Design, Synthesis, and Safener Activity of Novel Methyl (R)-N-Benzoyl/Dichloroacetyl-Thiazolidine-4-Carboxylates
Abstract
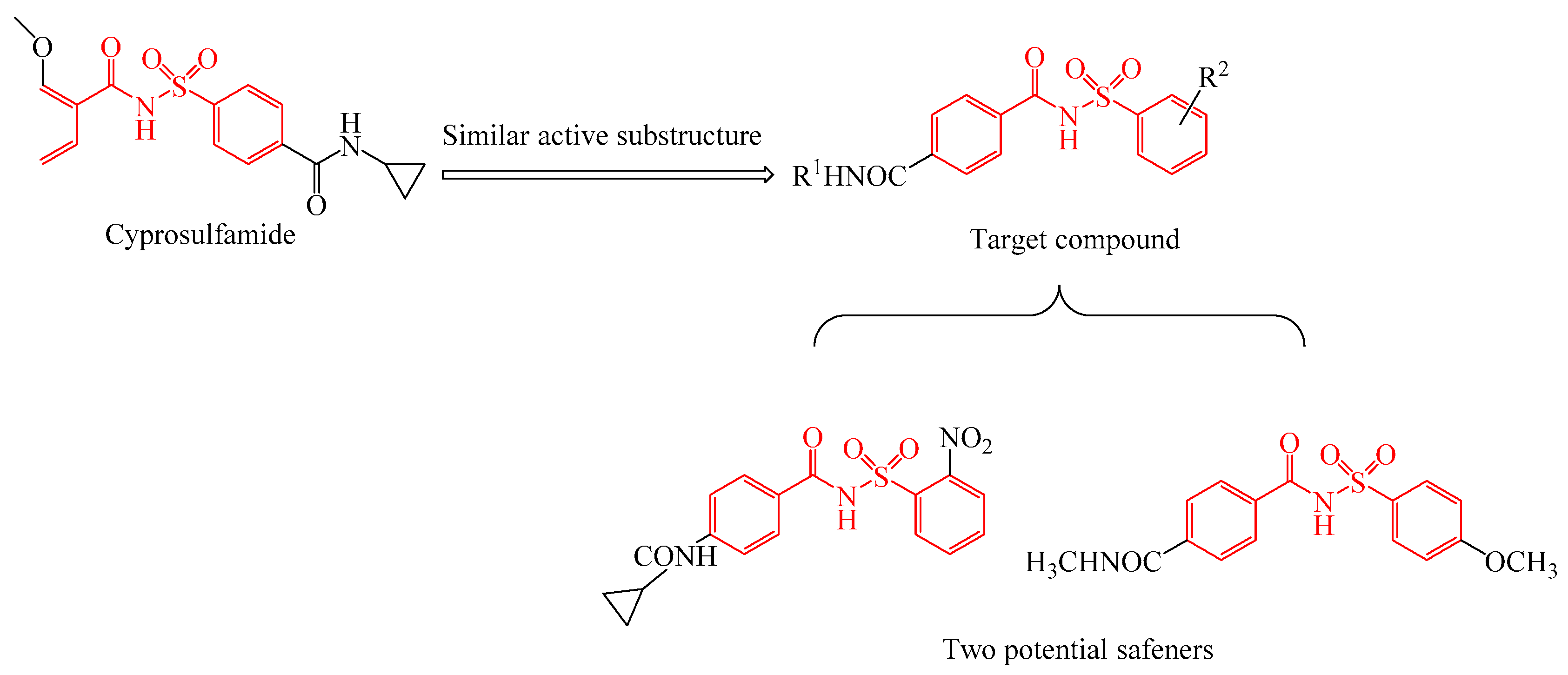
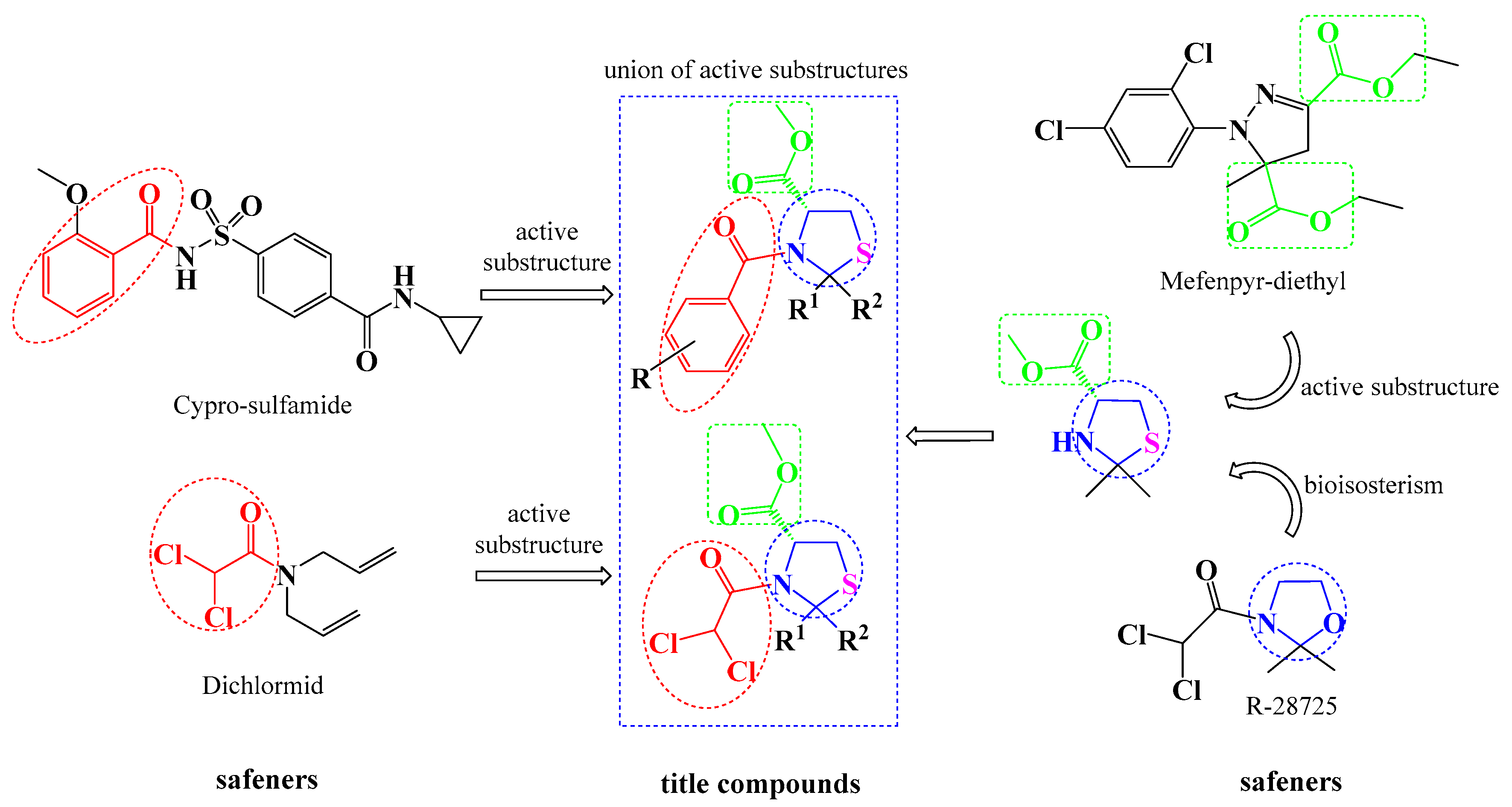
:1. Introduction
2. Results and Discussion
2.1. Chemistry
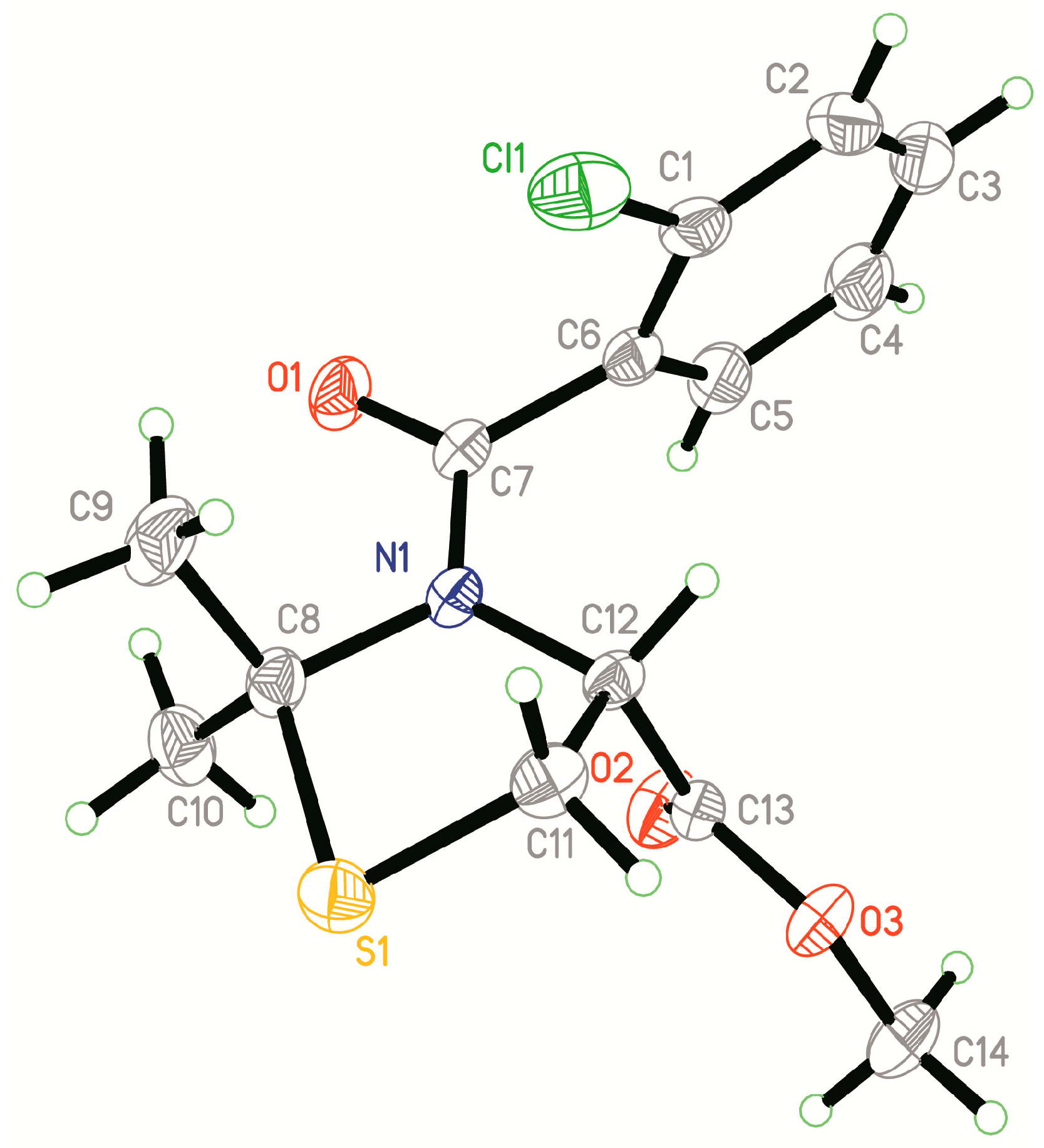
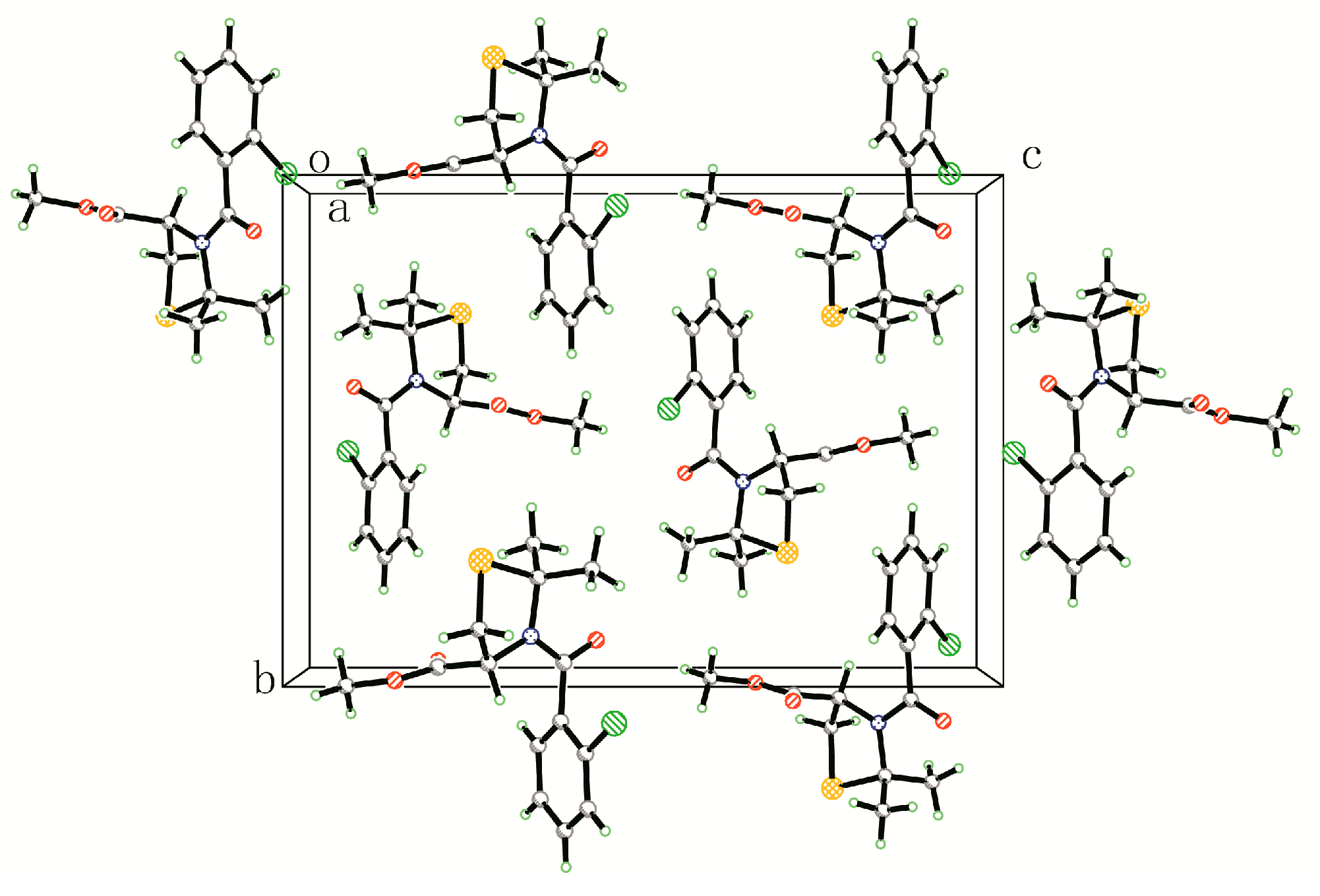
2.2. Crystal Structure of Compound 4q
2.3. Biological Activity Tests
2.4. Effect of Safeners on ALS Activity
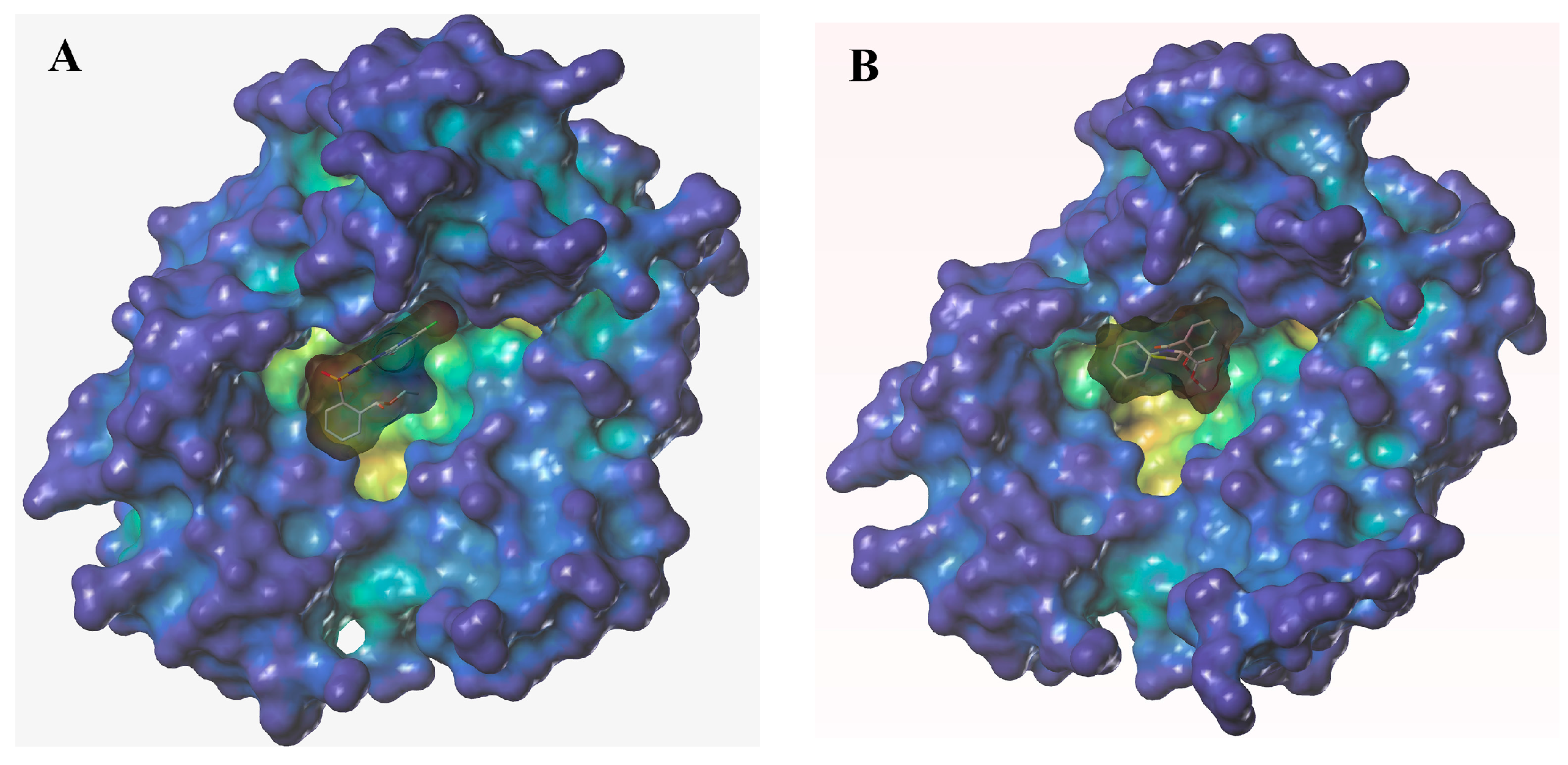
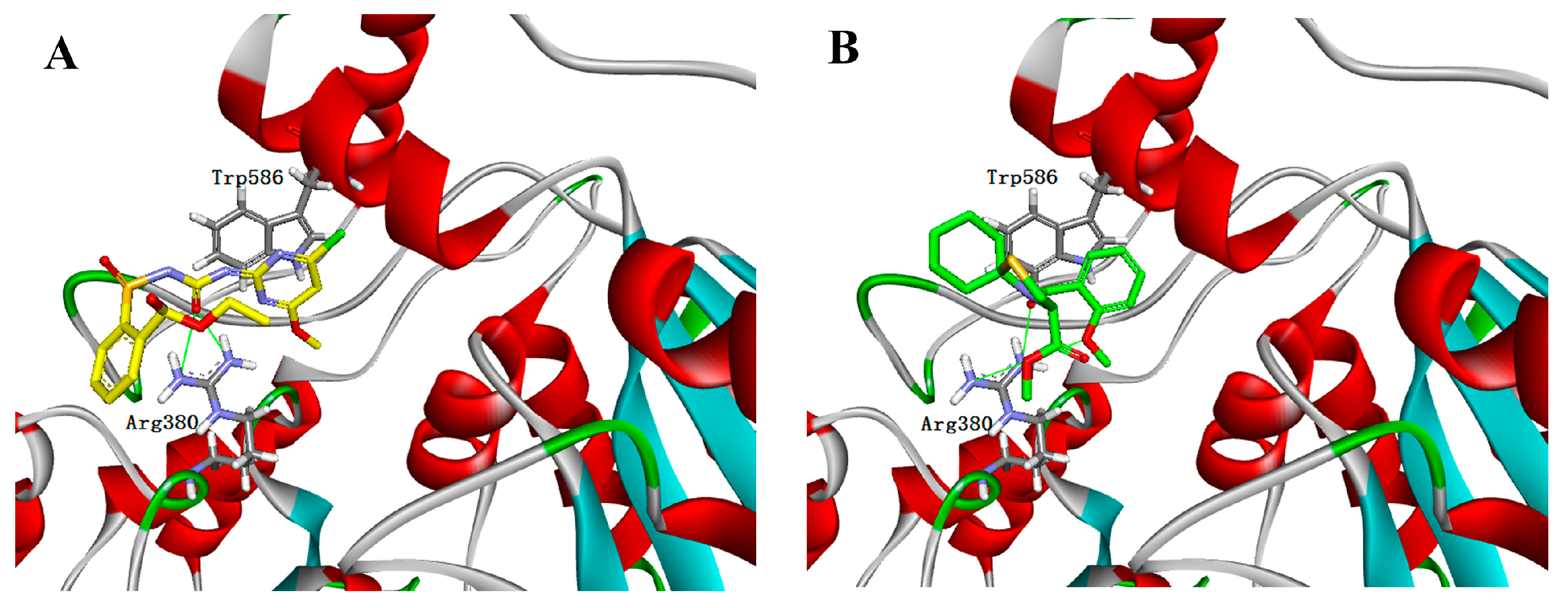
2.5. Molecular Docking Studies
3. Materials and Methods
3.1. Reagents and Analysis
3.2. General Procedure for the Preparation of 4
3.3. X-ray Diffraction
3.4. Biological Activity Texts
3.5. Determination of ALS Activity
3.6. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holmes, P.; Farquharson, R.; Hall, P.J.; Rolfe, B.G. Proteomic Analysis of Root Meristems and the Effects of Acetohydroxyacid Synthase-Inhibiting Herbicides in the Root of Medicago Truncatula. J. Proteome Res. 2006, 5, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yang, Q.; Zhang, Y.Z.; Jiao, H.T.; Mei, Y.; Li, X.F.; Zheng, M.Q. Cross-resistance patterns to acetolactate synthase (ALS)-inhibiting herbicides of flixweed (Descurainia sophia L.) conferred by different combinations of ALS isozymes with a Pro-197-Thr mutation or a novel Trp-574-Leu mutation. Pestic. Biochem. Physiol. 