Cytotoxic Effects and Anti-Angiogenesis Potential of Pistachio (Pistacia vera L.) Hulls against MCF-7 Human Breast Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Cell Viability Effect of PVLH against Cancer Cells
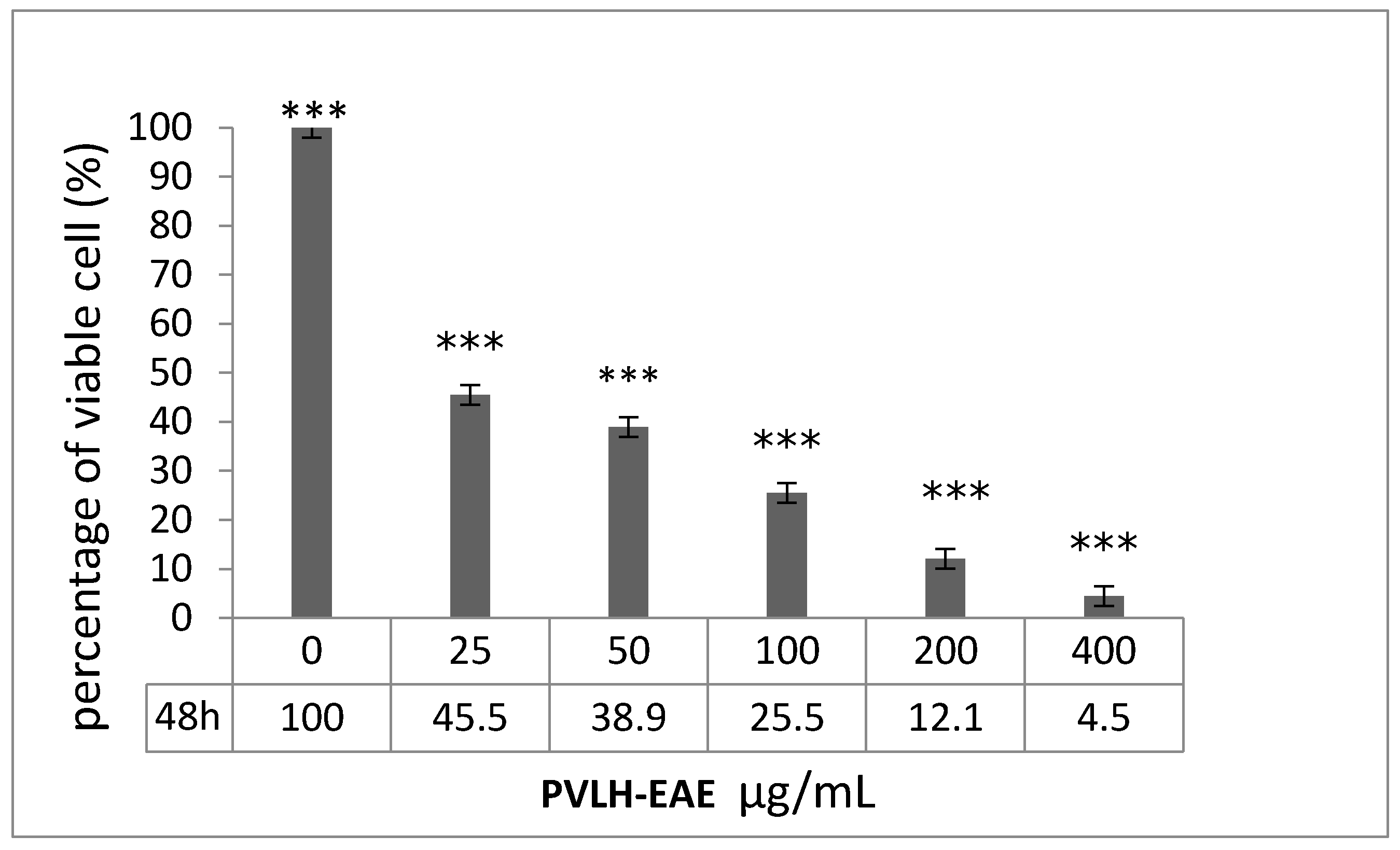
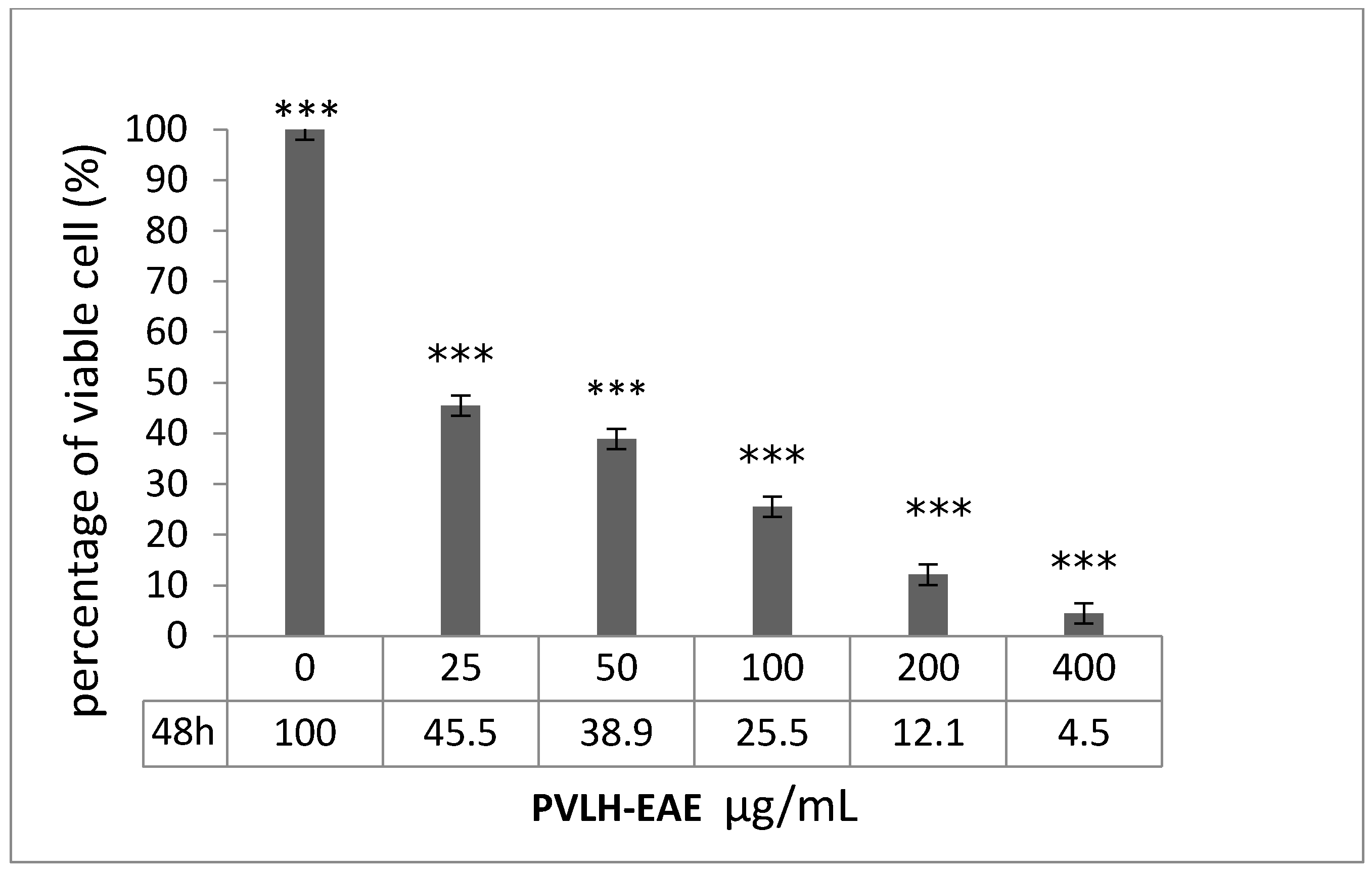
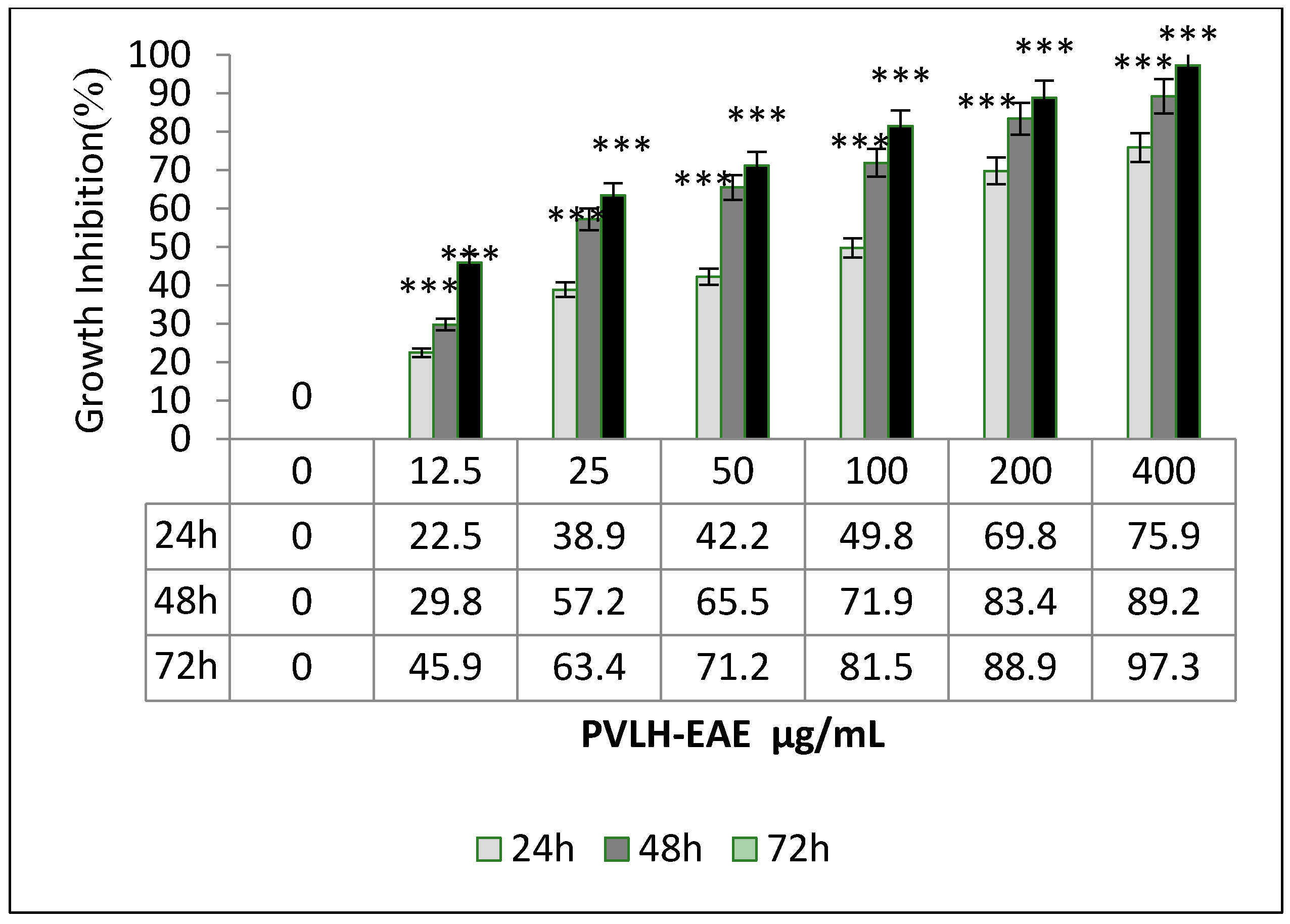
2.2. Cytotoxic and Cell Viability Effect of PVLH-EAE toward MCF-7
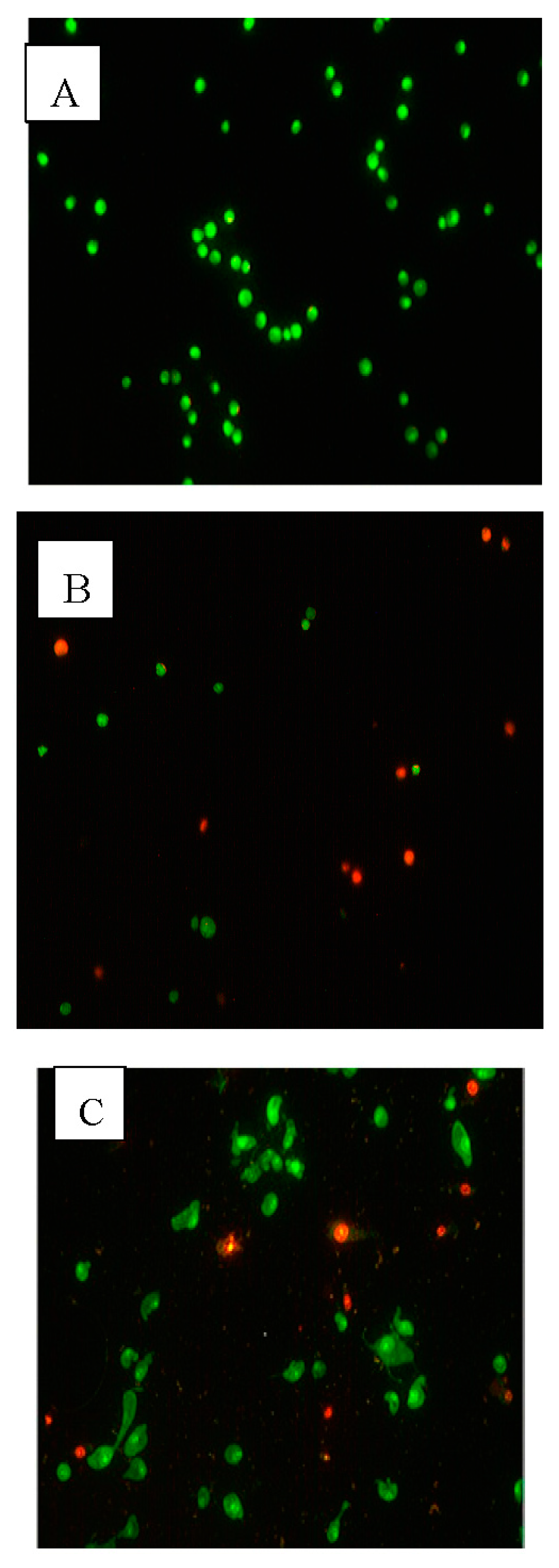
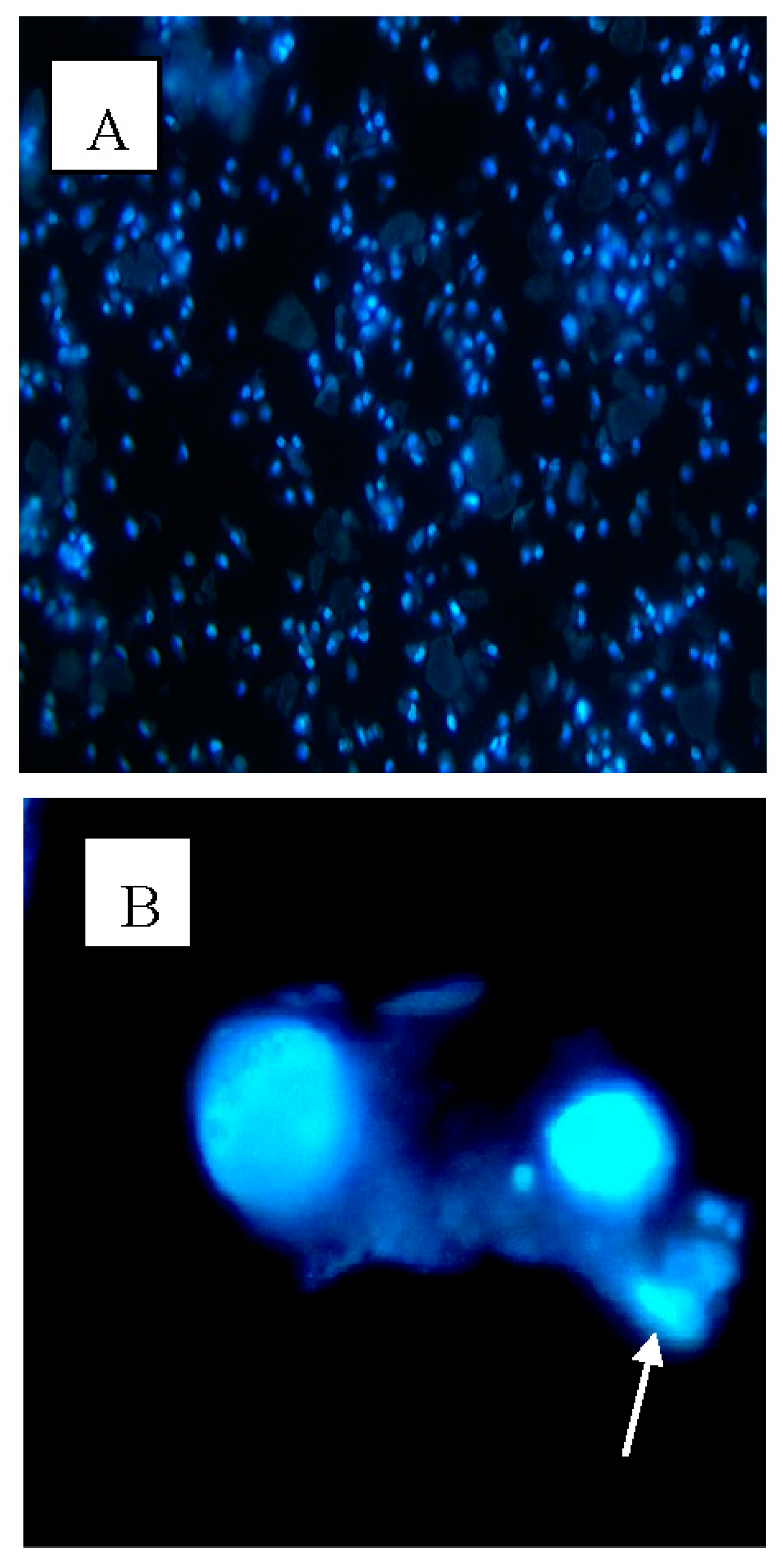
2.3. Apoptotic Morphological Changes
2.4. Flow Cytometry Analysis
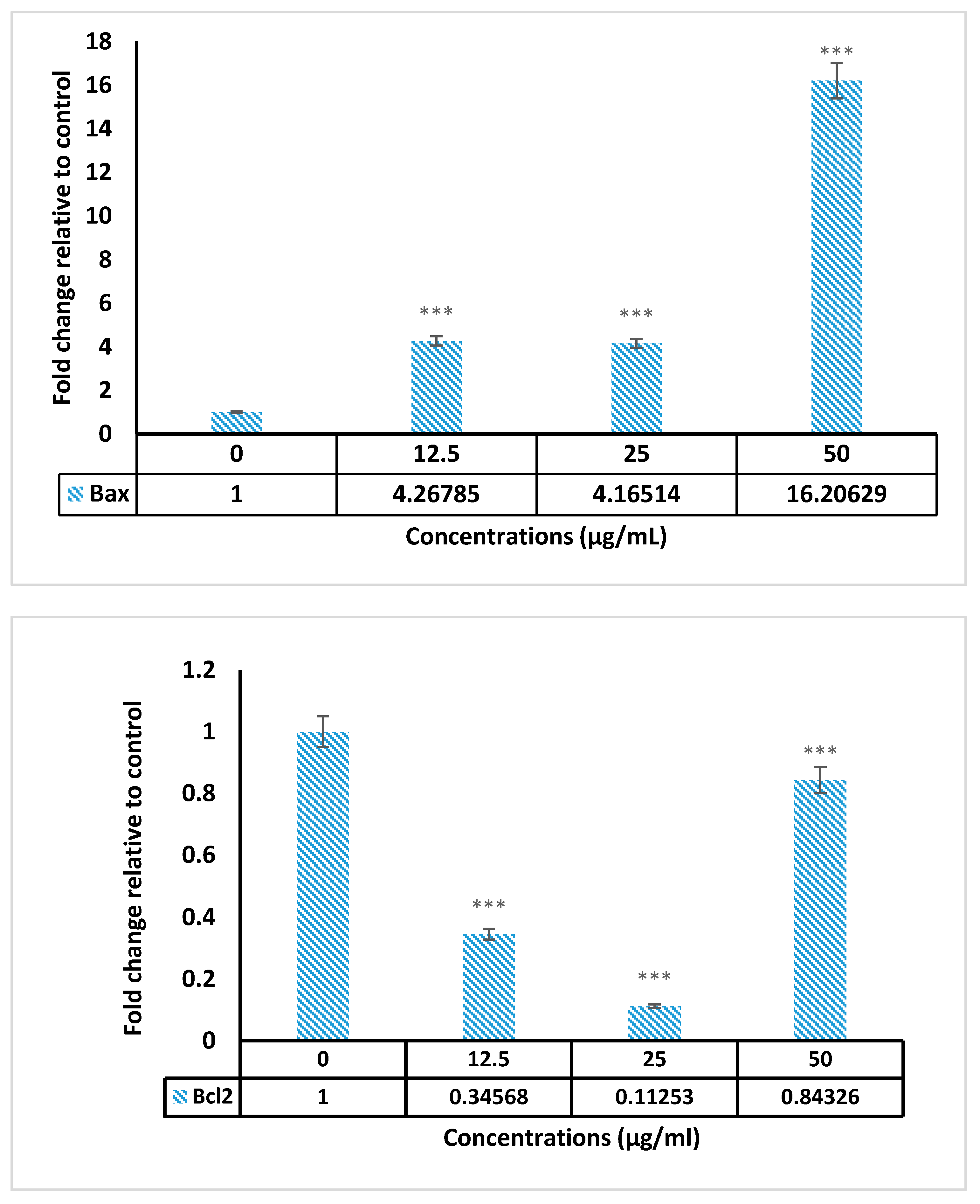
2.5. Real Time PCR Results
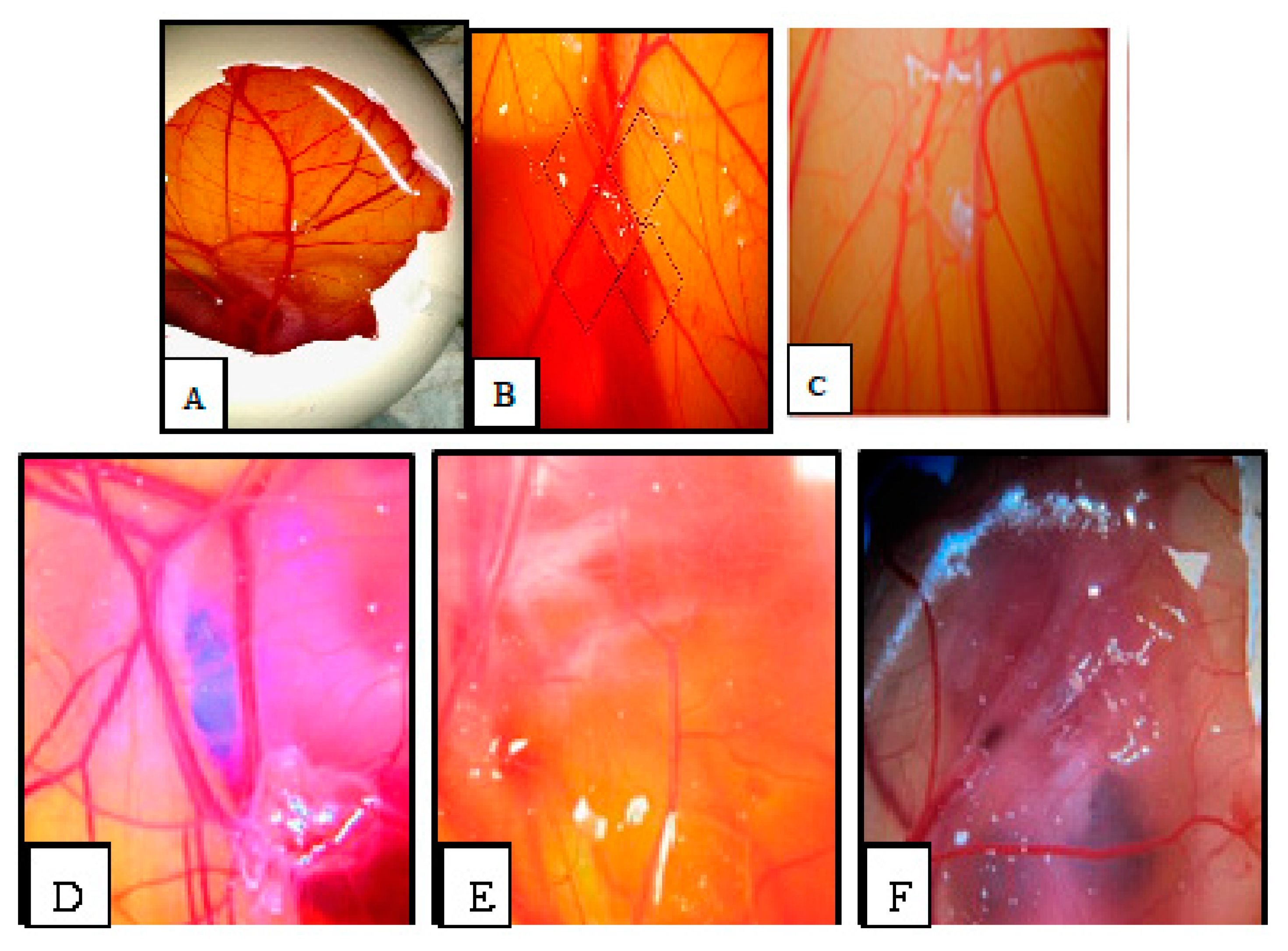
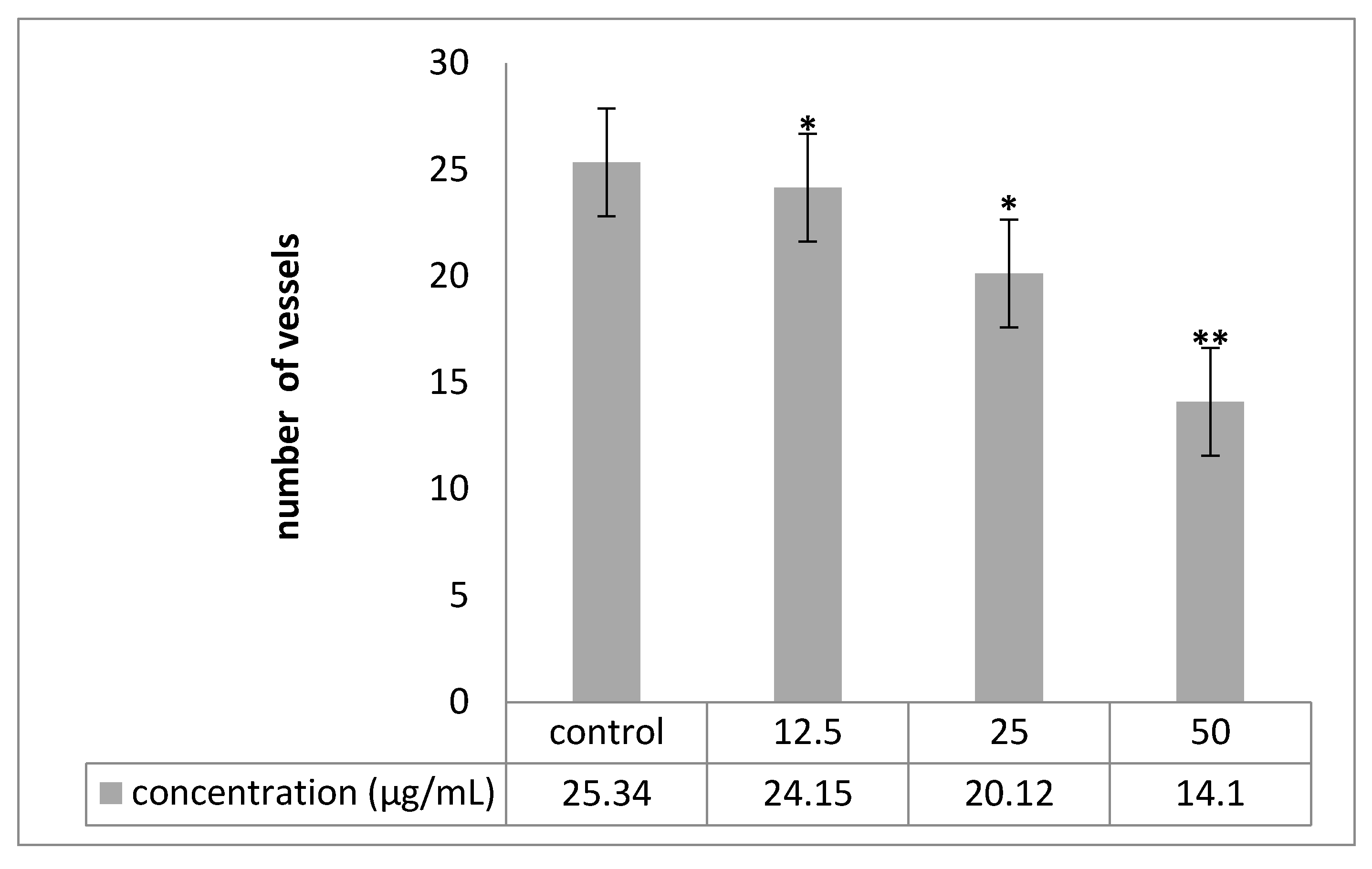
2.6. Chorioallantoic Membrane (CAM) Assay
3. Discussion
4. Materials and Methods
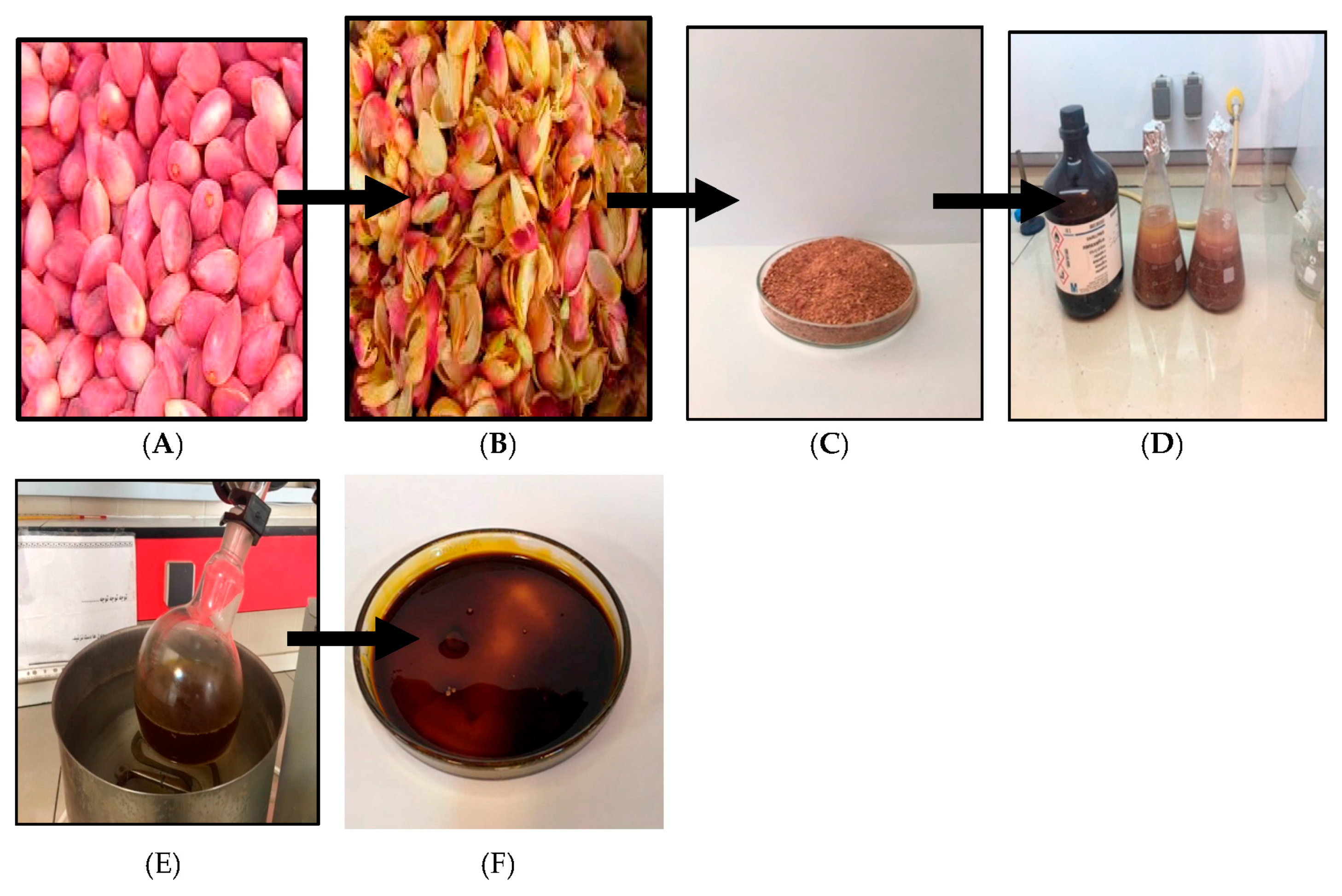
4.1. Plant Material and Extraction
4.2. Cell Lines and Cell Culture
4.3. MTT Cell Proliferation Assay
4.4. Trypan Blue Assay
4.5. Flow Cytometry Analysis
4.6. Acridine Orange/Propidium Iodide Staining (AO/PI)
4.7. Hoechst 33,342 Staining
4.8. Gene Expression Assay
4.9. Chick Chorioallantoic Membrane Assay
5. Statistical Analysis
