Crystal Structure Analysis of the First Discovered Stability-Enhanced Solid State of Tenofovir Disoproxil Free Base Using Single Crystal X-ray Diffraction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure Analysis of TD Free Base Crystal Using Single Crystal X-ray Crystallography
2.2. Characterization of TD Free Base Crystal
2.3. Hygroscopicity Evaluation of TD Free Base Crystal and TDF Form-I
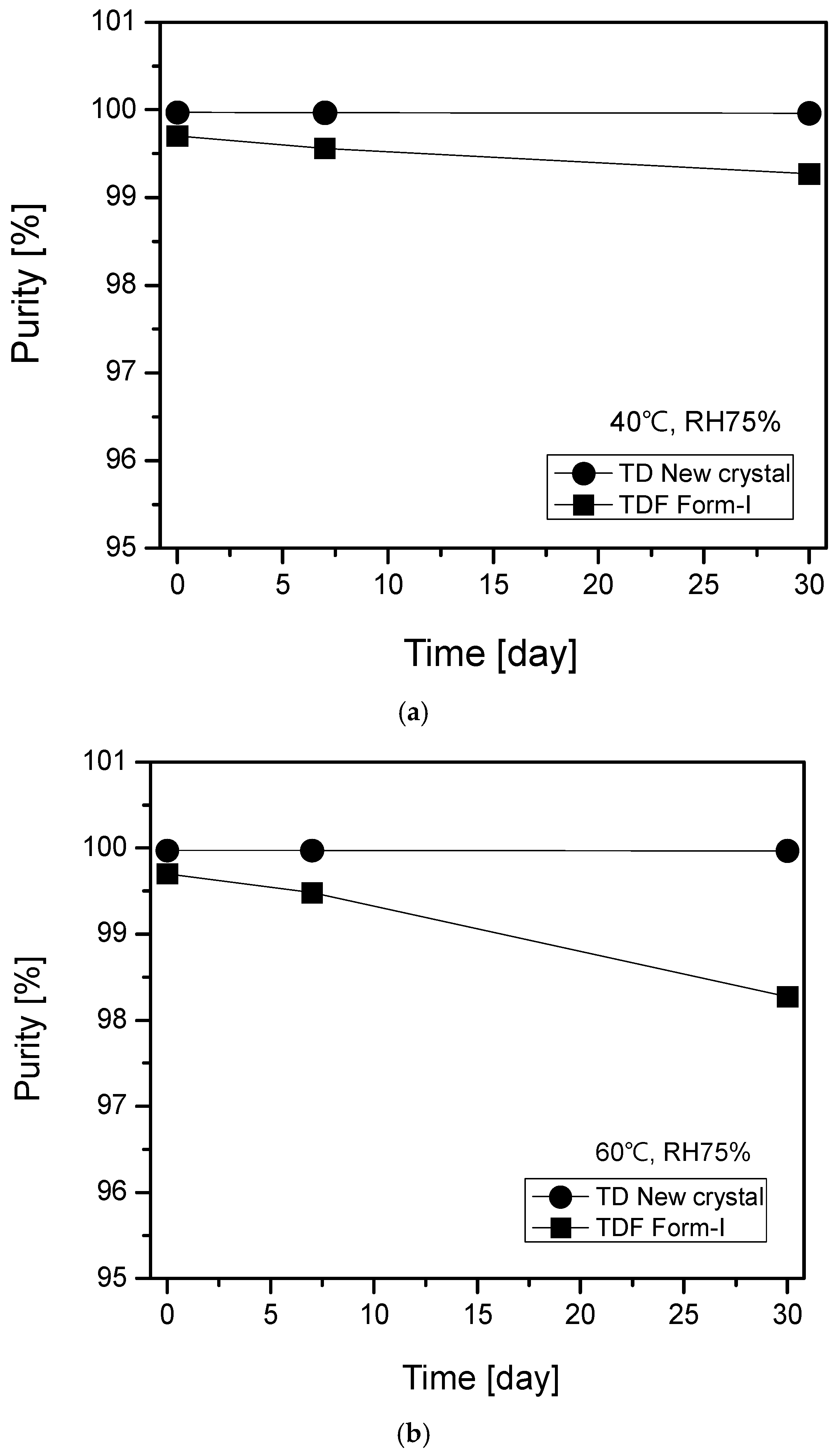
2.4. Stability Evaluation of TD Free Base Crystal and TDF Form-I under Accelerated and Stress Storage Conditions
2.5. Effect of Gastrointestinal pH on the Solubility of TD Free Base Crystal and TDF Form-I
3. Materials and Methods
3.1. Materials
3.2. Drowning-Out Crystallization Method
3.3. Differential Scanning Calorimetry (DSC)
3.4. Powder X-ray Diffraction (PXRD)
3.5. Single Crystal X-ray Diffraction (SXD)
3.6. Hygroscopicity Evaluation Using Hygroscopic Tester
3.7. Accelerated Stability and Stress Testing Analysis Using High-Performance Liquid Chromatography (HPLC)
3.8. Method for the Solubility Test at Distinct Gastrointestinal pH
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hilfiker, R. Polymorphism in the Pharmaceutical Industry; Wiley-Vch: Weinheim, Germany, 2006; Chapter 1; pp. 1–15. [Google Scholar]
- Sparma, B.; Chen, J.; His, H.Y.; Myerson, A.S. Solid form of pharmaceuticals: Polymorphs, salt and co-crystals. Korean J. Chem. Eng. 2011, 28, 315–322. [Google Scholar] [CrossRef]
- Chen, J.; Sarma, B.; Myerson, A.S. Pharmaceutical Crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Blagden, N.; Davey, R.J. Polymorph Selection: Challenges for the Future? Cryst. Growth Des. 2003, 3, 873–885. [Google Scholar] [CrossRef]
- Park, Y.; Lee, J.; Lee, S.H.; Choi, H.G.; Mao, C.; Kang, S.K.; Choi, S.E.; Lee, S.H. Crystal Structures of Tetramorphic Forms of Donepezil and Energy/Temperature Phase Diagram via Direct Heat Capacity Measurements. Cryst. Growth Des. 2013, 13, 5450–5458. [Google Scholar] [CrossRef]
- Prohens, R.; Barbas, R.; Portell, A.; Font-Bardia, M.; Puigjaner, C. Expanding the Crystal Form Landscape of the Antiviral Drug Adefovir Dipivoxil. Cryst. Growth Des. 2015, 15, 475–484. [Google Scholar] [CrossRef]
- Actins, A.; Arajs, R.; Belakovs, S.; Orola, L.; Veidis, M.V. The Crystal and Molecular Structure of a Polymorph and a Pseudo-Polymorph of Droperidol. J. Chem. Crystallogr. 2008, 38, 169–174. [Google Scholar] [CrossRef]
- Chandrappa, R.K.; Ochsenbein, P.; Martineau, C.; Bonin, M.; Althoff, G.; Engelke, F.; Malandrini, H.; Castro, B.; Hajji, M.E.; Taulelle, F. Polymorphism in Xaliproden (SR57746A): An X-ray Diffraction, Calorimetric, and Solid-State NMR Investigation. Cryst. Growth Des. 2013, 13, 4678–4687. [Google Scholar] [CrossRef]
