treatment of Hospital Wastewater with Powdered Activated Carbon, Ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, U.; Hastrup, C.; Klausen, M.M.; Pedersen, B.M.; Kristensen, G.H.; Jansen, J.L.C.; Bak, S.N.; Tuerk, J. Removal of APIs and bacteria from hospital wastewater by MBR plus O(3), O(3) + H(2)O(2), PAC or ClO(2). Water Sci. Technol. 2013, 67, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Sprehe, M.; Geissen, S.U.; Vogelpohl, A. Photochemical oxidation of iodized X-ray contrast media (XRC) in hospital wastewater. Water Sci.Technol. J. Int. Assoc. Water Pollut. Res. 2001, 44, 317–323. [Google Scholar]
- Carra, I.; Sánchez Pérez, J.A.; Malato, S.; Autin, O.; Jefferson, B.; Jarvis, P. Performance of different advanced oxidation processes for tertiary wastewater treatment to remove the pesticide acetamiprid. J. Chem. Technol. Biotechnol. 2016, 91, 72–81. [Google Scholar] [CrossRef]
- Rivas, G.; Carra, I.; García Sánchez, J.L.; Casas López, J.L.; Malato, S.; Sánchez Pérez, J.A. Modelling of the operation of raceway pond reactors for micropollutant removal by solar photo-Fenton as a function of photon absorption. Appl. Catal. B Environ. 2015, 178, 210–217. [Google Scholar] [CrossRef]
- Klamerth, N.; Malato, S.; Agüera, A.; Fernández-Alba, A.; Mailhot, G. Treatment of municipal wastewater treatment plant effluents with modified photo-Fenton as a tertiary treatment for the degradation of micro pollutants and disinfection. Enviro. Sci. Technol. 2012, 46, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Gamarra Vives, F.A.; Grandjean, D.; Magnet, A.; De Alencastro, L.F.; Pulgarin, C. Effect of advanced oxidation processes on the micropollutants and the effluent organic matter contained in municipal wastewater previously treated by three different secondary methods. Water Res. 2015, 84, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Voumard, M.; Grandjean, D.; Magnet, A.; De Alencastro, L.F.; Pulgarin, C. Micropollutant degradation, bacterial inactivation and regrowth risk in wastewater effluents: Influence of the secondary (pre) treatment on the efficiency of Advanced Oxidation Processes. Water Res. 2016, 102, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; De Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M. Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? Sci. Total Environ. 2013, 461, 480–498. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, N.; Esquius, L.; Grandjean, D.; Magnet, A.; Tungler, A.; de Alencastro, L.F.; Pulgarín, C. Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Res. 2013, 47, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Von Sperling, M. Performance evaluation and mathematical modelling of coliform die-off in tropical and subtropical waste stabilization ponds. Water Res. 1999, 33, 1435–1448. [Google Scholar] [CrossRef]
- Von Sperling, M. Modelling of coliform removal in 186 facultative and maturation ponds around the world. Water Res. 2005, 39, 5261–5273. [Google Scholar] [CrossRef] [PubMed]
- Canonica, S. Oxidation of aquatic organic contaminants induced by excited triplet states. CHIMIA Int. J. Chem. 2007, 61, 641–644. [Google Scholar] [CrossRef]
- Ndounla, J.; Spuhler, D.; Kenfack, S.; Wéthé, J.; Pulgarin, C. Inactivation by solar photo-Fenton in pet bottles of wild enteric bacteria of natural well water: Absence of re-growth after one week of subsequent storage. Appl. Catal. B Environ. 2013, 129, 309–317. [Google Scholar] [CrossRef]
- Giannakis, S.; Liu, S.; Carratalà, A.; Rtimi, S.; Bensimon, M.; Pulgarin, C. Effect of Fe (II)/Fe (III) species, pH, irradiance and bacterial presence on viral inactivation in wastewater by the photo-Fenton process: Kinetic modeling and mechanistic interpretation. Appl. Catal. B Environ. 2017, 204, 156–166. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Nguyen, D.A.; Baskaran, K. Performance evaluation of different ultrafiltration membranes for the reclamation and reuse of secondary effluent. Desalination 2011, 279, 383–389. [Google Scholar] [CrossRef]