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crane, J.C.; Iwakiri, B.T.; Dehiscence, B.E. Morphology and Reproduction of Pistachio. Hortic. Rev. 1981, 3, 376–393. [Google Scholar]
- Dreher, M.L. Pistachio nuts: Composition and potential health benefits. Nutr. Rev. 2011, 70, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.S.; Shrivastava, N.; Baghel, R.S.; Rajput, S. A rivew of quercetin: Antioxidant and anti cancer properties. World J. Pharm. Pharm. Sci. 2012, 1, 146–160. [Google Scholar]
- Müllauer, L.; Gruber, P.; Sebinger, D.; Buch, J.; Wohlfart, S.; Chott, A. Mutations in apoptosis genes: A pathogenetic factor for human disease. Mutat. Res. 2001, 488, 211–231. [Google Scholar] [CrossRef]
- Carle, R.; Schweiggert, R.M. Identification of Phenolic Compounds in Red and Green Pistachio (Pistacia vera L.) Hulls (Exo- and Mesocarp) by HPLC-DAD-ESI-(HR)-MS. Agric. Food Chem. 2016, 64, 5334–5344. [Google Scholar] [CrossRef]
- Namvar, F.; Suhaila, M.; Gasemi Fard, S.; Behravan, J. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012, 130, 376–382. [Google Scholar] [CrossRef]
