Inhibitory Effect of Bovine Lactoferrin on Catechol-O-Methyltransferase
Abstract
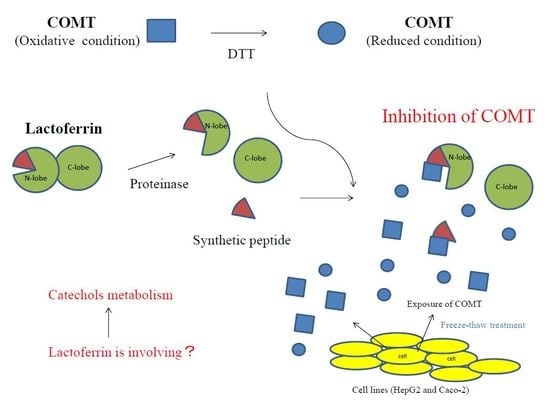
:1. Introduction
2. Results
2.1. Interaction (Inhibitory) Sites of bLF with COMT
2.2. Enzyme Kinetic Analysis: Inhibition of COMT by bLF
2.3. Inhibitory Potency of bLF on dithiothreitol (DTT)-Treated COMT
2.4. Effects of bLF on COMT Activity in Lysates from Human Cell Lines
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of bLF Fragments p36, p20, and p51 by Trypsin Digestion
4.3. COMT Assay
4.4. Inhibitory Activity of bLF against DTT-Pretreated COMT
4.5. Cell Culture and Preparation of Cell Lysates
4.6. Immunoblotting
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Masson, P.L.; Heremans, J.F. Lactoferrin in milk from different species. Comp. Biochem. Physiol. B 1971, 39, 119–129. [Google Scholar] [CrossRef]
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Haematologica 1995, 80, 252–267. [Google Scholar] [PubMed]
- Deriy, L.V.; Chor, J.; Thomas, L.L. Surface expression of lactoferrin by resting neutrophils. Biochem. Biophys. Res. Commun. 2000, 275, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Wakabayashi, H.; Yamauchi, K.; Teraguchi, S.; Tamura, Y.; Kurokawa, M.; Shiraki, K. Effects of orally administered bovine lactoferrin and lactoperoxidase on influenza virus infection in mice. J. Med. Microbiol. 2005, 54, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, T.; Yamauchi, K.; Tamura, Y.; Okamura, H. Oral administration of lactoferrin increases NK cell activity in mice via increased production of IL-18 and type I IFN in the small intestine. J. Interferon Cytokine Res. 2006, 26, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Akasu, T.; Saito, D.; Alexander, D.B.; Iigo, M.; Kakizoe, T.; Tsuda, H. Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial. Cancer Prev. Res. (Phila) 2009, 2, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.K.; Chierici, R.; Sawatzki, G.; Hill, M.J.; Volpato, S.; Vigi, V. Supplementation of an adapted formula with bovine lactoferrin: 1. Effect on the infant faecal flora. Acta Paediatr. 1992, 81, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J. Dairy Sci. 1991, 74, 4137–4142. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Shin, K.; Lönnerdal, B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry 2001, 40, 15771–15779. [Google Scholar] [CrossRef] [PubMed]
- Neels, J.G.; van Den Berg, B.M.; Lookene, A.; Olivecrona, G.; Pannekoek, H.; van Zonneveld, A.J. The second and fourth cluster of class A cysteine-rich repeats of the low density lipoprotein receptor-related protein share ligand-binding properties. J. Biol. Chem. 1999, 274, 31305–31311. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D.; Vigié, K.; Said, E.A.; Elass, E.; Masson, M.; Slomianny, M.C.; Carpentier, M.; Briand, J.P.; Mazurier, J.; Hovanessian, A.G. Surface nucleolin participates in both the binding and endocytosis of lactoferrin in target cells. Eur. J. Biochem. 2004, 271, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Abe, Y.; Inoue, N.; Kim, W.S.; Kumura, H.; Nagasawa, H.; Igarashi, I.; Shimazaki, K. The detection of bovine lactoferrin binding protein on Trypanosoma brucei. J. Vet. Med. Sci. 2004, 66, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.L.; Ishida, H.; Vogel, H.J. Structural characterization of the interaction of human lactoferrin with calmodulin. PLoS ONE 2012, 7, e51026. [Google Scholar] [CrossRef] [PubMed]
