Diarylethenes Display In Vitro Anti-TB Activity and Are Efficient Hits Targeting the Mycobacterium tuberculosis HU Protein
Abstract
:1. Introduction
2. Results
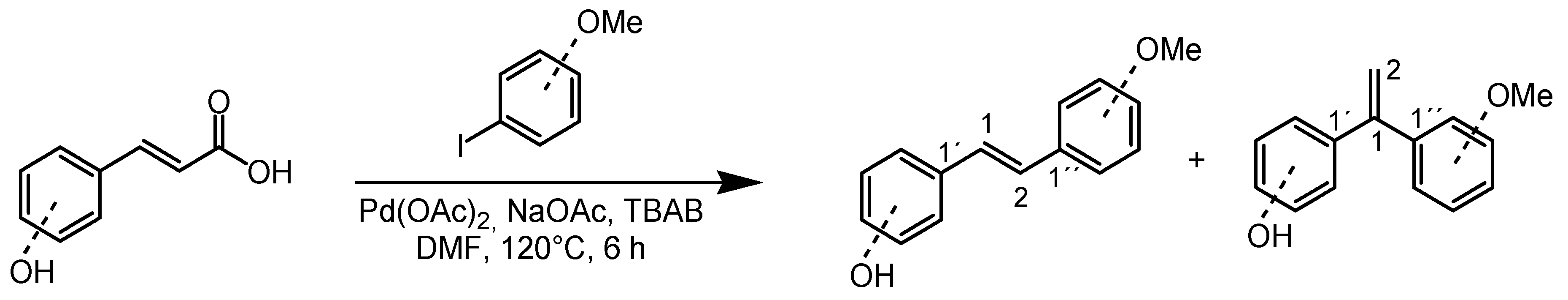
2.1. Synthesis of Diarylethenes 1–4
2.2. Antituberculosis Activity and Cytotoxicity of Diarylethenes 1–4
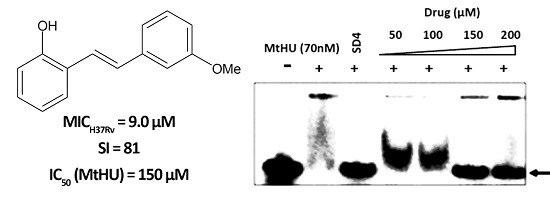
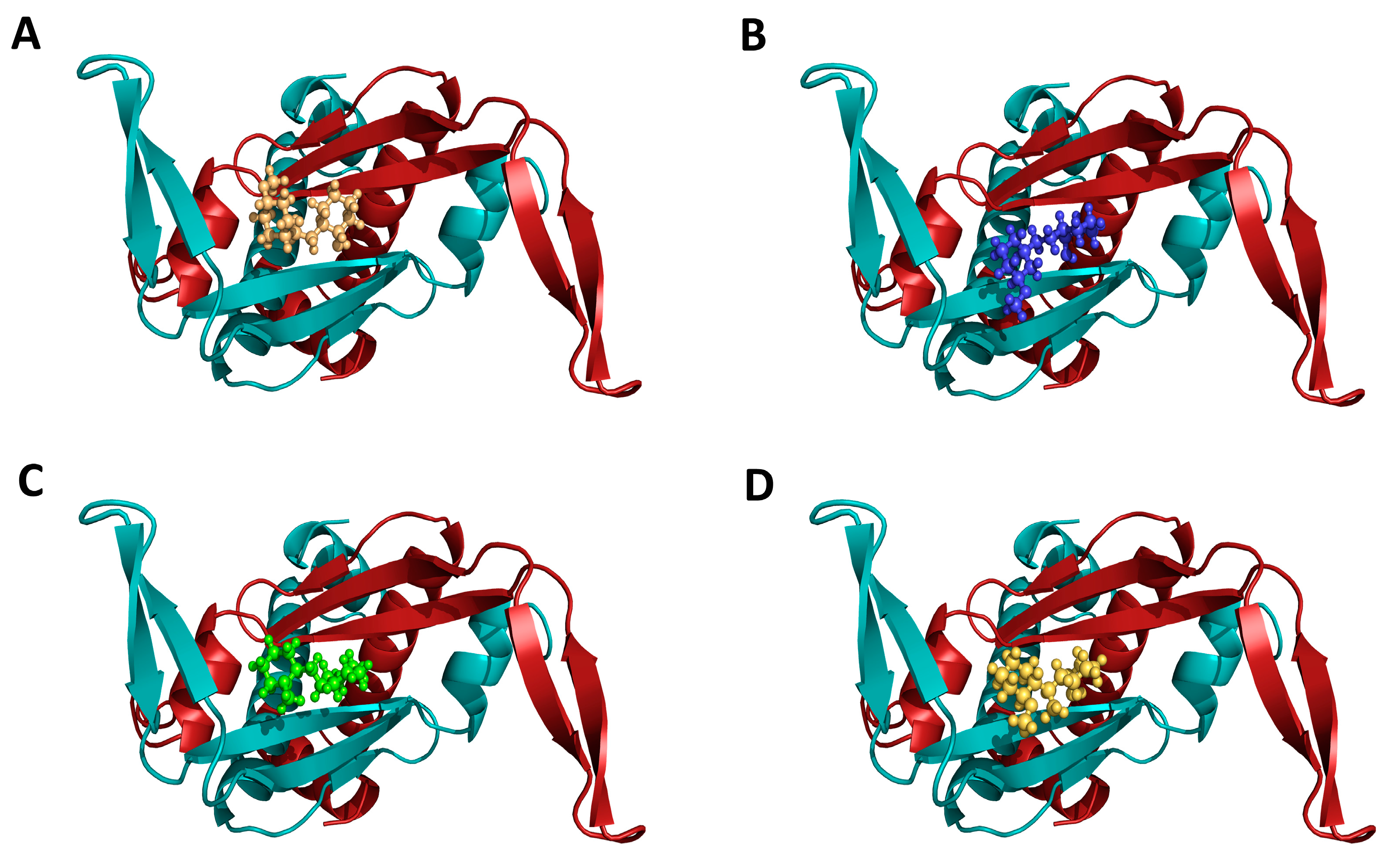
2.3. Inhibition of Mtb-HU Protein and Docking
3. Discussion
4. Materials and Methods
4.1. Reagents, Strains and Equipment
4.2. Synthesis of Diarylethenes
4.3. Antituberculosis Activity
4.4. Cytotoxicity
4.5. Mtb-HU Inhibition
4.6. In-silico Docking with Mtb-HU
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). Global Tuberculosis Report 2016; WHO Report; World Health Organization: Geneva, Switzerland, 2016; pp. 15–53. [Google Scholar]
- Getahun, H.; Matteelli, A.; Chaisson, R.E.; Raviglione, M. Latent Mycobacterium tuberculosis infection. N. Engl. J. Med. 2015, 372, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Jaggarajamma, K.; Sudha, G.; Chandrasekaran, V.; Nirupa, C.; Thomas, A.; Santha, T.; Muniyandi, M.; Narayanan, P.R. Reasons for non-compliance among patients treated under Revised National Tuberculosis Control Programme (RNTCP), Tiruvallur district, south India. Indian J. Tuberc. 2007, 54, 130–135. [Google Scholar] [PubMed]
- Velayati, A.A.; Farnia, P.; Masjedi, M.R.; Ibrahim, T.A.; Tabarsi, P.; Haroun, R.Z.; Kuan, H.O.; Ghanavi, J.; Varahram, M. Totally drug-resistant tuberculosis strains: Evidence of adaptation at the cellular level. Eur. Respir. J. 2009, 34, 1202–1203. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Douillet-Breuil, A.