- Eissa, S.; Labib, R.; Khalifa, A.; Swelam, N.; Khalil, F.; El-Shenawy, A.M. Regulators of apoptosis in human breast cancer. Clin. Biochem. 1999, 32, 321–326. [Google Scholar] [CrossRef]
- Kwon, K.H.; Barve, A.; Yu, S.; Huang, M.-T.; Kong, A.-N.T. Cancer chemoprevention by phytochemicals: potential molecular targets, biomarkers and animal models. Acta Pharmacol. Sin. 2007, 28, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Magkouta, S.; Stathopoulos, G.T.; Psallidas, I.; Kolisis, F.N.; Roussos, C.; Loutrari, H.; Magkouta, S.; Stathopoulos, G.T.; Psallidas, I. Protective Effects of Mastic Oil From Pistacia Lentiscus Variation Chia Against Experimental Growth of Lewis Lung Carcinoma Protective Effects of Mastic Oil From Pistacia Lentiscus Variation Chia Against Experimental Growth of Lewis Lung. Nutr. Cancer 2015, 37–41. [Google Scholar] [CrossRef]
- Fathi, P.; Fouladdel, S.; Hassani, S.; Yousefbeyk, F.; Mahmood, S.; Amin, G.; Azizi, E. Induction of apoptosis and cell cycle arrest by pericarp polyphenol-rich extract of Baneh in human colon carcinoma HT29 cells. Food Chem. Toxicol. 2012, 50, 1054–1059. [Google Scholar] [CrossRef]