- Elass-Rochard, E.; Roseanu, A.; Legrand, D.; Trif, M.; Salmon, V.; Motas, C.; Montreuil, J.; Spik, G. Lactoferrin-lipopolysaccharide interaction: Involvement of the 28–34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem. J. 1995, 312, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Corti, R.; Flammer, A.J.; Hollenberg, N.K.; Lüscher, T.F. Cocoa and cardiovascular health. Circulation 2009, 119, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Holt, R.R.; Orozco, T.J.; Schmitz, H.H.; Keen, C.L. Nutrition: Milk and absorption of dietary flavanols. Nature 2003, 426, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Roura, E.; Andrés-Lacueva, C.; Estruch, R.; Mata-Bilbao, M.L.; Izquierdo-Pulido, M.; Waterhouse, A.L.; Lamuela-Raventós, R.M. Milk does not affect the bioavailability of cocoa powder flavonoid in healthy human. Ann. Nutr. Metab. 2007, 51, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kartsova, L.A.; Alekseeva, A.V. Effect of milk caseins on the concentration of polyphenolic compounds in tea. J. Anal. Chem. 2008, 63, 1107–1111. [Google Scholar] [CrossRef]
- van der Burg-Koorevaar, M.C.; Miret, S.; Duchateau, G.S. Effect of milk and brewing method on black tea catechin bioaccessibility. J. Agric. Food Chem. 2011, 59, 7752–7758. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Yuan, J.M.; Yang, C.S.; Van Den Berg, D.J.; Lee, M.J.; Gao, Y.T.; Yu, M.C. Genetic Association between the COMT genotype and urinary levels of tea polyphenols and their metabolites among daily green tea drinkers. Int. J. Mol. Epidemiol. Genet. 2010, 1, 114–123. [Google Scholar] [PubMed]
- Miller, R.J.; Jackson, K.G.; Dadd, T.; Nicol, B.; Dick, J.L.; Mayes, A.E.; Brown, A.L.; Minihane, A.M. A preliminary investigation of the impact of catechol-O-methyltransferase genotype on the absorption and metabolism of green tea catechins. Eur. J. Nutr. 2012, 51, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012, 3, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J.; Tomchick, R. Enzymatic O-methylation of epinephrine and other catechols. J. Biol. Chem. 1958, 233, 702–705. [Google Scholar] [PubMed]
- Männistö, P.T.; Kaakkola, S. Catechol-O-methyltransferase (COMT): Biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol. Rev. 1999, 51, 593–628. [Google Scholar] [PubMed]
- Nissinen, E.; Tuominen, R.; Perhoniemi, V.; Kaakkola, S. Catechol-O-methyltransferase activity in human and rat small intestine. Life Sci. 1988, 42, 2609–2614. [Google Scholar] [CrossRef]
- Tilgmann, C.; Melen, K.; Lundström, K.; Jalanko, A.; Julkunen, I.; Kalkkinen, N.; Ulmanen, I. Expression of recombinant soluble and membrane-bound catechol O-methyltransferase in eukaryotic cells and identification of the respective enzymes in rat brain. Eur. J. Biochem. 1992, 207, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Lotta, T.; Vidgren, J.; Tilgmann, C.; Ulmanen, I.; Melén, K.; Julkunen, I.; Taskinen, J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995, 34, 4202–4210. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, M.J.; Palma, P.N.; Almeida, L.; Soares-da-Silva, P. Catechol-O-methyltransferase and its inhibitors in Parkinson’s disease. CNS Drug Rev. 2007, 13, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.T.; Liehr, J.G. Inhibition of catechol O-methyltransferase-catalyzed O-methylation of 2- and 4-hydroxyestradiol by quercetin. Possible role in estradiol-induced tumorigenesis. J. Biol. Chem. 1996, 271, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, C.Y.; Lambert, J.D.; Ai, N.; Welsh, W.J.; Yang, C.S. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: Structure-activity relationship and molecular-modeling studies. Biochem. Pharmacol. 2005, 69, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Assicot, M.; Bohuon, C. Production of antibodies to catechol-O-methyltransferase (EC 2.1.1.6) of rat liver. Biochem. Pharmacol. 1969, 18, 1893–1898. [Google Scholar] [CrossRef]
- Guldberg, H.C.; Marsden, C.A. Catechol-O-methyl transferase: Pharmacological aspects and physiological role. Pharmacol. Rev. 1975, 27, 135–206. [Google Scholar] [PubMed]
- Cotton, N.J.; Stoddard, B.; Parson, W.W. Oxidative inhibition of human soluble catechol-O-methyltransferase. J. Biol. Chem. 2004, 279, 23710–23718. [Google Scholar] [CrossRef] [PubMed]