2017, 136, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Mazur, B.J.; Falco Car, S. The development of herbicide resistant crops. Annu. Rev. Plant Biol. 1989, 40, 441–470. [Google Scholar] [CrossRef]
- Li, C.Y.; Lv, T.Y.; Liu, W.J.; Zang, H.L.; Cheng, Y.; Li, D.P. Efficient degradation of chlorimuron-ethyl by a bacterial consortium and shifts in the aboriginal microorganism community during the bioremediation of contaminated-soil. Ecotoxicol. Environ. Saf. 2017, 139, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zhang, H.W.; Zhang, X.L.; Qin, S.H.; Tan, H.B.; Li, X.Y. Effects of long-term chlorimuron-ethyl application on the diversity and antifungal activity of soil Pseudomonas spp. in a soybean field in Northeast China. Ann. Microbiol. 2013, 63, 335–341. [Google Scholar] [CrossRef]
- Procopio, S.O.; Braz, A.J.B.P.; Barroso, A.L.L.; Cargnelutti, A.; Cruvinel, K.L.; Betta, M.; Braz, G.B.P.; Fraga, J.J.S.; Cunha, L.D. Potential Use of Chlorimuron-Ethyl, Imazethapyr and Cloransulam-Methyl in Common Bean Crop. Planta Daninha 2009, 27, 327–336. [Google Scholar] [CrossRef]