- Germain, M.; D’Amours, D.; Dixit, V. Cleavage of automodified poly (ADP-ribose) polymerase during apoptosis. J. Biol. Chem. 1999, 274, 28379–28384. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Imai, T.; Tanaka, Y.; Tsukamura, K.; Hayakawa, Y.; Kikumori, T.; Mase, T.; Itoh, T.; Nishikawa, M.; Hayashi, H.; et al. Wakame seaweed suppresses the proliferation of 7,12-dimethylbenz(a)-anthracene-induced mammary tumors in rats. Jpn. J. Cancer Res. 1999, 90, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.E.; Honselman, C.; Halvorson, A.; Rhodes, K.; Skwir, K. The effect of pistachio shells as a visual cue in reducing caloric consumption. Appetite 2011, 57, 418–420. [Google Scholar] [CrossRef]
- Goli, A.H.; Barzegar, M.; Sahari, M.A. Food Chemistry Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chem. 2005, 92, 521–525. [Google Scholar] [CrossRef]
- Garavand, F.; Madadlou, A.; Moini, S. Determination of Phenolic Profile and Antioxidant Activity of Pistachio Hull Using HPLC-DAD-ESI-MS as affected by Ultrasound and Microwave. Int. J. Food Prop. 2015, 2912. [Google Scholar] [CrossRef]