- Teniola, D.; Ayoola, E.A.; Arigbabu, A.O. Lactic dehydrogenase levels in patients with duodenal ulcer, gastric ulcer, gastric polys and gastric carcinoma. Scand. J. Gastroenterol. Suppl. 1986, 124, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.M.; Watson, A.J. Apoptosis and gastrointestinal pharmacology. Pharmacol Ther. 1996, 72, 149–169. [Google Scholar] [CrossRef]
- Kuwata, H.; Yip, T.T.; Tomita, M.; Hutchens, T.W. Direct evidence of the generation in human stomach of an antimicrobial peptide domain (lactoferricin) from ingested lactoferrin. Biochim. Biophys. Acta 1998, 1429, 129–141. [Google Scholar] [CrossRef]
- Troost, F.J.; Steijns, J.; Saris, W.H.; Brummer, R.J. Gastric digestion of bovine lactoferrin in vivo in adults. J. Nutr. 2001, 131, 2101–2104. [Google Scholar] [PubMed]
- Karthikeyan, S.; Yadav, S.; Paramasivam, M.; Srinivasan, A.; Singh, T.P. Structure of buffalo lactoferrin at 3.3 A resolution at 277 K. Acta Crystallogr. Sect. D 2000, 56, 684–689. [Google Scholar] [CrossRef]
- Moorse, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-dimensional structure of diferric bovine lactoferrin at 2.8 A resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.M.; Zhou, N.; Shan, X.; Arrowsmith, C.H.; Vogel, H.J. Three-dimensional solution structure of lactoferricin B, an antimicrobial peptide derived from bovine lactoferrin. Biochemistry 1998, 37, 4288–4298. [Google Scholar] [CrossRef] [PubMed]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, H.; Wu, B. Structure-based drug design of catechol-O-methyltransferase inhibitors for CNS disorders. Br. J. Clin. Pharmacol. 2014, 77, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Kiyosawa, I.; Kuwahara, K. Amounts of lactoferrin in human colostrum and milk. J. Dairy Sci. 1972, 55, 1651–1659. [Google Scholar] [CrossRef]
- Ballow, M.; Donshik, P.C.; Rapacz, P.; Samartino, L. Tear lactoferrin levels in patients with external inflammatory ocular disease. Investig. Ophthalmol. Vis. Sci. 1987, 28, 543–545. [Google Scholar]
- Cheng, J.B.; Wang, J.Q.; Bu, D.P.; Liu, G.L.; Zhang, C.G.; Wei, H.Y.; Zhou, L.Y.; Wang, J.Z. Factors affecting the lactoferrin concentration in bovine milk. J. Dairy Sci. 2008, 91, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Wakabayashi, H.; Yamauchi, K.; Teraguchi, S.; Hayasawa, H. Bovine lactoferrin and lactoferricin derived from milk: Production and applications. Biochem. Cell Biol. 2002, 80, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Mizumachi, K.; Takezawa, T. The bovine lactoferrin region responsible for promoting the collagen gel contractile activity of human fibroblasts. Biochem. Biophys. Res. Commun. 2002, 299, 813–817. [Google Scholar] [CrossRef]
- Shimazaki, K.; Tanaka, T.; Kon, H.; Oota, K.; Kawaguchi, A.; Maki, Y.; Sato, T. Separation and characterization of the C-terminal half molecule of bovine lactoferrin. J. Dairy Sci. 1993, 76, 946–955. [Google Scholar] [CrossRef]
- Floderus, Y.; Sääf, J.; Ross, S.B.; Wetterberg, L. Catechol-O-methyltransferase activity in human erythrocytes: Methodological aspects. Upsala J. Med. Sci. 1981, 86, 309–318. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, M.; Iijima, H.; Shinoda, I.; Iwamoto, H.; Takeda, Y. Inhibitory Effect of Bovine Lactoferrin on Catechol-O-Methyltransferase. Molecules 2017, 22, 1373. https://doi.org/10.3390/molecules22081373
Ikeda M, Iijima H, Shinoda I, Iwamoto H, Takeda Y. Inhibitory Effect of Bovine Lactoferrin on Catechol-O-Methyltransferase. Molecules. 2017; 22(8):1373. https://doi.org/10.3390/molecules22081373
Chicago/Turabian StyleIkeda, Masayuki, Hiroshi Iijima, Ichizo Shinoda, Hiroshi Iwamoto, and Yasuhiro Takeda. 2017. "Inhibitory Effect of Bovine Lactoferrin on Catechol-O-Methyltransferase" Molecules 22, no. 8: 1373. https://doi.org/10.3390/molecules22081373