- Carvalho, S.J.P.; Soares, D.J.; Lopez-ovejero, R.F.; Christoffoleti, P.J. Soil persistence of chlorimuron-ethyl and metsulfuron-methyl and phytotxicity to corn seeded as a succeeding crop. Planta Daninha 2015, 33, 331–339. [Google Scholar] [CrossRef]
- Lindell, S.D. HPPD Herbicide-Safener Combinations as Resistance Breaking Solutions for 21st Century Agriculture; American Chemical Society: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Zhao, L.X.; Qu, H.T.; Fu, Y.; Gao, S.; Ye, F. Alleviation of injury from chlorimuron-ethyl in maize treated with safener 3-dichloroacetyl oxazolidine. Can. J. Plant Sci. 2015, 95, 897–903. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-Shady, A.M.; El-Nagar, M.M.F. Cyanobacterial Arthrospira (Spirulina platensis) as safener against harmful effects of fusilade herbicide on faba bean plant. Rend. Lincei 2016, 27, 455. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, J.Y.; Zhang, D.; Chen, Y.F.; Gao, S.; Zhao, L.X.; Ye, F. Solvent-Free Synthesis and Safener Activity of Sulfonylurea Benzothiazolines. Molecules 2017, 22, 1601. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, B.; Gou, Z.P.; Li, Y.; Zhang, X.; Wang, Y.Q.; Yu, S.J.; Li, Y.H.; Sun, D.Q. Design of novel CSA analogues as potential safeners and fungicides. Bioorg. Med. Chem. Lett. 2015, 25, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, W.G.; Hou, Y.W.; Wang, B.; Zhao, L.X.; Ye, F. One-pot Synthesis, Crystal structure, and Bioactivity of N-Phenoxyacetyl-2,4,5-trisubstituted-1,3-oxazolidines. J. Heterocycl. Chem. 2017, 54, 1660–1664. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [PubMed]
- Scott-Craig, J.S.; Casida, J.E.; Poduje, L.; Walton, J.D. Herbicide Safener-Binding Protein of Maize. Plant Physiol. 1998, 116, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R. Advances in Heterocyclic Chemistry; ACADEMIC PRESS: London, UK, 1982; pp. 75–77. ISBN 0-12-020630-7. [Google Scholar]
- Zhao, L.X.; Fu, Y.; Ye, F.; Gao, S. A mild and highly efficient synthesis of chiral N-dichloroacetyl-1,3-oxazolidines. J. Heterocycl. Chem. 2012, 49, 943–946. [Google Scholar] [CrossRef]
- Braga, A.L.; Milani, P.; Vargas, F.; Paixao, M.W.; Sehnem, J.A. Modular chiral thiazolidine catalysts in asymmetricaryl transfer reactions. Tetrahedron Asymmetry 2006, 17, 2793–2797. [Google Scholar] [CrossRef]
- Meng, Q.L.; Li, Y.L.; He, Y.; Guan, Y.D. Novel thiazolidine derivatives as chiral catalysts in the enantioselective addition of diethylzinc to aldehydes. Tetrahedron Asymmetry 2000, 11, 4255–4261. [Google Scholar] [CrossRef]