- Rajaei, A.; Barzegar, M.; Mohabati, A.; Ali, M.; Hamidi, Z. Antioxidant, anti-microbial and antimutagenicity activities of pistachio (Pistachia vera) green hull extract. Food Chem. Toxicol. 2010, 48, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Biochimie Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 2010, 92, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- He, M. Mechanisms of antiprostate cancer by gum mastic: NF-κB signal as target. Acta Pharmacol. Sin. 2007, 28, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, C.; Su, A.; Zhang, Y.; Yuan, J.; Ju, X. Structural characterization of phenolic compounds and antioxidant activity of the phenolic-rich fraction from defatted adlay (Coix lachryma-jobi L. var. ma-yuen Stapf ) seed meal. Food Chem. 2016, 196, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Laganà, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Fathalizadeh, J.; Bagheri, V.; Khorramdelazad, H.; Kazemi, M.; Jafarzadeh, A. Induction of apoptosis by pistachio (Pistacia vera L.) hull extract and its molecular mechanisms of action in human. Cell. Mol. Biol. 2016, 61, 128–134. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Bae, S.-Y.; Kim, K.-H.; Han, C.-H.; Cho, S.-H.; Nam, S.-W.; Choi, Y.H.; Kim, B.-W. Induction of apoptosis in HeLa cells by ethanolic extract of Corallina pilulifera. Food Chem. 2007, 104, 196–201. [Google Scholar] [CrossRef]
