Synthesis and Antimicrobial Activity of Sulfur Derivatives of Quinolinium Salts
Abstract
:1. Introduction
2. Results and Discussion
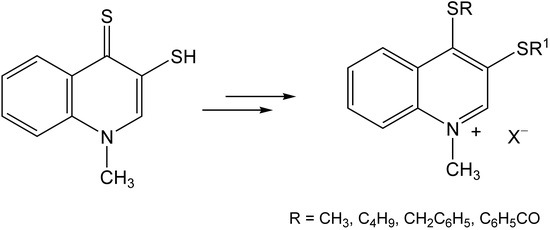
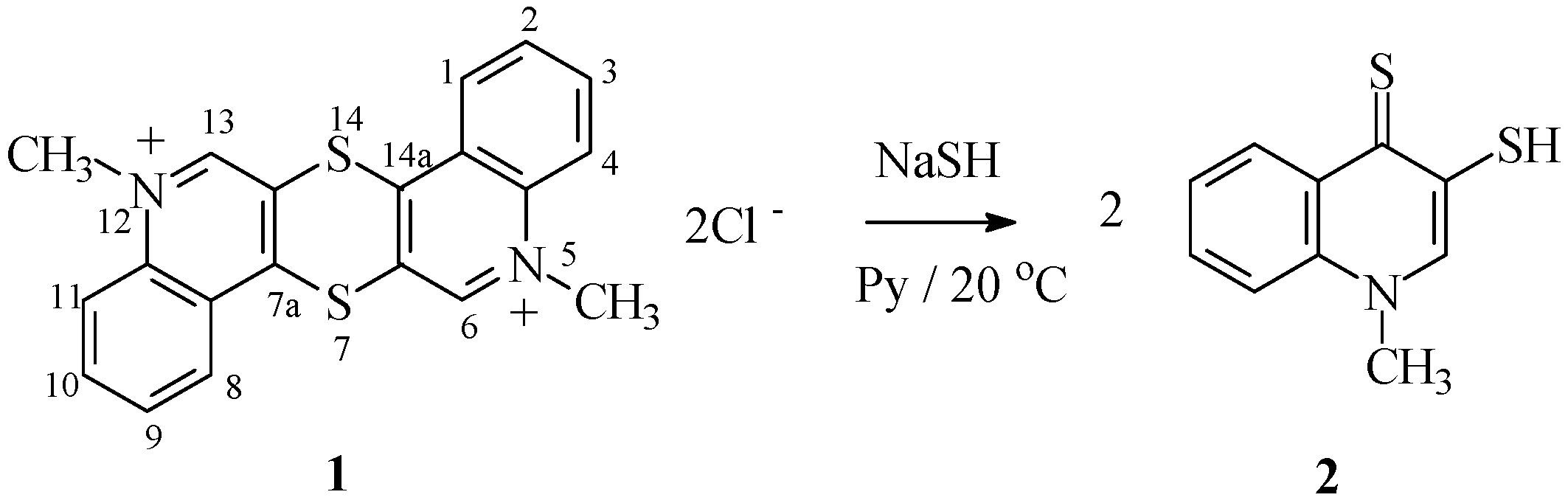
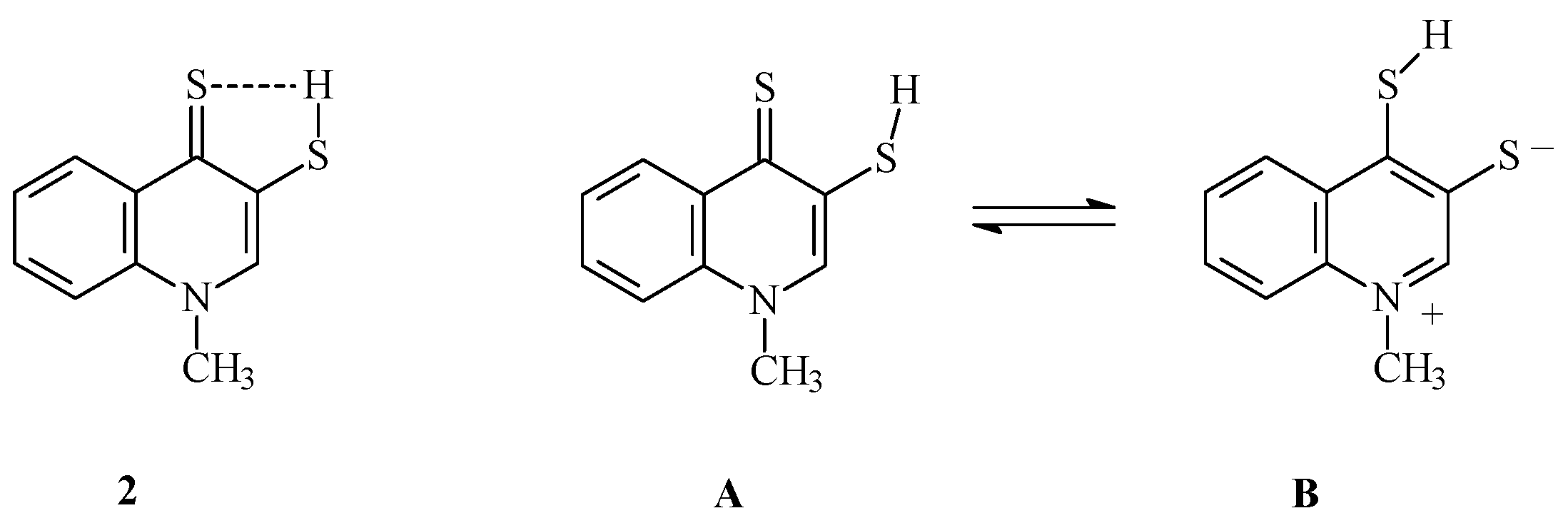
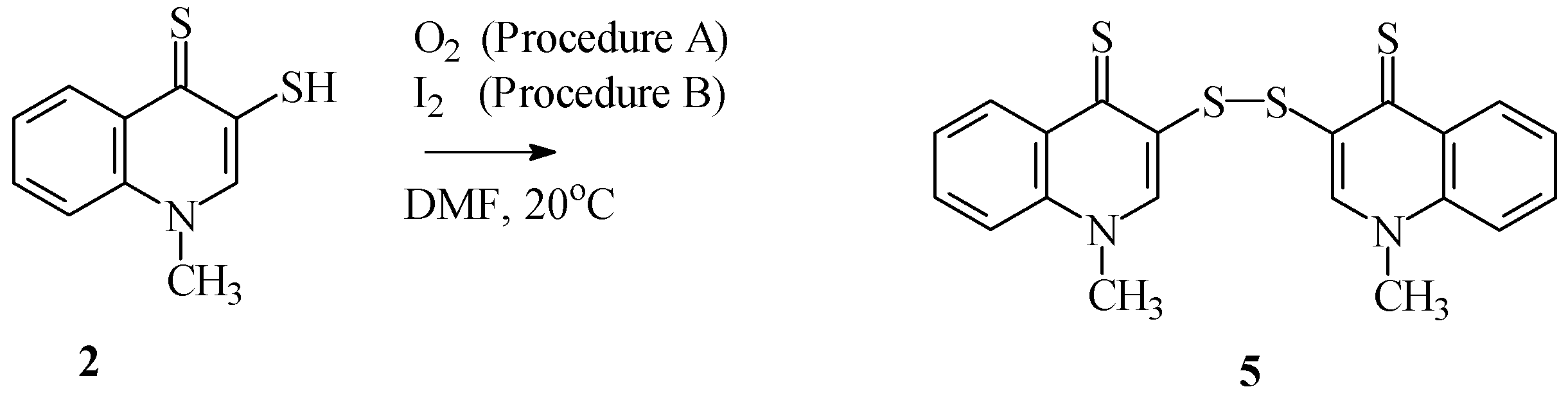
2.1. Chemistry
2.2. Biological Activity
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 1-Methyl-3-(mercapto)quinoline-4(1H)-thione 2
3.1.2. General Procedure for Synthesis of 1-Methyl-3-thioquinoline-4(1H)-thione Derivatives 3
3.1.3. General Procedure for Synthesis of 1-Methyl-3-thio-4-thioquinolinium Derivatives Salts (4)
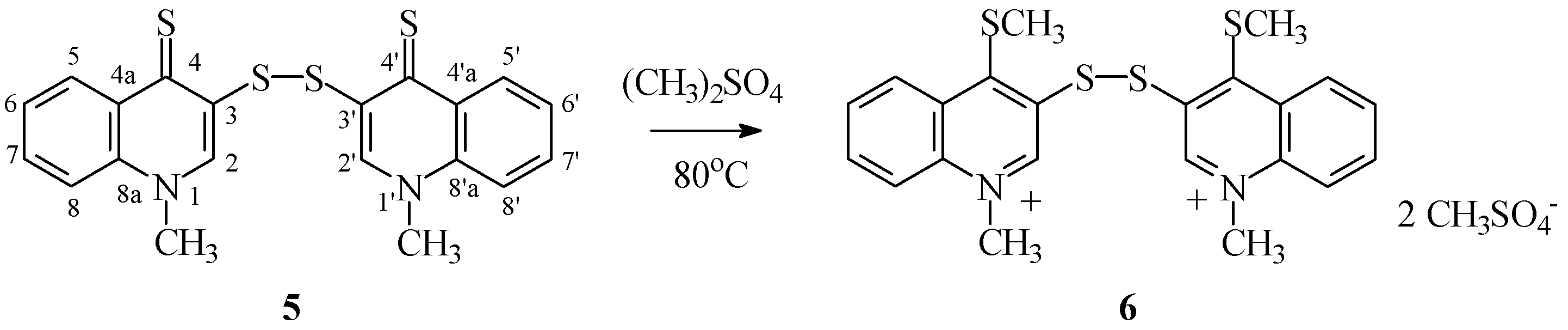
3.1.4. Synthesis of Di(1-methylquinoline-4(1H)-thione-3-yl) Disulfide (5)
3.1.5. Synthesis of Bis-Methyl Sulfate di(1-methyl-4-(methylthio)quinolinium-3-yl) Disulfide 6
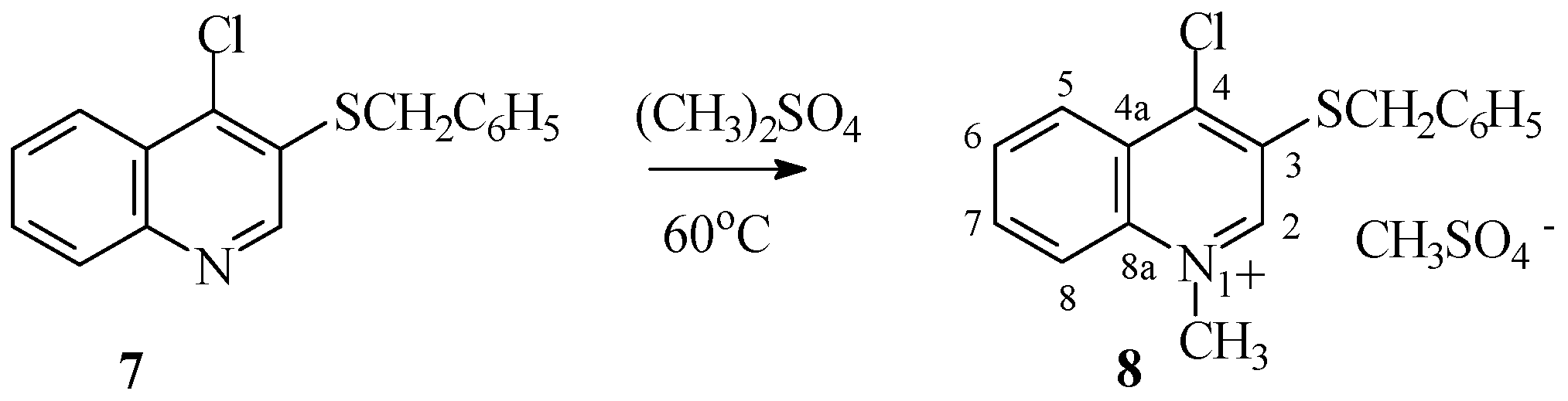
3.1.6. Synthesis of 1-Methyl-4-chloro-3-(benzylthio)quinolinium Methyl Sulfate (8)
3.2. Biological Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Emori, T.G.; Culver, D.H.; Horan, T.C.; Jarvis, W.R.; White, J.W.; Olson, D.R.; Banerjee, S.; Edwards, J.R.; Martone, W.J.; Gaynes, R.P.; et al. National Nosocomial Infections Surveillance System (NNIS): Description of Surveillance Methods. Am. J. Infect. Control. 2004, 32, 470–485. [Google Scholar] [CrossRef]
- CDC. Staphylococcus aureus resistant to vancomycin—United States. Morb. Mortal. Wkly. Rep. 2002, 51, 565–567. [Google Scholar]