- Takata, T.; Tamura, Y.; Ando, W. New synthetic utility of singlet oxygen in sulphide photo-oxidation: Selective and stereospecific hydroxylation α to sulphur of 4-substituted 3-benzoyl-2,2-dimethylthiazolidines. Tetrahedron 1985, 41, 2133–2137. [Google Scholar] [CrossRef]
- Song, Z.C.; Ma, G.Y.; Zhu, H.L. Synthesis, Characterization and Antibacterial Activities of N-tert-Butoxycarbonyl-Thiazolidine Carboxylic Acid. RSC Adv. 2015, 5, 24824–24833. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Sugiyama, H. Selective action of pyrazosulfuron-ethyl on growth and acetolactate synthase activity between rice and Cyperus serotinus. Weed Res. 1991, 36, 251–256. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors’ lab. |

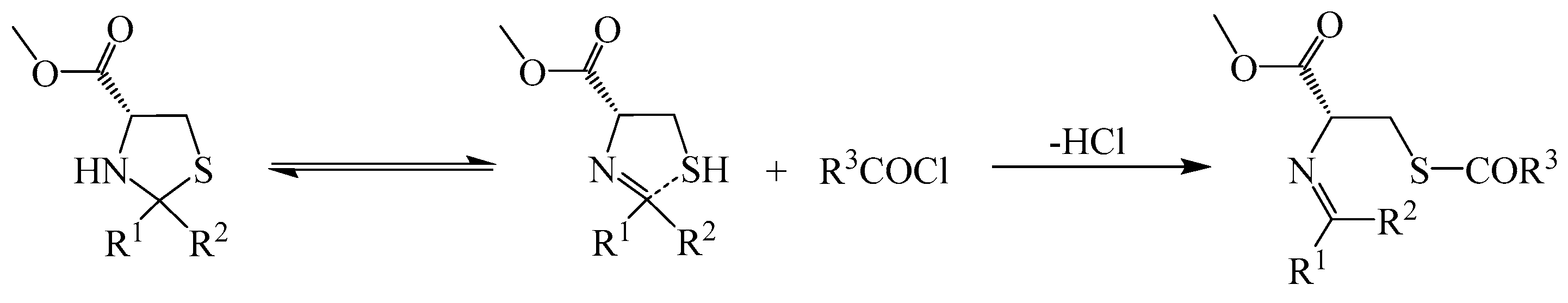
| Compound | R1 | R2 | R3 | Yield/% | Compound | R1 | R2 | R3 | Yield/% |
|---|---|---|---|---|---|---|---|---|---|
| 4a | (CH2)5 | p-NO2C6H4 | 91 | 4k | (CH2)4 | o-ClC6H4 | 70 | ||
| 4b | (CH2)5 | 2,4-Cl2C6H3 | 81 | 4l | CH3 | CH3 | 2,4-Cl2C6H3 | 78 | |
| 4c | (CH2)5 | p-ClC6H4 | 77 | 4m | CH3 | CH3 | p-ClC6H4 | 72 | |
| 4d | (CH2)5 | m-CH3C6H4 | 69 | 4n | CH3 | CH3 | p-NO2C6H4 | 87 | |
| 4e | (CH2)5 | o-OCH3C6H4 | 71 | 4o | CH3 | CH3 | m-CH3C6H4 | 65 | |