- Giannakis, S.; Ruales-Lonfat, C.; Rtimi, S.; Thabet, S.; Cotton, P.; Pulgarin, C. Castles fall from inside: Evidence for dominant internal photo-catalytic mechanisms during treatment of Saccharomyces cerevisiae by photo-Fenton at near-neutral pH. Appl. Catal. B Environ. 2016, 185, 150–162. [Google Scholar] [CrossRef]
- Giannakis, S.; Jovic, M.; Gasilova, N.; Pastor Gelabert, M.; Schindelholz, S.; Furbringer, J.-M.; Girault, H.; Pulgarin, C. Iohexol degradation in wastewater and urine by UV-based Advanced Oxidation Processes (AOPs): Process modeling and by-products identification. J. Environ. Manag. 2017, 195, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Bisesi, J.H.; Bridges, W.; Klaine, S.J. Effects of the antidepressant venlafaxine on fish brain serotonin and predation behavior. Aquat. Toxicol. 2014, 148, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.P.; Ford, A.T. The biological effects of antidepressants on the molluscs and crustaceans: A review. Aquat. Toxicol. 2014, 151, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.P.; Molnar, N. Antidepressants cause foot detachment from substrate in five species of marine snail. Mar. Environ. Res. 2013, 84, 24–30. [Google Scholar] [CrossRef] [PubMed]
- García-Galán, M.J.; Anfruns, A.; Gonzalez-Olmos, R.; Rodríguez-Mozaz, S.; Comas, J. UV/H2O2 degradation of the antidepressants venlafaxine and O-desmethylvenlafaxine: Elucidation of their transformation pathway and environmental fate. J. Hazard. Mater. 2016, 311, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Lambropoulou, D.; Evgenidou, E.; Saliverou, V.; Kosma, C.; Konstantinou, I. Degradation of venlafaxine using TiO2/UV process: Kinetic studies, RSM optimization, identification of transformation products and toxicity evaluation. J. Hazard. Mater. 2017, 323, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Hendaoui, I.; Jovic, M.; Grandjean, D.; De Alencastro, L.F.; Girault, H.; Pulgarin, C. Solar photo-Fenton and UV/H2O2 processes against the antidepressant Venlafaxine in urban wastewaters and human urine. Intermediates formation and biodegradability assessment. Chem. Eng. J. 2017, 308, 492–504. [Google Scholar] [CrossRef]

| Parameter | Details | Municipal WW | Hospital WW | Units |
|---|---|---|---|---|
| BOD | 60 | 160 | mg/L | |
| COD | 110 | 280 | mg/L | |
| SS | 80 | 135 | mg/L | |
| pH | 7.5 | 8 | ||
| TKN | 20–70 | 33 | mg/L | |
| Chlorides | 50 | 200 | mg/L | |
| Total P | 4 | 7 | mg/L | |
| Bacteria | Total Coliforms | 106 | 7.7 × 109 | CFU/mL |
| Viruses | Norovirus | 1.6 × 102 | 2.4 × 106 | PFU/mL |
| Hepatitis A virus | 102 | 104 | PFU/mL | |
| Adenovirus | 1.6 × 102 | 2.8 × 106 | PFU/mL |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakis, S.; Rtimi, S.; Pulgarin, C. Light-Assisted Advanced Oxidation Processes for the Elimination of Chemical and Microbiological Pollution of Wastewaters in Developed and Developing Countries. Molecules 2017, 22, 1070. https://doi.org/10.3390/molecules22071070
Giannakis S, Rtimi S, Pulgarin C. Light-Assisted Advanced Oxidation Processes for the Elimination of Chemical and Microbiological Pollution of Wastewaters in Developed and Developing Countries. Molecules. 2017; 22(7):1070. https://doi.org/10.3390/molecules22071070
Chicago/Turabian StyleGiannakis, Stefanos, Sami Rtimi, and Cesar Pulgarin. 2017. "Light-Assisted Advanced Oxidation Processes for the Elimination of Chemical and Microbiological Pollution of Wastewaters in Developed and Developing Countries" Molecules 22, no. 7: 1070. https://doi.org/10.3390/molecules22071070