- Kumar, S.; Bawa, S.; Gupta, H. Biological Activities of Quinoline Derivatives. Mini-Rev. Med. Chem. 2009, 9, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.N.; Remers, W.A. (Eds.) Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 10th ed.; Lippincott-Raven Publishers: Philadelphia, Pennsylvania, 1998; pp. 235–252. ISBN 978-0-397-51583-7. [Google Scholar]
- Bringmann, G.; Reichert, Y.; Kane, V. The total synthesis of streptonigrin and related antitumor antibiotic natural products. Tetrahedron 2004, 60, 3539–3574. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.I.; Sim, G.A. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminate. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Sharma, P.C.; Jain, A.; Jain, S. Fluoroquinolone antibacterials: A review on chemistry, microbiology and therapeutic prospects. Acta Poloniae Pharm. Drug Res. 2009, 66, 587–604. [Google Scholar]
- Patrick, G.L. Antibacterial agents. In An Introduction to Medicinal Chemistry, 2nd ed.; Oxford University Press: Oxford, UK, 2003; pp. 379–435. ISBN 978-0-19-969739-7. [Google Scholar]
- Vittorio, C.; Guyen, B.; Opoku-Boahen, Y.; Mann, J.; Gowan, S.M.; Lloyd, M.; Martin, A.R.; Stephen, N. A novel inhibitor of human telomerase derived from 10H-indolo[3,2-b]quinoline. Bioorg. Med. Chem. Lett. 2000, 10, 2063–2066. [Google Scholar] [CrossRef]
- Mikata, Y.; Mika, Y.; Shun-Ichiro, O.; Ichiro, O.; Kawasaki, M.; Maeda, M.; Shigenobu, Y. Effect of side chain location in (2-aminoethyl)aminomethyl-2-phenylquinolines as antitumor agents. Bioorg. Med. Chem. Lett. 1998, 8, 1243–1248. [Google Scholar] [CrossRef]