| 4f | (CH2)5 | C6H5 | 75 | 4p | CH3 | CH3 | o-OCH3C6H4 | 69 | |
| 4g | (CH2)4 | p-ClC6H4 | 67 | 4q | CH3 | CH3 | o-ClC6H4 | 71 | |
| 4h | (CH2)4 | p-NO2C6H4 | 83 | 4r | (CH2)5 | CHCl2 | 48 | ||
| 4i | (CH2)4 | 2,4-Cl2C6H3 | 72 | 4s | CH3 | CH3 | CHCl2 | 41 | |
| 4j | (CH2)4 | m-CH3C6H4 | 65 | ||||||
| Compound | Recovery of Plant Height (%) | Recovery of Root Length (%) | Recovery of Plant Weight (%) | Recovery of Root Weight (%) |
|---|---|---|---|---|
| R-28725 | 86.22 ± 1.37 | 84.73 ± 0.98 | 87.47 ± 1.12 | 83.61 ± 1.98 |
| 4a | 42.90 ± 1.22 | 60.52 ± 1.37 | 45.96 ± 1.11 | 68.11 ± 1.82 |
| 4b | 59.96 ± 1.09 | 45.53 ± 1.27 | 47.16 ± 1.24 | 68.48 ± 0.63 |
| 4c | 80.33 ± 0.82 | 86.48 ± 0.87 | 94.61 ± 1.43 | 85.79 ± 1.62 |
| 4d | 67.97 ± 1.02 | 41.97 ± 1.31 | 78.25 ± 0.98 | 41.98 ± 1.05 |
| 4e | 89.08 ± 0.65 | 91.20 ± 0.89 | 98.92 ± 1.09 | 93.70 ± 1.22 |
| 4f | 62.05 ± 0.21 | 64.07 ± 0.33 | 73.75 ± 1.39 | 51.56 ± 0.52 |
| 4g | 85.86 ± 0.97 | 87.53 ± 1.43 | 85.62 ± 1.26 | 83.39 ± 0.92 |
| 4h | 57.08 ± 0.69 | 46.83 ± 1.07 | 41.26 ± 0.73 | 59.74 ± 0.62 |
| 4i | 45.64 ± 1.62 | 55.60 ± 0.55 | 53.93 ± 1.29 | 57.56 ± 0.54 |
| 4j | 57.41 ± 1.26 | 66.61 ± 1.92 | 71.15 ± 1.42 | 67.59 ± 1.65 |
