Synthesis and Study of Fe-Doped Bi2S3 Semimagnetic Nanocrystals Embedded in a Glass Matrix
Abstract
:1. Introduction
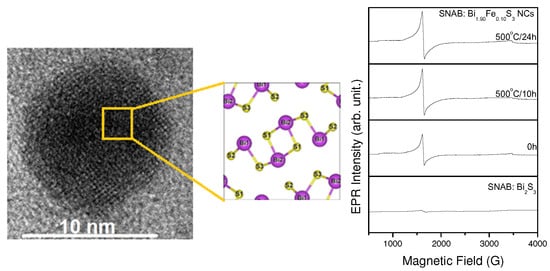
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Erwin, S.C.; Zu, L.; Haftel, M.I.; Efros, A.L.; Kennedy, T.A.; Norris, D.J. Doping semiconductor nanocrystals. Nature 2005, 436, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Karan, N.S.; Sarma, D.D.; Kadam, R.M.; Pradhan, N. Doping Transition Metal (Mn or Cu) Ions in Semiconductor Nanocrystals. J. Phys. Chem. Lett. 2010, 1, 2863–2866. [Google Scholar] [CrossRef]
- Silva, R.S.; Neto, E.S.F.; Dantas, N.O. Optical, Magnetic, and Structural Properties of Semiconductor and Semimagnetic Nanocrystals. In Nanocrystals—Synthesis, Characterization and Applications, 26th ed.; Neralla, S., Ed.; InTech: Rijeka, Croatia, 2012; Volume 3, pp. 61–80. [Google Scholar] [CrossRef]
- Jang, D.M.; Kwak, I.H.; Kwon, E.L.; Jung, C.S.; Im, H.S.; Park, K.; Park, J. Transition-Metal Doping of Oxide Nanocrystals for Enhanced Catalytic Oxygen Evolution. J. Phys. Chem. C 2015, 119, 1921–1927. [Google Scholar] [CrossRef]
- Furdyna, J.K. Diluted Magnetic Semiconductors. J. Appl. Phys. 1988, 64, R29–R64. [Google Scholar] [CrossRef]
- Archer, P.I.; Santangelo, S.A.; Gamelin, D.R. Direct Observation of sp-d exchange interactions in colloidal Mn2+- and Co2+-doped CdSe quantum dots. Nano Lett. 2007, 7, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.D.; Liu, W.; Baker, T.A.; Sinitsyn, N.A.; Klimov, V.I.; Crooker, S.A. Revealing giant internal magnetic fields due to spin fluctuations in magnetically doped colloidal nanocrystals. Nat. Nanotechnol. 2016, 11, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.S.; Silva, J.T.T.; Rocha, V.R.; Cano, N.F.; Silva, A.C.A.; Dantas, N.O. Synthesis Process Controlled of Semimagnetic Bi2–xMnxS3 Nanocrystals in a Host Glass Matrix. J. Phys. Chem. C 2014, 118, 18730–18735. [Google Scholar] [CrossRef]
- Lourenço, S.A.; Silva, R.S.; Dantas, N.O. Growth kinetic on the optical properties of the Pb1−xMnxSe nanocrystals embedded in a glass matrix: Thermal annealing and Mn2+ concentration. Phys. Chem. Chem. Phys. 2012, 14, 11040–11047. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Chatti, M.; Adusumalli, V.N.K.B.; Mahalingam, V. Highly Selective and Sensitive Detection of Cu2+ Ions Using Ce(III)/Tb(III)-Doped SrF2 Nanocrystals as Fluorescent Probe. ACS Appl. Mater. Interfaces 2015, 7, 25702–25708. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.A.; Silva, R.S.; Dantas, N.O. Tunable dual emission in visible and near-infrared spectra using Co -doped PbSe nanocrystals embedded in a chalcogenide glass matrix. Phys. Chem. Chem. Phys. 2016, 18, 23036–23043. [Google Scholar] [CrossRef] [PubMed]
- Farvid, S.S.; Ju, L.; Worden, M.; Radovanovic, P.V. Colloidal Chromium-Doped In2O3 Nanocrystals as Building Blocks for High-TC Ferromagnetic Transparent Conducting Oxide Structures. J. Phys. Chem. C 2008, 112, 17755–17759. [Google Scholar] [CrossRef]
- Zhou, D.; Kittilstved, K.R. Electron trapping on Fe3+ sites in photodoped ZnO colloidal nanocrystals. Chem. Commun. 2016, 52, 9101–9104. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Xu, G.; Wei, X.; Liu, Y.; Hou, X.; Du, P.; Weng, W.; Shen, G.; Han, G. Room-temperature ferromagnetism in Fe-doped PbTiO3 nanocrystals. Appl. Phys. Lett. 2007, 91, 063106. [Google Scholar] [CrossRef]