- Mirian, M.; Behrooeian, M.; Ghanadian, M.; Dana, N.; Sadeghi-Aliabadi, H. Cytotoxicity and antiangiogenic effects of Rhus coriaria, Pistacia vera and Pistacia khinjuk oleoresin methanol extracts. Res. Pharm. Sci. 2015, 10, 233–240. [Google Scholar] [PubMed]
- Namvar, F.; Baharara, J.; Mahdi, A. Antioxidant and Anticancer Activities of Selected Persian Gulf Algae. Indian J. Clin. Biochem. 2014, 29, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Price, P.; Mcmillan, T.J. Use of the Tetrazolium Assay in Measuring the Response of Human Tumor Cells to Ionizing Radiation Use of the Tetrazolium Assay in Measuring the Response of Human Tumor Cells to Ionizing Radiation. Cancer Res. 1990, 50, 1392–1396. [Google Scholar] [PubMed]
Sample Availability: Samples of the Pistacia vera L. Hulls extracts are available from the authors. |



| Cancer Cell Line | Classification | Hexane Ex. | Ethyl acetate Ex. | Methanol Ex. | Water Ex. |
|---|---|---|---|---|---|
| BJ-5ta | Normal fibroblast | ˃100 | ˃100 | ˃100 | ˃100 |