- Sharples, D.; Spengler, C.; Molnar, J.; Zsuzsanna, A.; Molnar, A.; Kiss, J.T.; Szabo, J.A.; Hilgeroth, A.; Gallo, S.; Mohamoud, A.; et al. The interaction between resistance modifiers such as pyrido[3,2-g]quinoline, aza-oxafluorene and pregnane derivatives with DNA, plasmid DNA and tRNA. Eur. J. Med. Chem. 2005, 40, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, P.; Dinakaran, M.; Yogeeswari, P.; Sriram, D.; China, A.; Nagaraja, V. Synthesis and antimycobacterial activities of novel 6-nitroquinolone-3-carboxylic acids. Eur. J. Med. Chem. 2009, 44, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, A.; Monga, V.; Malde, A.K.; Coutinho, E.; Jain, R. Synthesis, anti-tuberculosis activity, and 3D-QSAR study of 4-(adamantan-1-yl)-2-substituted quinolones. Bioorg. Med. Chem. 2007, 15, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Sadana, A.K.; Mirza, Y.; Om, P. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo[4,3-a] pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo[4,3-a]quinolines as antibacterial agents. Eur. J. Med. Chem. 2003, 38, 533–536. [Google Scholar] [CrossRef]
- Narender, P.; Srinivas, U.; Ravinder, M.; Rao, B.A.; Ramesh, C.; Harakishore, K.; Gangadasu, B.; Murthy, U.S.N.; Rao, V.J. Synthesis of multisubstituted quinolines from Baylis-Hillman adducts obtained from substituted 2-chloronicotinaldehydes and their antimicrobial activity. Bioorg. Med. Chem. 2006, 14, 4600–4609. [Google Scholar] [CrossRef] [PubMed]
- Murugananthamn, N.; Sivakumar, R.; Anbalagan, N.; Gunasekaran, V.; Leonard, J.T. Synthesis, anticonvulsant and antihypertensive activities of 8-substituted quinoline derivatives. Biol. Pharm. Bull. 2004, 27, 1683–1687. [Google Scholar] [CrossRef]