- Yu, S.; Yun, H.J.; Lee, D.M.; Yi, J. Preparation and characterization of Fe-doped TiO2 nanoparticles as a support for a high performance CO oxidation catalyst. J. Mater. Chem. 2012, 22, 12629–12635. [Google Scholar] [CrossRef]
- Jo, D.Y.; Kim, D.; Kim, J.H.; Chae, H.; Seo, H.J.; Do, Y.R.; Yang, H. Tunable White Fluorescent Copper Gallium Sulfide Quantum Dots Enabled by Mn Doping. ACS Appl. Mater. Interfaces 2016, 8, 12291–12297. [Google Scholar] [CrossRef] [PubMed]
- Halder, G.; Bhattacharyya, S. Plight of Mn Doping in Colloidal CdS Quantum Dots To Boost the Efficiency of Solar Cells. J. Phys. Chem. 2015, 119, 13404–13412. [Google Scholar] [CrossRef]
- Huang, G.; Wang, C.; Xu, X.; Cui, Y. An optical ratiometric temperature sensor based on dopant-dependent thermal equilibrium in dual-emitting Ag & Mn:ZnInS quantum dots. RSC Adv. 2016, 6, 58113–58117. [Google Scholar] [CrossRef]
- Žutić, I.; Petukhov, A. Spintronics: Shedding light on nanomagnets. Nat. Nanotechnol. 2009, 4, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.H.; Zhang, B.P.; Li, J.F. Microstructure composite-like Bi2S3 polycrystals with enhanced thermoelectric properties. J. Mater. Chem. 2012, 22, 17589–17594. [Google Scholar] [CrossRef]
- Chmielowski, R.; Péré, D.; Bera, C.; Opahle, I.; Xie, W.; Jacob, S.; Capet, F.; Roussel, P.; Weidenkaff, A.; Madsen, G.K.H.; et al. Theoretical and experimental investigations of the thermoelectric properties of Bi2S3. J. Appl. Phys. 2015, 117, 125103. [Google Scholar] [CrossRef]
- Black, J.; Conwell, E.M.; Seigle, L.; Spencer, C.W. Electrical and Optical Properties of some M2V−BN3VI−B Semiconductors. J. Phys. Chem. Solids 1957, 2, 240–251. [Google Scholar] [CrossRef]
- Filip, M.R.; Patrick, C.E.; Giustino, F. GW quasiparticle band structures of stibnite, antimonselite, bismuthinite, and guanajuatite. Phys. Rev. B 2013, 87, 205125. [Google Scholar] [CrossRef]
- Stavila, V.; Whitmire, K.H.; Rusakova, I. Synthesis of Bi2S3 nanostructures from bismuth (III) thiourea and thiosemicarbazide complexes. Chem. Mater. 2009, 21, 5456–5465. [Google Scholar] [CrossRef]
- Sharma, Y.; Srivastava, P.; Dashora, A.; Vadkhiya, L.; Bhayani, M.K.; Jain, R.; Jani, A.R.; Ahuja, B.L. Electronic structure, optical properties and Compton profiles of Bi2S3 and Bi2Se3. Solid State Sci. 2012, 14, 241–249. [Google Scholar] [CrossRef]
- Lundegaard, L.F.; Makovicky, E.; Boffa-Ballaran, T.; Balic-Zunic, T. Crystal structure and cation lone electron pair activity of Bi2S3 between 0 and 10 GPa. Phys. Chem. Miner. 2005, 32, 578–584. [Google Scholar] [CrossRef]
- Ge, Z.H.; Zhang, B.P.; Liu, Y.; Li, J.F. Nanostructured Bi2−xCuxS3 bulk materials with enhanced thermoelectric performance. Phys. Chem. Chem. Phys. 2012, 14, 4475–4481. [Google Scholar] [CrossRef] [PubMed]
- Panmand, R.P.; Kumar, G.; Mahajan, S.M.; Kulkarni, M.V.; Kale, B.B.; Gosavic, S.W. Novel and stable Mn2+@Bi2S3 quantum dots–glass system with giant magneto optical Faraday rotations. J. Mater. Chem. C 2013, 1, 1203–1210. [Google Scholar] [CrossRef]
- Racen, H.; Schneider, H. EPR study of Fe3+ centers in cristobalite and tridymite. Am. Mineral. 1986, 71, 105–110. Available online: http://www.minsocam.org/ammin/AM71/AM71_105.pdf (accessed on 12 May 2017).