| 4k | 41.82 ± 0.68 | 46.24 ± 0.23 | 55.83 ± 1.05 | 55.13 ± 0.13 |
| 4l | 20.60 ± 0.69 | 15.09 ± 0.32 | 15.04 ± 1.22 | 25.27 ± 1.24 |
| 4m | 48.99 ± 0.57 | 66.04 ± 1.13 | 48.27 ± 1.06 | 47.83 ± 0.36 |
| 4n | 25.02 ± 0.92 | 29.16 ± 0.91 | 17.01 ± 0.61 | 24.94 ± 0.85 |
| 4o | 34.12 ± 1.32 | 38.30 ± 0.45 | 39.58 ± 1.02 | 56.35 ± 1.13 |
| 4p | 76.00 ± 1.16 | 61.09 ± 1.75 | 68.93 ± 1.26 | 55.57 ± 1.44 |
| 4q | 23.20 ± 0.53 | 14.56 ± 0.32 | 19.04 ± 1.42 | 25.25 ± 0.54 |
| 4r | 57.74 ± 1.31 | 39.46 ± 1.44 | 31.46 ± 0.88 | 46.34 ± 1.32 |
| 4s | 33.22 ± 1.65 | 36.30 ± 1.44 | 45.75 ± 1.54 | 37.89 ± 0.74 |
| Treatment | ALS Activity (nmol h−1 mg−1 Protein) a | Treatment | ALS Activity (nmol h−1 mg−1 Protein) a | Treatment | ALS Activity (nmol h−1 mg−1 Protein) a |
|---|---|---|---|---|---|
| Control b | 0.091 ± 0.002 | chlorimuron-ethyl + 4f | 0.075 ± 0.003 | chlorimuron-ethyl + 4n | 0.059 ± 0.003 |
| chlorimuron-ethyl | 0.046 ± 0.003 | chlorimuron-ethyl + 4g | 0.079 ± 0.003 | chlorimuron-ethyl + 4o | 0.064 ± 0.001 |
| chlorimuron-ethyl + R-28725 | 0.084 ± 0.002 | chlorimuron-ethyl + 4h | 0.071 ± 0.002 | chlorimuron-ethyl + 4p | 0.071 ± 0.001 |
| chlorimuron-ethyl + 4a | 0.064 ± 0.001 | chlorimuron-ethyl + 4i | 0.068 ± 0.002 | chlorimuron-ethyl + 4q | 0.055 ± 0.002 |
