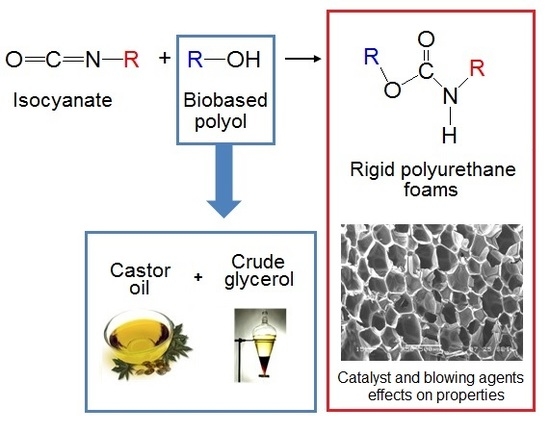
Polyurethane Foams for Thermal Insulation Uses Produced from Castor Oil and Crude Glycerol Biopolyols
Abstract
:1. Introduction
2. Materials and Methods
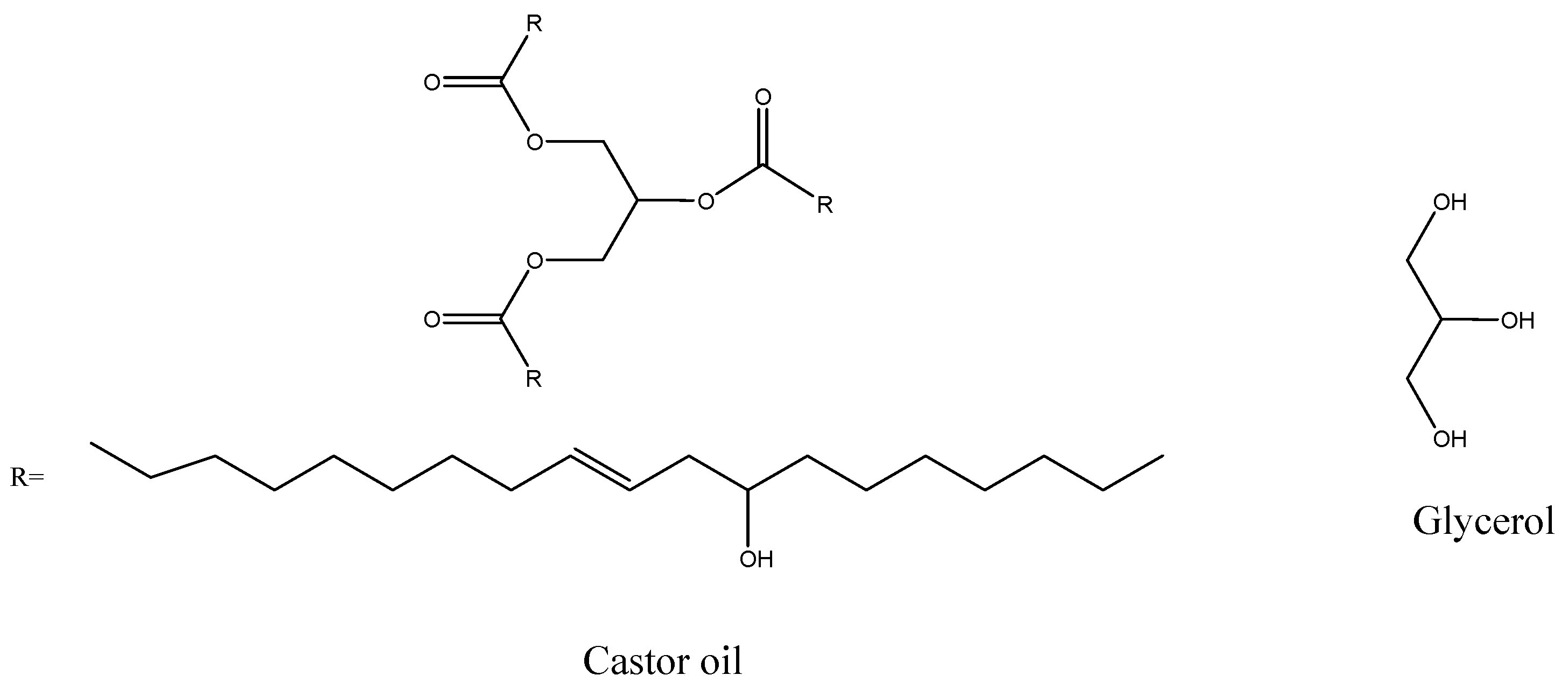
2.1. Materials
2.2. Polyol Preparation
2.3. Foam Synthesis