- Calhoun, W.; Carlson, R.P.; Crossley, R.; Datko, L.J.; Dietrich, S.; Healtheringtoj, K.; Marshall, L.A.; Meade, P.J.; Opalko, A.; Shephard, R.G. Synthesis and antiinflammatory activity of certain 5,6,7,8-tetrahydroquinolines and related compounds. J. Med. Chem. 1995, 38, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Kohno, Y.; Awano, K.; Miyashita, M.; Fujimori, S.; Kuriyama, K.; Sakoe, Y.; Kudoh, S.; Saito, K.; Kojima, E. Synthesis and antirheumatic activity of novel tetrahydroquinoline-8-carboxylic acid derivatives. Bioorg. Med. Chem. Lett. 1997, 7, 1515–1518. [Google Scholar] [CrossRef]
- McCall, J.M.; Brink, R.E.; Kamdar, B.V.; Ska-Letzky, L.L.; Perricone, S.C.; Piper, R.C.; Delehonty, P.J. 7-(Trifluoromethyl)-4-aminoquinoline hypotensives: Novel peripheral sympatholytics. J. Med. Chem. 1986, 29, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Sircar, I.; Haleen, S.J.; Burke, S.E.; Barth, H. Synthesis and biological activity of 4-(diphenylmethyl)-[(4-quinolinyloxy-)methyl]-1-piperazineethanol and related compounds. J. Med. Chem. 1992, 35, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Zięba, A.; Wojtyczka, R.D.; Kępa, M.; Idzik, D. Antimicrobial activity of novel 1-methyl-3-thio-4-aminoquinolinium salts. Folia Microbiol. 2010, 55, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zięba, A.; Wojtyczka, R.D.; Kępa, M.; Idzik, D. Synthesis and in vitro antimicrobial activity of 1-methyl-3-sulfothio-4-aminoquinolinium chlorides. Acta Poloniae Pharm. Drug Res. 2013, 70, 163–166. [Google Scholar]