| HepG2 | Liver cancer | ˃100 | 92.24 ± 5.82 | ˃100 | ˃100 |
| CasKi | Cervical cancer | 93.44 ± 2.5 | 81.17 ± 2.87 | ˃100 | ˃100 |
| H23 | Lung cancer | 80.31 ± 1.63 | 67.63 ± 5.54 | 89.21 ± 1.30 | ˃100 |
| MCF-7 | Breast cancer | 44.88 ± 1.47 | 21.20 ± 1.35 | ˃100 | ˃100 |
| HCT116 | Colon cancer | 53.20 ± 2.69 | 25.15 ± 1.85 | 84.27 ± 1.29 | ˃100 |
| HT-29 | Colon cancer | 36.17 ± 1.22 | 23.00 ± 1.2 | ˃100 | ˃100 |
| Group | Time | % Viable | % Apoptotic | % Necrotic |
|---|---|---|---|---|
| Control MCF-7 + 0.1% DMSO | 0 | 96 + 0.5 | 0.7 + 0.3 | 0.3 + 0.2 |
| 24 | 93.7 ± 2.1 | 4.5 ± 1.7 | 1.8 ± 0.7 | |
| 48 | 92.3 ± 1.4 | 6.8 ± 3.2 | 2.9 ± 0.5 | |
| 72 | 87.3 ± 0.4 | 8.9 ± 2.4 | 4.1 ± 1.1 | |
| Treatment MCF-7 + 25 μg/mL PVLH-EAE | 0 | 98.9 + 1.4 | 0.4 + 0.3 | 0.7 + 0.4 |
| 24 | 68.9 ± 1.2 | 27.87 ± 6.6* | 2.1 ± 0.9 | |
| 48 | 49.8 ± 2.6 | 43.5 ± 3.3* | 6.7 ± 1.2 | |
| 72 | 32.1 ± 3.7 | 57.7 ± 2.7* | 8.9 ± 2.5 |
| BCL-2 | 5 CATGTGTGTGGAGAGCGTCAAC 3 F 5 CAGATAGGCACCCAGGGTGAT 3 R |