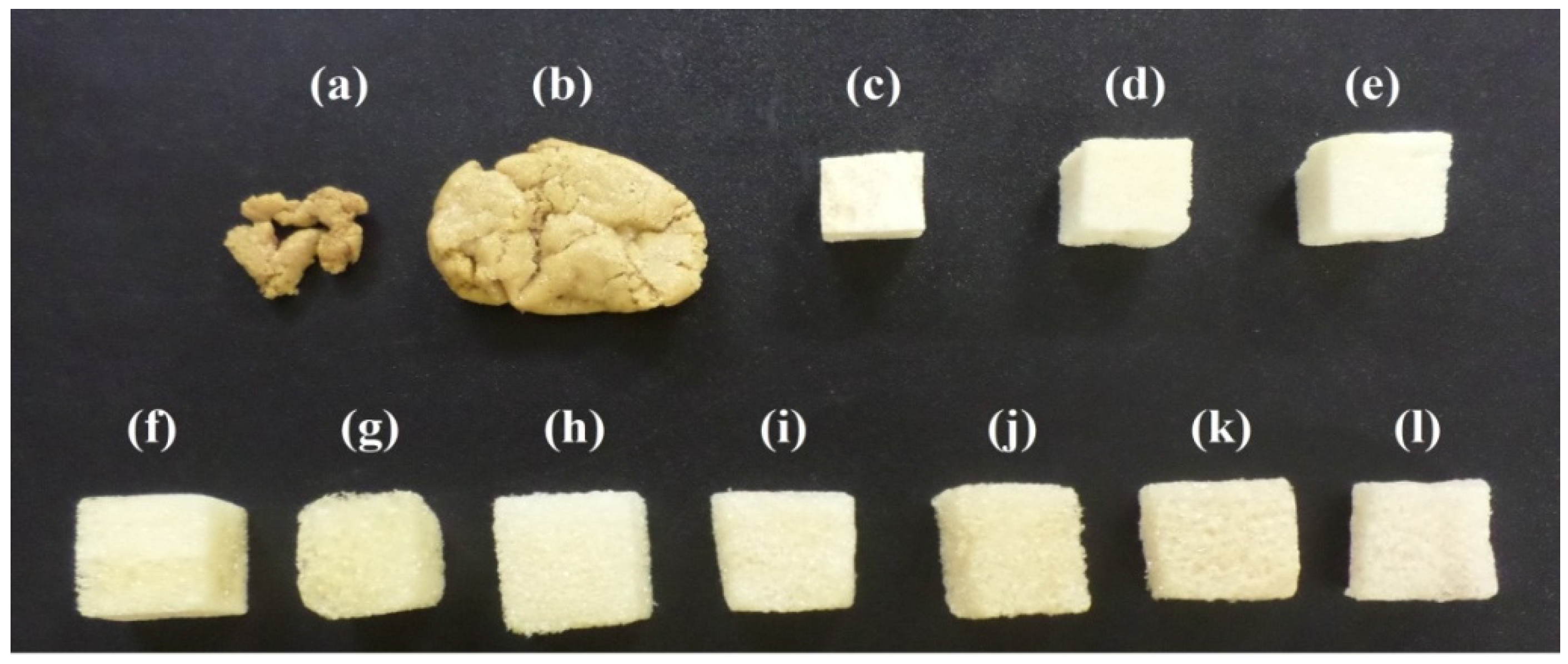
2.3.1. Study of the Best Binary Mixture to Produce Polyurethane Foams
2.3.2. Study of Catalyst and Blowing Agent Effect on the Foams Properties
2.4. Characterization
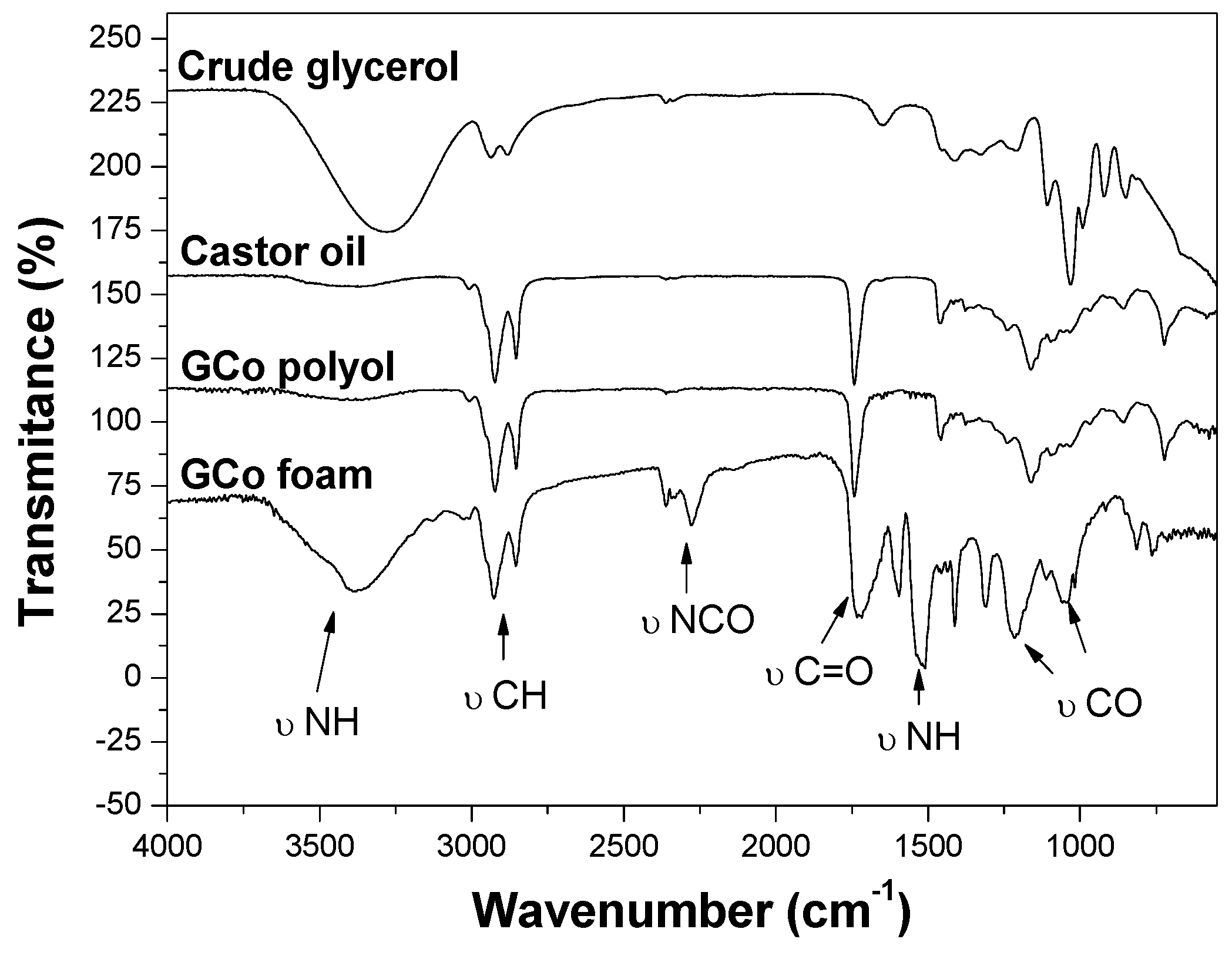
2.4.1. FTIR Analyses
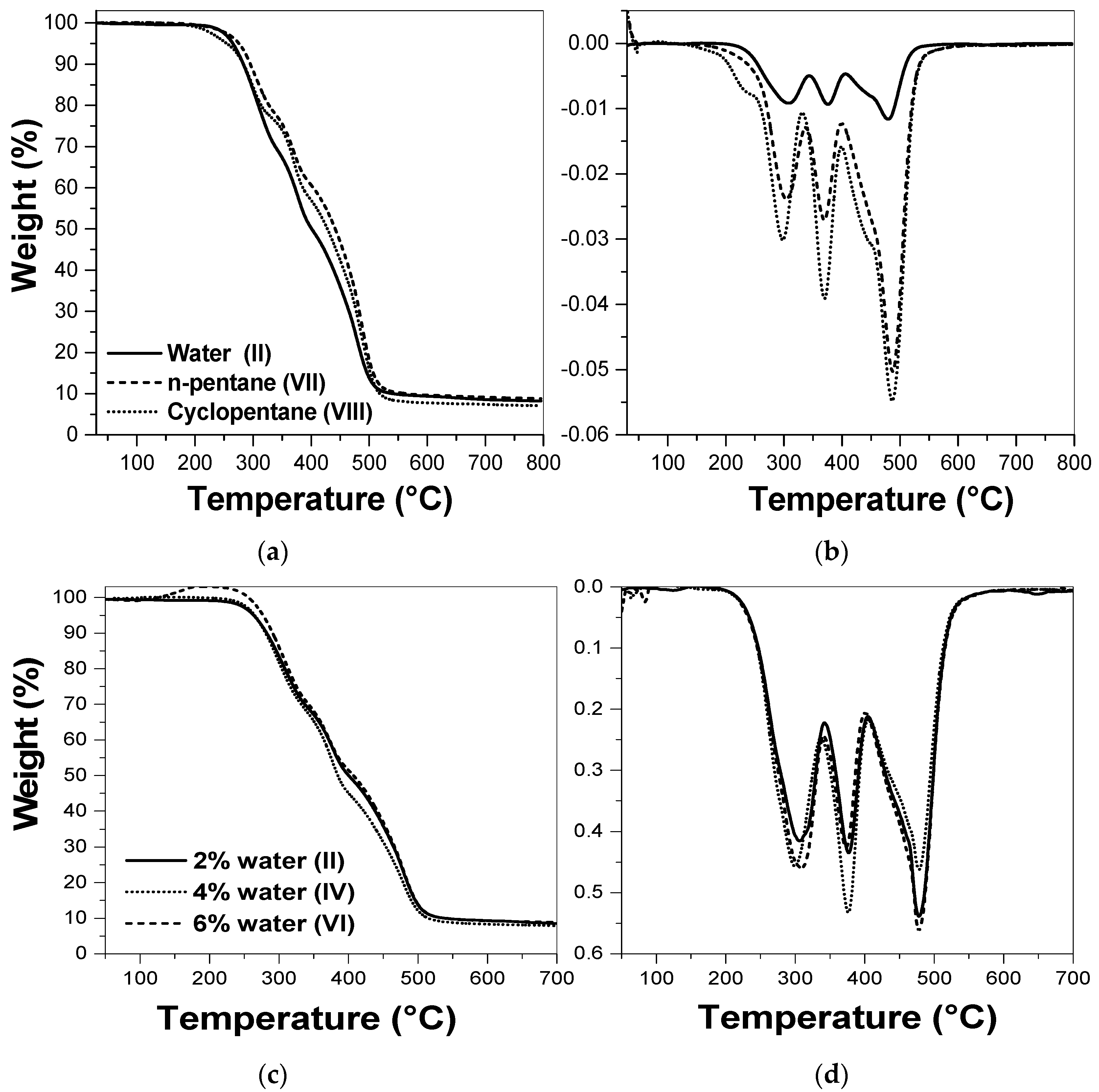