- Wojtyczka, R.D.; Zięba, A.; Dziedzic, A.; Kępa, M.; Idzik, D. An activity of thioacyl derivatives of 4-aminoquinolinium salts towards biofilm producing and planktonic forms of coagulase-negative staphylococci. BioMed Res. Int. 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hagrs, M.; Bayoumi, H.A.; El-Gamal, K.M.; Mayhoub, A.S.; Hamada, S.; Abulkhair, H.S. Synthesis and preliminary antimicrobial evaluation of some new 6-methoxyquinoline-3-carbonitrile derivatives. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 338–345. [Google Scholar] [CrossRef]
- Maślankiewicz, A.; Zięba, A. 1-Alkyl-3,4-di(alkylthio)quinolinium salts. Pol. J. Chem. 1994, 68, 1957–1971. [Google Scholar]
- Zięba, A. 3-Acylsulfanyl-1-methyl-4-methylsulfanyl-quinolinium salts and their transformation into 4-aminoquinolinium-3-thiolates and azaphenothiazine derivatives. Pol. J. Chem. 2008, 82, 1399–1402. [Google Scholar]
- Maślankiewicz, A.; Zięba, A. 5,12-Di-(1-alkyl)thioquinanthrenediinium bis-salts and 1-alkyl-3-alkylthio-1,4-dihydro-4-thiooxoquinolines. Heterocycles 1992, 34, 247–258. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure determination of organic compounds. In Tables of Spectral Data, 4th ed.; Revised and Enlarged Edition; Springer: Berlin/Heidelberg, Germany, 2009; p. 127. ISBN 978-3-540-93810-1. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentration (MIC) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar]
Sample Availability: Samples of the compounds described herein are available from the authors. |

| 4a | 4b | 4c | 4d | 4e | 4f | 6 | 8 | CIP | |
|---|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | 32 | 32 | 16 | 0.5 | 1 | 1 | 16 | 64 | 0.25 |
| S. aureus ATCC 43300 | 128 | 128 | 16 | 1 | 32 | 4 | 32 | 32 | 0.5 |
| S. epidermidis ATCC 12228 | 32 | 128 | 32 | 0.5 | 2 | 1 | 32 | 128 | 0.25 |