- Munger, J.D., Jr.; Rohloff, J.C.; Schultze, L.M. Nucleotide Analog Composition and Synthesis Method. U.S. Patent 5,935,946 A, 10 August 1999. [Google Scholar]
- Gomes, E.C.L.; Mussel, W.N.; Resende, J.M.; Fialho, S.L.; Barbosa, J.; Carignani, E.; Geppi, M.; Yoshida, M.I. Characterization of Tenofovir Disoproxil Fumarate and Its Behavior under Heating. Cryst. Growth Des. 2015, 15, 1915–1922. [Google Scholar] [CrossRef]
- Lee, E.H.; Smith, D.T.; Fanwick, P.E.; Byrn, S.R. Characterization and Anisotropic Lattice Expansion/Contraction of Polymorphs of Tenofovir Disoproxil Fumarate. Cryst. Growth Des. 2010, 10, 2314–2322. [Google Scholar] [CrossRef]
- Dova, E.; Mazurek, J.M.; Anker, J. Tenofovir Disoproxil Hemi-Fumaric Acid Co-Crystal. U.S. Patent 20,090,270,352 A1, 29 October 2009. [Google Scholar]
- Dova, E.; Mazurek, J.M. Polymorphic Forms of Tenofovir Disoproxil Fumarate. Eur. Pat. WO 2008140302 A1, 20 November 2008. [Google Scholar]
- Yuan, L.; Dahl, T.C.; Oliyai, R. Degradation kinetics of oxycarbonyloxymethyl prodrugs of phosphonates in solution. Pharm. Res. 2001, 18, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Dahl, T.C.; Oliyai, R. Effect of carbonate salts on the kinetics of acid-catalyzed dimerization of adefovir dipivoxil. Pharm. Res. 2000, 17, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.H.; Woo, D.K.; Kang, S.K.; Chon, M.H.; Park, D.J.; Lee, S.K.; Choi, S.J. The New Tenofovir Disoproxil Salt and the Preparation Method Thereof. Kr. Patent Application KR20140028790 A, 2014. [Google Scholar]
- Phull, M.S.; Kankan, R.N.; Rao, D.R. Crystalline form Tenofovir Disoproxil and Process for It’s Preparation. U.S. Patent 2011/0112292 A1, 12 May 2011. [Google Scholar]
- Rigaku. PROCESS-AUTO, Rigaku Corporation: Tokyo, Japan, 2006.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Tenofovir disoproxil are available from the authors. |







| Form-A [11,12] | Form-B [11] | Form-I [10] | ULT-11 [13] | |
|---|---|---|---|---|
| TDF crystals | TD/FA 2:1 Co-crystal | TD/FA 1:1 Co-crystal MeOH solvate | TD/FA 1:1 Salt form | TD/FA 1:1 Salt form |
| Formula weight (g/mol) | 1153.963 | 651.543 | 635.52 | 635.52 |
| Crystal system | monoclinic | orthorhombic | monoclinic | monoclinic |
| Space group | P21 | P21212 | P21 | P21 |
| a (Å) | 9.7774 (2) | 18.438 (10) | 9.835 (2) | 9.7440 (3) |
| b (Å) | 22.2104 (5) | 34.057 (2) | 22.315 (5) | 18.0330 (5) |
| c (Å) | 12.5000 (3) | 9.907 (6) | 12.545 (3) | 17.4080 (6) |
| β (°) | 95.1689 (1) | 90 | 95.042 (3) | 102.759 (2) |
| Cell volume (Å3) | 2703.46 | 6221.03 | 2744.88 | 2983.3 |