2.4.2. Thermogravimetric Analyses (TGA and DTG)
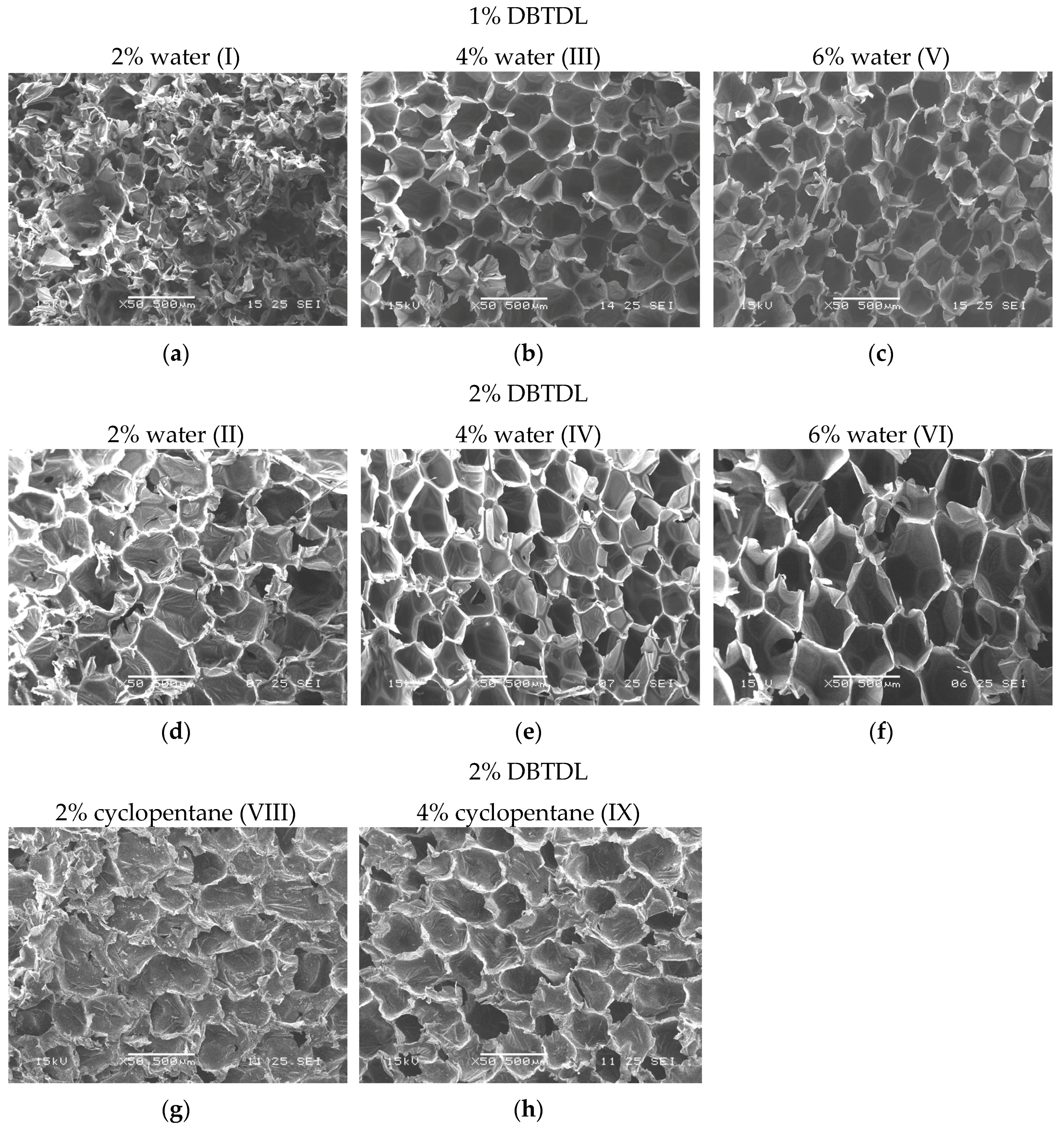
2.4.3. Morphology Analyses
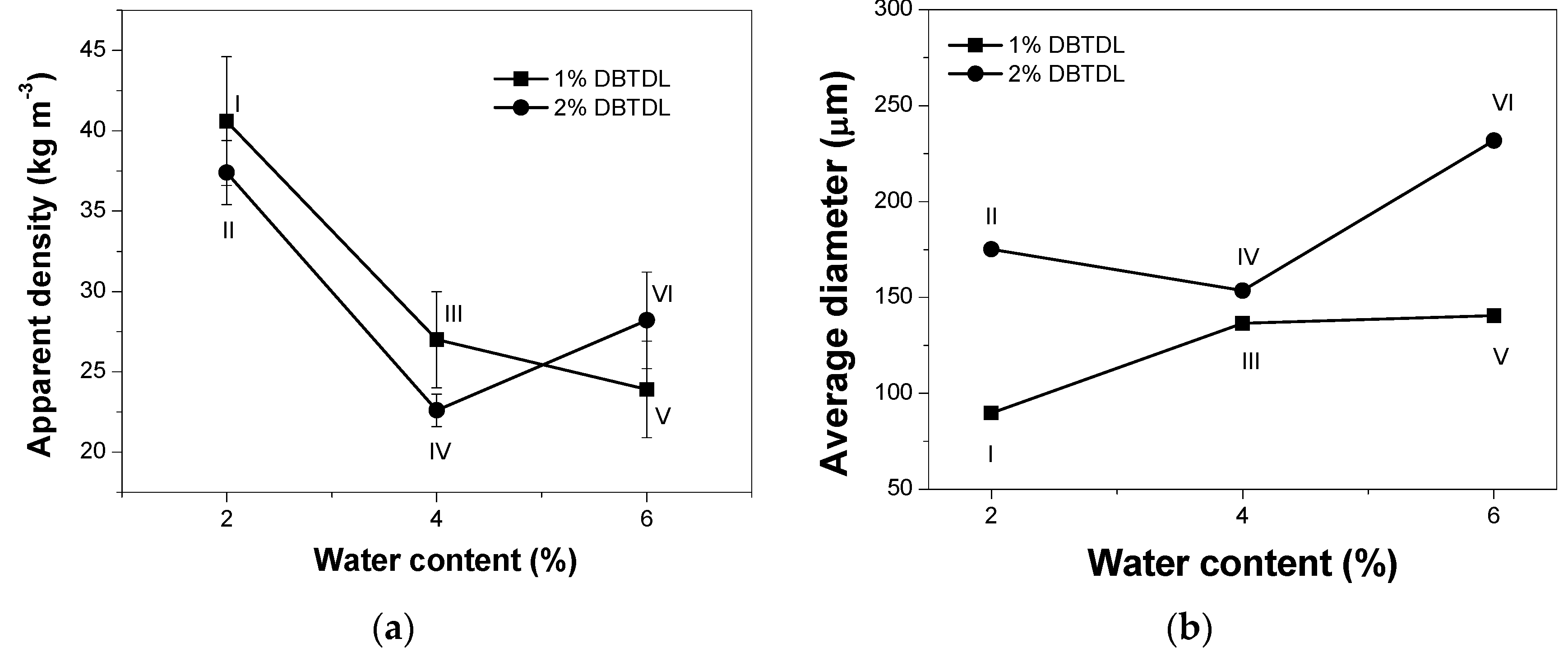
2.4.4. Determination of Apparent Density
2.4.5. Mechanical Properties
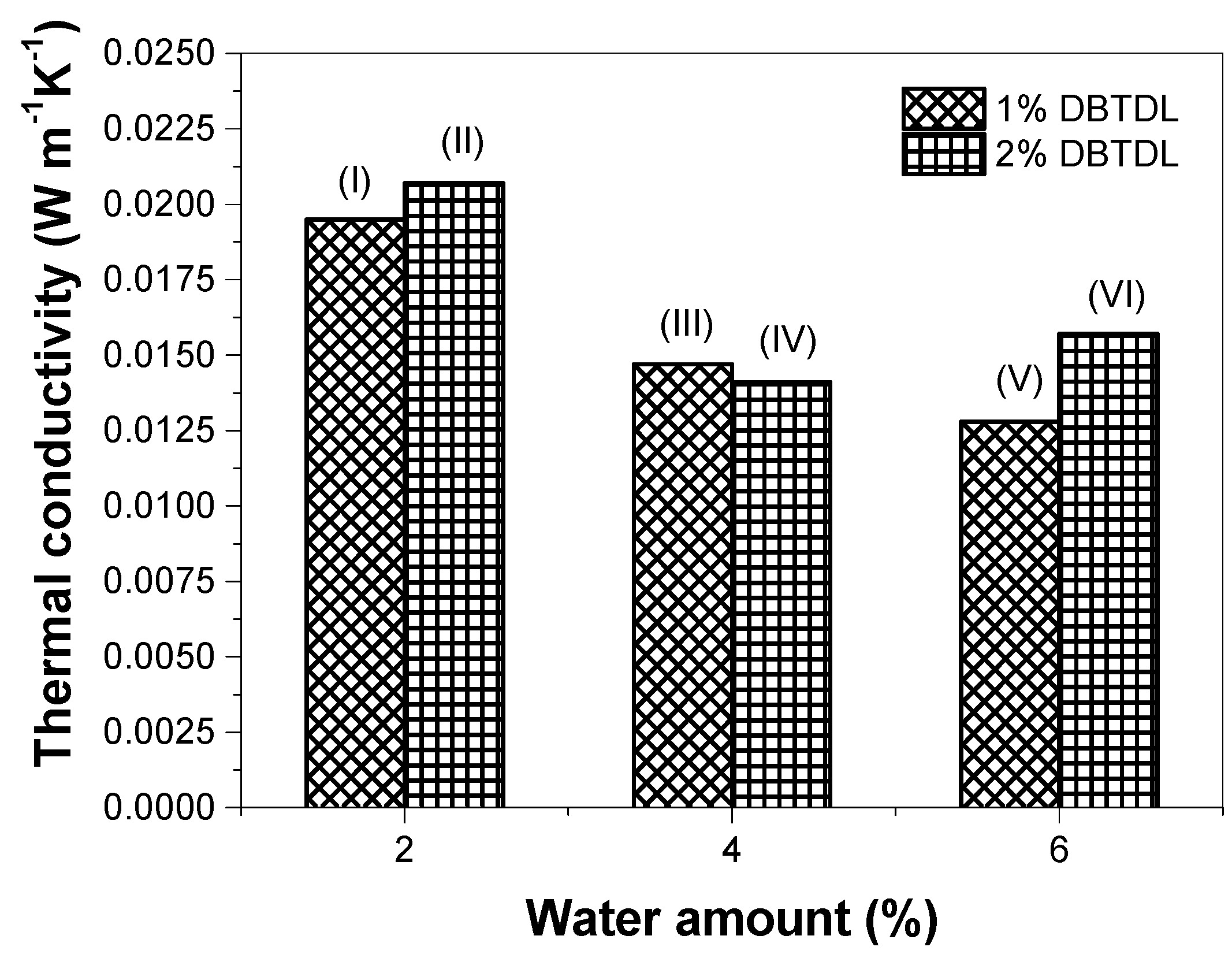
2.4.6. Thermal Conductivity
3. Results and Discussion
3.1. Study of the Best Binary Mixture to Produce Polyurethane Foams
3.2. Study of Catalyst and Blowing Agent Effect in the Foams Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grand View Research Global Rigid Polyurethane (PU) Foams Industry Trends And Market Segment Forecasts To 2020–Worldwide Rigid Polyurethane (PU) Foams Market, Product (Molded Foam Parts, Slabstock Polyether, Slabstock Polyester), and Segment Forecast to 2020. Available online: http://www.grandviewresearch.com/industry-analysis/rigid-polyurethane-pu-foams-industry (accessed on 3 October 2016).