| E. faecalis ATCC 29212 | 128 | 128 | 32 | 0.5 | 1 | 1 | 32 | 32 | 0.5 |
| M. luteus ATCC 10240 | 64 | 128 | 32 | 0.5 | 1 | 0.5 | 64 | 16 | 1 |
| B. subtilis ATCC 11774 | 64 | 128 | 32 | 0.5 | 4 | 0.5 | 16 | 256 | 2 |
| E. coli ATCC 11776 | 64 | 128 | 128 | 16 | 64 | 32 | 32 | 128 | 0.016 |
| K. pneumoniae ATCC 27736 | 64 | 128 | 128 | 32 | 128 | 64 | 256 | 256 | 0.5 |
| P. mirabilis ATCC 7002 | 128 | 128 | 128 | 8 | 128 | 32 | 128 | 128 | 0.25 |
| P. aeruginosa ATCC 27853 | 256 | 256 | 128 | 64 | 512 | 256 | 256 | 256 | 0.5 |
| A. baumannii ATCC 19606 | 128 | 128 | 64 | 8 | 16 | 8 | 16 | 256 | 0.25 |
| S. marcescens ATCC 8100 | 64 | 64 | 128 | 16 | 64 | 8 | 64 | 128 | 1 |
| C. albicans ATCC 60193 | 64 | 64 | 64 | 1 | 16 | 1 | 16 | 128 | nt |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Empel, A.; Kisiel, E.; Wojtyczka, R.D.; Kępa, M.; Idzik, D.; Sochanik, A.; Wąsik, T.J.; Zięba, A. Synthesis and Antimicrobial Activity of Sulfur Derivatives of Quinolinium Salts. Molecules 2018, 23, 218. https://doi.org/10.3390/molecules23010218
Empel A, Kisiel E, Wojtyczka RD, Kępa M, Idzik D, Sochanik A, Wąsik TJ, Zięba A. Synthesis and Antimicrobial Activity of Sulfur Derivatives of Quinolinium Salts. Molecules. 2018; 23(1):218. https://doi.org/10.3390/molecules23010218
Chicago/Turabian StyleEmpel, Anna, Ewa Kisiel, Robert D. Wojtyczka, Małgorzata Kępa, Danuta Idzik, Aleksander Sochanik, Tomasz J. Wąsik, and Andrzej Zięba. 2018. "Synthesis and Antimicrobial Activity of Sulfur Derivatives of Quinolinium Salts" Molecules 23, no. 1: 218. https://doi.org/10.3390/molecules23010218