| chlorimuron-ethyl + 4b | 0.062 ± 0.004 | chlorimuron-ethyl + 4j | 0.077 ± 0.002 | chlorimuron-ethyl + 4r | 0.064 ± 0.002 |
| chlorimuron-ethyl + 4c | 0.081 ± 0.002 | chlorimuron-ethyl + 4k | 0.068 ± 0.001 | chlorimuron-ethyl + 4s | 0.062 ± 0.001 |
| chlorimuron-ethyl + 4d | 0.065 ± 0.002 | chlorimuron-ethyl + 4l | 0.058 ± 0.001 | ||
| chlorimuron-ethyl + 4e | 0.085 ± 0.002 | chlorimuron-ethyl + 4m | 0.068 ± 0.003 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.-X.; Wu, H.; Zou, Y.-L.; Wang, Q.-R.; Fu, Y.; Li, C.-Y.; Ye, F. Design, Synthesis, and Safener Activity of Novel Methyl (R)-N-Benzoyl/Dichloroacetyl-Thiazolidine-4-Carboxylates. Molecules 2018, 23, 155. https://doi.org/10.3390/molecules23010155
Zhao L-X, Wu H, Zou Y-L, Wang Q-R, Fu Y, Li C-Y, Ye F. Design, Synthesis, and Safener Activity of Novel Methyl (R)-N-Benzoyl/Dichloroacetyl-Thiazolidine-4-Carboxylates. Molecules. 2018; 23(1):155. https://doi.org/10.3390/molecules23010155
Chicago/Turabian StyleZhao, Li-Xia, Hao Wu, Yue-Li Zou, Qing-Rui Wang, Ying Fu, Chun-Yan Li, and Fei Ye. 2018. "Design, Synthesis, and Safener Activity of Novel Methyl (R)-N-Benzoyl/Dichloroacetyl-Thiazolidine-4-Carboxylates" Molecules 23, no. 1: 155. https://doi.org/10.3390/molecules23010155