-C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Puntumchai, A.; Kittakoop, P.; Rajviroongit, S.; Vimuttipong, S.; Likhitwitayawuid, K.; Thebtaranonth, Y. Lakoochins A and B, New Antimycobacterial stilbene derivatives from Artocarpus lakoocha. J. Nat. Prod. 2004, 67, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.R.; de Carvalho, G.S.G.; da Silva, A.D.; Leite, C.Q. Synthesis and anti-Mycobacterium tuberculosis evaluation of aza-Stilbene derivatives. Sci. World J. 2011, 11, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, T.; Ghosh, S.; Dixit, K.; Ganesan, V.; Ramagopal, U.; Dey, D.; Sarma, S.; Ramakumar, S.; Nagaraja, V. Targeting Mycobacterium tuberculosis nucleoid-associated protein HU with structure-based inhibitors. Nat. Commun. 2014, 5, 4124. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D.; Mortazavi, P.N.; Munshi, T.; Evangelopoulos, D.; McHugh, T.D.; Gibbons, S.; Malkinson, J.; Bhakta, S. 2-Hydroxy-substituted cinnamic acids and acetanilides are selective growth inhibitors of Mycobacterium tuberculosis. MedChemComm 2014, 5, 47–50. [Google Scholar] [CrossRef]
- Jeffery, T. Palladium-catalysed vinylation of organic halides under solid–liquid phase transfer conditions. J. Chem. Soc. Chem. Commun. 1984, 1287–1289. [Google Scholar] [CrossRef]
- Lion, C.J.; Matthews, C.S.; Stevens, M.F.; Westwell, A.D. Synthesis, antitumor evaluation, and apoptosis-inducing activity of hydroxylated (E)-stilbenes. J. Med. Chem. 2005, 48, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.; Best, D.; Burns, D.J.; Lam, H.W. Synthesis of spirocyclic enones by rhodium-catalyzed dearomatizing oxidative annulation of 2-alkenylphenols with alkynes and enynes. Chem. Eur. J. 2014, 20, 8599–8602. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D.; Evangelopoulos, D.; Gupta, A.; Birchall, K.; Mwaigwisya, S.; Saxty, B.; McHugh, T.D.; Gibbons, S.; Malkinson, J.; Bhakta, S. Antitubercular specific activity of ibuprofen and the other 2-arylpropanoic acids using the HT-SPOTi whole-cell phenotypic assay. BMJ Open 2013, 3, e002672–e002685. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Orme, I. Search for new drugs for treatment of tuberculosis. Antimicrob. Agents Chemother. 2001, 45, 1943–1946. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.H.M.; Mulders, J.M.C.A.; Mommers, J.H.M.; Henderickx, H.J.W.; de Vries, J.G. Homeopathic ligand-free palladium as a catalyst in the Heck reaction. A comparison with a palladacycle. Org. Lett. 2003, 5, 3285–3288. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, E.S.; Santos, J.A.; Lima, L.L.; Machado, P.A.; Campos, D.L.; Pavan, F.R.; Silva, A.D. Synthesis, antitubercular and leishmanicidal evaluation of resveratrol analogues. J. Braz. Chem. Soc. 2016, 27, 2161–2169. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Groom, C.R.; Alex, A. Ligand efficiency: A useful metric for lead selection. Drug Discov. Today 2004, 9, 430–431. [Google Scholar] [CrossRef]
- Adamo, C.; Cammi, R.; Ochterski, J.; Martin, R.; Morokuma, K.; Farkas, O.; Foresman, J.; Fox, D. Gaussian 09; Revision A. 02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Parr, R.G. Density functional theory of atoms and molecules. In Horizons of Quantum Chemistry; Springer: Berlin, Germany, 1980; pp. 5–15. [Google Scholar]