- Zhang, H.; Fang, W.-Z.; Li, Y.-M.; Tao, W.-Q. Experimental study of the thermal conductivity of polyurethane foams. Appl. Therm. Eng. 2017, 115, 528–538. [Google Scholar] [CrossRef]
- Tibério Cardoso, G.; Claro Neto, S.; Vecchia, F. Rigid foam polyurethane (PU) derived from castor oil (Ricinus communis) for thermal insulation in roof systems. Front. Archit. Res. 2012, 1, 348–356. [Google Scholar] [CrossRef]
- Vilar, W. Química e Tecnologia dos Poliuretanos, 3rd ed.; Vilar Consultoria: Rio de Janeiro, Brazil, 2004. [Google Scholar]
- Xue, B.L.; Wen, J.L.; Sun, R.C. Lignin-based rigid polyurethane foam reinforced with pulp fiber: Synthesis and characterization. ACS Sustain. Chem. Eng. 2014, 2, 1474–1480. [Google Scholar] [CrossRef]
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green synthesis of flexible polyurethane foams from liquefied lignin. Eur. Polym. J. 2013, 49, 1174–1184. [Google Scholar] [CrossRef]
- Araújo, R.C.S.; Pasa, V.M.D.; Melo, B.N. Effects of biopitch on the properties of flexible polyurethane foams. Eur. Polym. J. 2005, 41, 1420–1428. [Google Scholar] [CrossRef]
- Melo, B.N.; Pasa, V.M.D. Thermal and morphological study of polyurethanes based on eucalyptus tar pitch and castor oil. J. Appl. Polym. Sci. 2004, 92, 3287–3291. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y. Polyols and polyurethane foams from acid-catalyzed biomass liquefaction by crude glycerol: Effects of crude glycerol impurities. J. Appl. Polym. Sci. 2014, 131, 9054–9062. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y. Two-step sequential liquefaction of lignocellulosic biomass by crude glycerol for the production of polyols and polyurethane foams. Bioresour. Technol. 2014, 161, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wan, C.; Li, Y. Production and characterization of biopolyols and polyurethane foams from crude glycerol based liquefaction of soybean straw. Bioresour. Technol. 2012, 103, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Narine, S.S.; Kong, X.; Bouzidi, L.; Sporns, P. Physical properties of polyurethanes produced from polyols from seed oils: I. Elastomers. J. Am. Oil Chem. Soc. 2007, 84, 55–63. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Matsumura, H.; Hatakeyama, T. Glass transition and thermal degradation of rigid polyurethane foams derived from castor oil-molasses polyols. J. Therm. Anal. Calorim. 2013, 111, 1545–1552. [Google Scholar] [CrossRef]
- Cangemi, J.M.; dos Santos, A.M.; Neto, S.C.; Chierice, G.O. Biodegradation of polyurethane derived from castor oil. Polímeros 2008, 18, 201–206. [Google Scholar] [CrossRef]
- Chethana, M.; Madhukar, B.S.; Siddaramaiah; Somashekar, R. Structure-property relationship of biobased polyurethanes obtained from mixture of naturally occurring vegetable oils. Adv. Polym. Technol. 2014, 33, 1–11. [Google Scholar] [CrossRef]
- Li, Q.F.; Feng, Y.L.; Wang, J.W.; Yin, N.; Zhao, Y.H.; Kang, M.Q.; Wang, X.W. Preparation and properties of rigid polyurethane foam based on modified castor oil. Plast. Rubber Compos. 2016, 45, 16–21. [Google Scholar] [CrossRef]