- Dantas, N.O.; Paula, P.M.N.; Silva, R.S.; López-Richard, V.; Marques, G.E. Radiative versus nonradiative optical processes in PbS nanocrystals. J. Appl. Phys. 2011, 109, 024308. [Google Scholar] [CrossRef]
- Silva, R.S.; Mikhail, H.D.; Pavani, R.; Cano, N.F.; Silva, A.C.A.; Dantas, N.O. Synthesis of diluted magnetic semiconductor Bi2−xMnxTe3 nanocrystals in a host glass matrix. J. Alloy. Compd. 2015, 648, 778–782. [Google Scholar] [CrossRef]
- Dantas, N.O.; Neto, E.S.F.; Silva, R.S.; Jesus, D.R.; Pelegrini, F. Evidence of Cd1-xMnxS Nanocrystal Growth in a Glass Matrix by the Fusion Method. Appl. Phys. Lett. 2008, 93, 193115-1–193115-3. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ, U.S. National Institutes of Health: Bethesda, ML, USA, 1997–2016. Available online: http://imagej.nih.gov/ij/ (accessed on 14 May 2017).
- Dantas, N.O.; Silva, A.S.; Silva, A.C.A.; Neto, E.S.F. Atomic and Magnetic Force Microscopy of Semiconductor and Semimagnetic Nanocrystals Grown in Colloidal Solutions and Glass Matrices. In Optical Imaging: Technology, Methods and Applications, 1st ed.; Tanaka, A., Nakamura, B., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2012; Volume 1, pp. 109–132. [Google Scholar]
- Dantas, N.O.; Wayta, W.E.F.; Silva, A.C.A.; Cano, N.F.; Rodriguez, A.F.R.; Oliveira, A.C.; Garg, V.K.; Morais, P.C. Magnetic and optical investigation of SiO2·30Na2O·1Al2O3·(29 − x)B2O3·xFe2O3 glass matrix. Solid State Sci. 2012, 14, 1169–1174. [Google Scholar] [CrossRef]
- Iwamoto, N.; Makino, Y.; Kasahara, S. State of Fe3+ ion and Fe3+-F- Interaction in Calcium Fluorosilicate Glasses. J. Non-Cryst. Solids 1983, 55, 113–124. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Troullier, N.; Martins, J.L. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B 1991, 43, 1993–2006. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junqueira, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter 2002, 14, 2745–2779. Available online: stacks.iop.org/JPhysCM/14/2745 (accessed on 15 May 2017).
- Bader, R.F. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 064111. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds not are available from the authors. |





| Structural Parameters | Bi2S3 Exp.[26] | Bi2S3 | Bi2S3:FeBi1 | Bi2S3:FeBi2 |
|---|---|---|---|---|
| a (Å) | 11.282 | 11.249 dev. −0.29% | 11.419 | 11.229 |
| b (Å) | 3.9728 | 4.0296 dev. 1.43% | 3.9540 | 3.9318 |
| c (Å) | 11.131 | 11.004 dev. −1.14% | 10.652 | 11.077 |
| Vuc (Å3) | 498.4 | 498.8 dev. 0.08% | 480.9 | 489.1 |
| Structure | Atom | Bader Charge | Pseudo Valency Charge |
|---|---|---|---|
| Bi2S3 | Bi1 | 3.546 | 1.454 |
| Bi2 | 3.400 | 1.600 | |
| S1 | 7.108 | −1.108 | |
| S2 | 6.986 | −0.986 | |
| S3 | 6.960 | −0.960 | |
| Bi2S3:FeBi1 | Fe1 | 6.678 | 1.322 |
| Bi2S3:FeBi2 | Fe2 | 6.694 | 1.306 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.S.; Mikhail, H.D.; Guimarães, E.V.; Gonçalves, E.R.; Cano, N.F.; Dantas, N.O. Synthesis and Study of Fe-Doped Bi2S3 Semimagnetic Nanocrystals Embedded in a Glass Matrix. Molecules 2017, 22, 1142. https://doi.org/10.3390/molecules22071142
Silva RS, Mikhail HD, Guimarães EV, Gonçalves ER, Cano NF, Dantas NO. Synthesis and Study of Fe-Doped Bi2S3 Semimagnetic Nanocrystals Embedded in a Glass Matrix. Molecules. 2017; 22(7):1142. https://doi.org/10.3390/molecules22071142
Chicago/Turabian StyleSilva, Ricardo S., Hanna D. Mikhail, Eder V. Guimarães, Elis R. Gonçalves, Nilo F. Cano, and Noelio O. Dantas. 2017. "Synthesis and Study of Fe-Doped Bi2S3 Semimagnetic Nanocrystals Embedded in a Glass Matrix" Molecules 22, no. 7: 1142. https://doi.org/10.3390/molecules22071142