- Gill, P.M.; Johnson, B.G.; Pople, J.A.; Frisch, M.J. The performance of the Becke—Lee—Yang—Parr (B—LYP) density functional theory with various basis sets. Chem. Phys. Lett. 1992, 197, 499–505. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein−ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
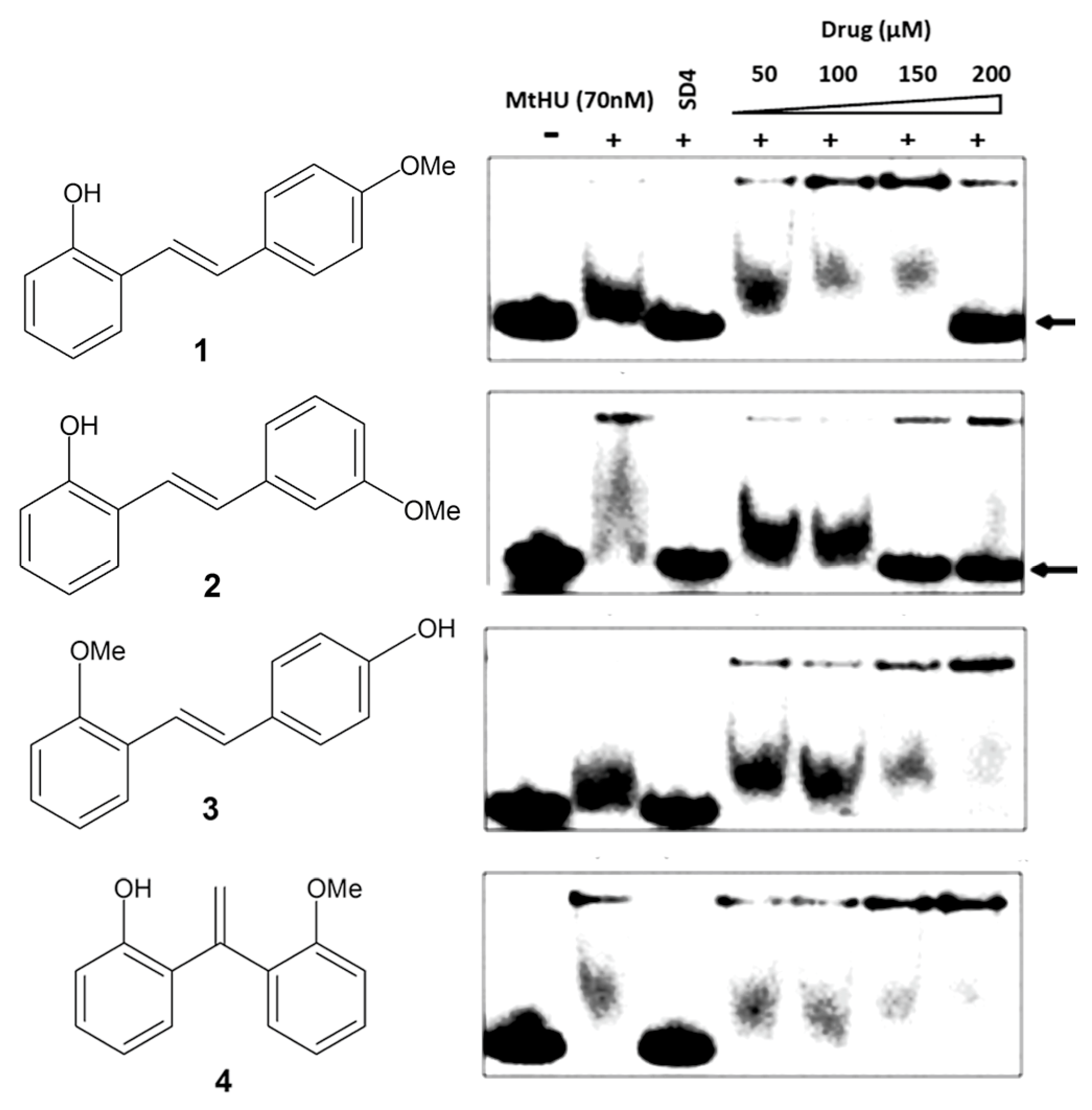
| Compound | MIC H37Rv (µM) | GIC50 BHK21 (µM) | Selectivity Index (SI) | IC90 Mtb-HU (µM) | Glide Docking Score |
|---|---|---|---|---|---|
| 1 | 9.0 | 522 | 58 | 200 | −3.26 |
| 2 | 9.0 | 729 | 81 | 150 | −3.29 |
| 3 | 22 | 98 | 4.4 | >200 | −2.86 |
| 4 | 9.0 | 499 | 55 | >200 | −2.96 |
| Isoniazid | 3.6 | nd | nd | nd | nd |
| Mitmab | nd | 8.8 | nd | nd | nd |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suarez, M.A.; Valencia, J.; Cadena, C.C.; Maiti, R.; Datta, C.; Puerto, G.; Isaza, J.H.; San Juan, H.; Nagaraja, V.; Guzman, J.D. Diarylethenes Display In Vitro Anti-TB Activity and Are Efficient Hits Targeting the Mycobacterium tuberculosis HU Protein. Molecules 2017, 22, 1245. https://doi.org/10.3390/molecules22081245
Suarez MA, Valencia J, Cadena CC, Maiti R, Datta C, Puerto G, Isaza JH, San Juan H, Nagaraja V, Guzman JD. Diarylethenes Display In Vitro Anti-TB Activity and Are Efficient Hits Targeting the Mycobacterium tuberculosis HU Protein. Molecules. 2017; 22(8):1245. https://doi.org/10.3390/molecules22081245
Chicago/Turabian StyleSuarez, María Angélica, Jhesua Valencia, Christian Camilo Cadena, Raktim Maiti, Chandreyee Datta, Gloria Puerto, José Hipólito Isaza, Homero San Juan, Valakunja Nagaraja, and Juan David Guzman. 2017. "Diarylethenes Display In Vitro Anti-TB Activity and Are Efficient Hits Targeting the Mycobacterium tuberculosis HU Protein" Molecules 22, no. 8: 1245. https://doi.org/10.3390/molecules22081245