- Ristić, I.S.; Bjelović, Z.D.; Holló, B.; Mészáros Szécsényi, K.; Budinski-Simendić, J.; Lazić, N.; Kićanović, M. Thermal stability of polyurethane materials based on castor oil as polyol component. J. Therm. Anal. Calorim. 2013, 111, 1083–1091. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Hu, L.; Zhou, Y. Synthesis of rigid polyurethane foams with castor oil-based flame retardant polyols. Ind. Crops Prod. 2014, 52, 380–388. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, H.; Zhang, L.; Hu, L.; Zhou, Y. Study of the mechanical, thermal properties and flame retardancy of rigid polyurethane foams prepared from modified castor-oil-based polyols. Ind. Crops Prod. 2014, 59, 135–143. [Google Scholar] [CrossRef]
- Badri, K.H. Biobased polyurethane from palm kernel oil-based polyol: Polyurethane. In Polyurethane; InTech: Rijeka, Croatia, 2012; pp. 447–470. [Google Scholar]
- Chuayjuljit, S.; Maungchareon, A.; Saravari, O. Preparation and Properties of Palm Oil-Based Rigid Polyurethane Nanocomposite Foams. J. Reinf. Plast. Compos. 2010, 29, 218–225. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; MacOsko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Yoshioka, M.; Nishio, Y.; Saito, D.; Ohashi, H.; Hashimoto, M.; Shiraishi, N. Synthesis of biopolyols by mild oxypropylation of liquefied starch and its application to polyurethane rigid foams. J. Appl. Polym. Sci. 2013, 130, 622–630. [Google Scholar] [CrossRef]
- Kim, D.; Kwon, O.; Yang, S.; Park, J.; Chun, B.C. Structural, Thermal, and Mechanical Properties of Polyurethane Foams Prepared with Starch as the Main Component of Polyols. Fibers Polym. 2007, 8, 155–162. [Google Scholar] [CrossRef]
- Veronese, V.B.; Menger, R.K.; Madalena, M.; Forte, D.C.; Petzhold, C.L. Rigid Polyurethane Foam Based on Modified Vegetable Oil. J. Appl. Polym. Sci. 2011, 120, 530–537. [Google Scholar] [CrossRef]
- Ionescu, M.; Radojči, D.; Wan, X.; Shrestha, M.L.; Petrovi, Z.S.; Upshaw, T.A. Highly functional polyols from castor oil for rigid polyurethanes. Eur. Polym. J. 2016, 84, 736–749. [Google Scholar] [CrossRef]
- Stirna, U.; Lazdina, B.; Vilsone, D.; Lopez, M.J.; Vargas-Garcia, M.D.C.; Suarez-Estrella, F.; Moreno, J. Structure and properties of the polyurethane and polyurethane foam synthesized from castor oil polyols. J. Cell. Plast. 2012, 48, 476–488. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, L. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Carvalho, V.E. Contrução de um Comparador Térmico de Leitura Direta; Universidade Federal de Minas Gerais: Belo Horizonte, Minas Gerais, Brazil, 1978. [Google Scholar]
- Hu, S.; Li, Y. Polyols and polyurethane foams from base-catalyzed liquefaction of lignocellulosic biomass by crude glycerol: Effects of crude glycerol impurities. Ind. Crop. Prod. 2014, 57, 188–194. [Google Scholar] [CrossRef]
- Corcuera, M.A.; Rueda, L.; Fernandez d’Arlas, B.; Arbelaiz, A.; Marieta, C.; Mondragon, I.; Eceiza, A. Microstructure and properties of polyurethanes derived from castor oil. Polym. Degrad. Stab. 2010, 95, 2175–2184. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Pasa, V.M.D. Production and characterization of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur. Polym. J. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- Modesti, M.; Adriani, V.; Simioni, F. Chemical and physical blowing agents in structural polyurethane foams: Simulation and characterization. Polym. Eng. Sci. 2000, 40, 2046–2057. [Google Scholar] [CrossRef]