| Parameter | TD Free Base Crystal |
|---|---|
| Chemical formula | C19 H30 N5 O10 P |
| Formula weight, amu | 519.45 |
| Crystal system | Orthorhombic |
| Space group | P212121 |
| a, Å | 8.7039 (5) |
| b, Å | 12.2787 (8) |
| c, Å | 23.8682 (16) |
| Cell volume (Å3) | 2550.9 (3) |
| Z | 4 |
| Temperature, K | 290 (1) |
| Radiation | Graphite monochromated MoKα (λ = 0.71073 Å) |
| Linear absorption coefficient, mm−1 | 0.168 |
| Crystal size, mm3 | 0.24 × 0.10 × 0.08 |
| Scan type | ω |
| θ limits, ° | 3.0° < θ < 25.00° |
| No. unique data | 4478 |
| No. unique data with I >2σ(I) | 2541 |
| wR2 (all data) | 0.1706 |
| R (on for I >2σ(I)) | 0.0667 |
| Goodness-of-fit on F2 | 1.015 |
| Flack x parameter | −0.13 (11) |
| Minimum and Maximum residual electron density (e/Å3) | −0.317 and 0.376 |
| Type | Donor–H…Acceptor | D–H (Å) | H…A (Å) | D…A (Å) | D–H…A (°) |
|---|---|---|---|---|---|
| Inter | N(1)–H(1A)…N(9) i | 0.86 | 2.22 | 3.083(8) | 177 |
| N(1)–H(1B)…N(3) ii | 0.86 | 2.26 | 3.099(8) | 166 | |
| C(8)–H(8)…O(17) iii | 0.93 | 2.38 | 3.152(9) | 141 | |
| C(33)–H(33)…N(9) iv | 0.98 | 2.58 | 3.510(11) | 159 | |
| Intra | C(15)–H(15B)…N(5) | 0.97 | 2.60 | 2.293(9) | 129 |
| C(19)–H(19A)…O(17) | 0.97 | 2.55 | 2.990(10) | 108 | |
| C(24)–H(24)…O(22) | 0.98 | 2.39 | 2.745(14) | 100 | |
| C(28)–H(28A)…O(17) | 0.97 | 2.54 | 3.006(10) | 110 |
| Solubility (mg/mL) | TD Free Base | TDF |
|---|---|---|
| DW | 5.88 mg/mL | 6.2 mg/mL |
| pH 1.2 | 34.88 mg/mL | 35.0 mg/mL |
| pH 4.0 | 5.12 mg/mL | 5.12 mg/mL |
| pH 6.8 | 6.91 mg/mL | 6.91 mg/mL |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.-H.; Kiyonga, A.N.; Yoon, W.; Ryu, H.C.; Kim, J.-S.; Kang, C.; Park, M.; Yun, H.; Jung, K. Crystal Structure Analysis of the First Discovered Stability-Enhanced Solid State of Tenofovir Disoproxil Free Base Using Single Crystal X-ray Diffraction. Molecules 2017, 22, 1182. https://doi.org/10.3390/molecules22071182
An J-H, Kiyonga AN, Yoon W, Ryu HC, Kim J-S, Kang C, Park M, Yun H, Jung K. Crystal Structure Analysis of the First Discovered Stability-Enhanced Solid State of Tenofovir Disoproxil Free Base Using Single Crystal X-ray Diffraction. Molecules. 2017; 22(7):1182. https://doi.org/10.3390/molecules22071182
Chicago/Turabian StyleAn, Ji-Hun, Alice Nguvoko Kiyonga, Woojin Yoon, Hyung Chul Ryu, Jae-Sun Kim, Chaeri Kang, Minho Park, Hoseop Yun, and Kiwon Jung. 2017. "Crystal Structure Analysis of the First Discovered Stability-Enhanced Solid State of Tenofovir Disoproxil Free Base Using Single Crystal X-ray Diffraction" Molecules 22, no. 7: 1182. https://doi.org/10.3390/molecules22071182