| BAX | 5 TTTGCTTCAGGGTTTCATCCA 3 F 5 CTCCATGTTACTGTCCAGTTCGT 3 R |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seifaddinipour, M.; Farghadani, R.; Namvar, F.; Mohamad, J.; Abdul Kadir, H. Cytotoxic Effects and Anti-Angiogenesis Potential of Pistachio (Pistacia vera L.) Hulls against MCF-7 Human Breast Cancer Cells. Molecules 2018, 23, 110. https://doi.org/10.3390/molecules23010110
Seifaddinipour M, Farghadani R, Namvar F, Mohamad J, Abdul Kadir H. Cytotoxic Effects and Anti-Angiogenesis Potential of Pistachio (Pistacia vera L.) Hulls against MCF-7 Human Breast Cancer Cells. Molecules. 2018; 23(1):110. https://doi.org/10.3390/molecules23010110
Chicago/Turabian StyleSeifaddinipour, Maryam, Reyhaneh Farghadani, Farideh Namvar, Jamaludin Mohamad, and Habsah Abdul Kadir. 2018. "Cytotoxic Effects and Anti-Angiogenesis Potential of Pistachio (Pistacia vera L.) Hulls against MCF-7 Human Breast Cancer Cells" Molecules 23, no. 1: 110. https://doi.org/10.3390/molecules23010110
APA StyleSeifaddinipour, M., Farghadani, R., Namvar, F., Mohamad, J., & Abdul Kadir, H. (2018). Cytotoxic Effects and Anti-Angiogenesis Potential of Pistachio (Pistacia vera L.) Hulls against MCF-7 Human Breast Cancer Cells. Molecules, 23(1), 110. https://doi.org/10.3390/molecules23010110