- Lim, H.; Kim, E.Y.; Kim, B.K. Polyurethane foams blown with various types of environmentally friendly blowing agents. Plast. Rubber Compos. 2010, 39, 364–369. [Google Scholar] [CrossRef]
- Choe, K.H.; Lee, D.S.; Seo, W.J.; Kim, W.N. Properties of Rigid Polyurethane Foams with Blowing Agents and Catalysts. Polym. J. 2004, 36, 368–373. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Effect of Foam Density on the Properties of Water Blown Rigid Polyurethane Foam. J. Appl. Polym. Sci. 2008, 108, 1810–1817. [Google Scholar] [CrossRef]
- Mills, N.J. Polymer Foams Handbook : Engineering and Biomechanics Applications and Design Guide; Butterworth Heinemann: Oxford, UK, 2007. [Google Scholar]
- Jarfelt, U.; Ramnas, O. Thermal conductivity of polyurethane foam–best performance. In Proceedings of the 10th International Symposium on District Heating and Cooling, Göteborg, Sweden, 3–5 September 2006. [Google Scholar]
- Ribeiro Da Silva, V.; Mosiewicki, M.A.; Yoshida, M.I.; Coelho Da Silva, M.; Stefani, P.M.; Marcovich, N.E. Polyurethane foams based on modified tung oil and reinforced with rice husk ash I: Synthesis and physical chemical characterization. Polym. Test. 2013, 32, 438–445. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-based polyurethane foams toward applications beyond thermal insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
Sample Availability: Not Available. |
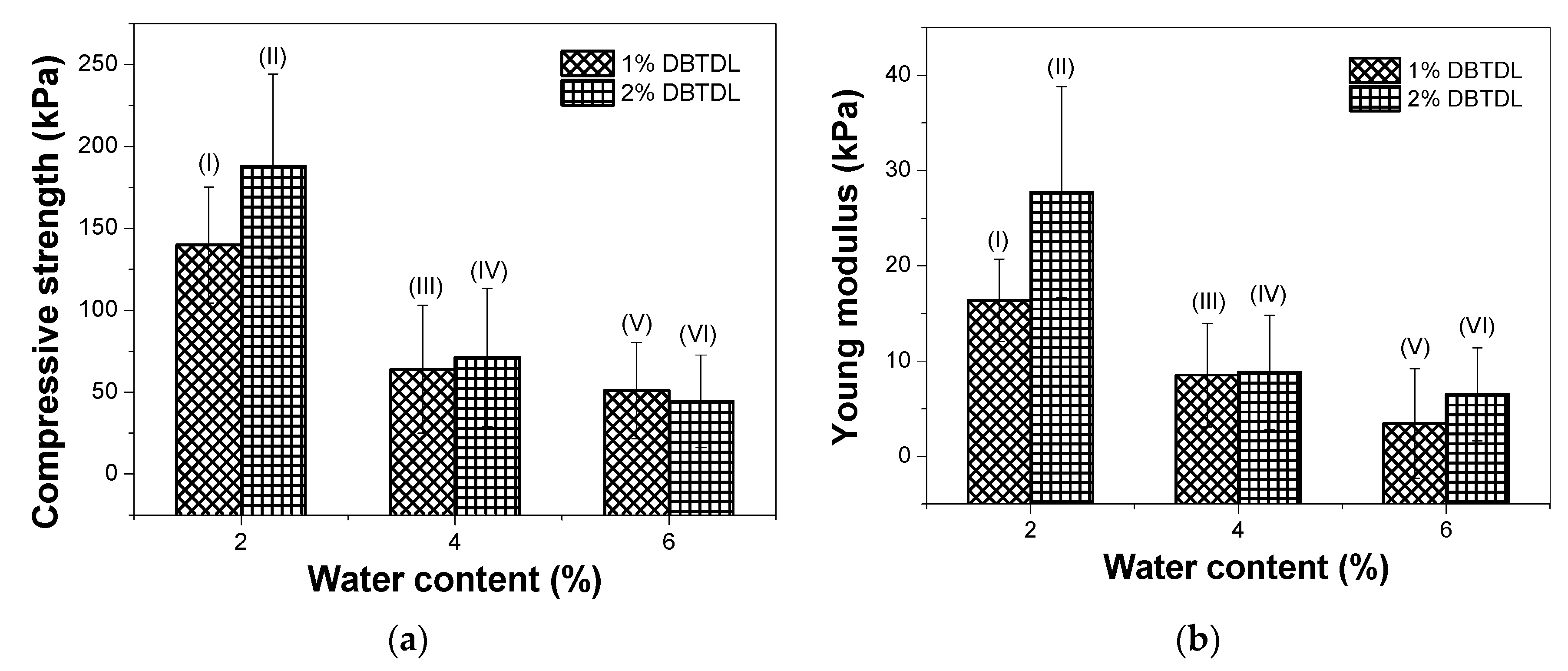
| Formulations | Blowing Agent (Type) | Blowing Agent (%) | Catalyst DBTDL (%) |
|---|---|---|---|
| I | Water | 2 | 1 |
| II | Water | 2 | 2 |
| III | Water | 4 | 1 |
| IV | Water | 4 | 2 |
| V | Water | 6 | 1 |
| VI | Water | 6 | 2 |
| VII | n-pentane | 2 | 2 |
| VIII | ciclopentane | 2 | 2 |
| IX | ciclopentane | 4 | 2 |
| Formulation | Blowing Agent | Apparent Density (kg·m−3) |
|---|---|---|
| II | Water | 37.4 |
| VII | n-pentane | 61.3 |
| VIII | Cyclopentane | 99.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carriço, C.S.; Fraga, T.; Carvalho, V.E.; Pasa, V.M.D. Polyurethane Foams for Thermal Insulation Uses Produced from Castor Oil and Crude Glycerol Biopolyols. Molecules 2017, 22, 1091. https://doi.org/10.3390/molecules22071091
Carriço CS, Fraga T, Carvalho VE, Pasa VMD. Polyurethane Foams for Thermal Insulation Uses Produced from Castor Oil and Crude Glycerol Biopolyols. Molecules. 2017; 22(7):1091. https://doi.org/10.3390/molecules22071091
Chicago/Turabian StyleCarriço, Camila S., Thaís Fraga, Vagner E. Carvalho, and Vânya M. D. Pasa. 2017. "Polyurethane Foams for Thermal Insulation Uses Produced from Castor Oil and Crude Glycerol Biopolyols" Molecules 22, no. 7: 1091. https://doi.org/10.3390/molecules22071091
APA StyleCarriço, C. S., Fraga, T., Carvalho, V. E., & Pasa, V. M. D. (2017). Polyurethane Foams for Thermal Insulation Uses Produced from Castor Oil and Crude Glycerol Biopolyols. Molecules, 22(7), 1091. https://doi.org/10.3390/molecules22071091