HnRNPA1 Specifically Recognizes the Base of Nucleotide at the Loop of RNA G-Quadruplex
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, Y.; Kaminaga, K.; Komiyama, M. G-Quadruplex Formation by Human Telomeric Repeats-Containing RNA in Na+ Solution. J. Am. Chem. Soc. 2008, 130, 11179–11184. [Google Scholar] [CrossRef] [PubMed]
- Martadinata, H.; Phan, A.T. Structure of Propeller-Type Parallel-Stranded RNA G-Quadruplexes, Formed by Human Telomeric RNA Sequences in K+ Solution. J. Am. Chem. Soc. 2009, 131, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Suzuki, Y.; Ito, K.; Komiyama, M. Telomeric Repeat-Containing RNA Structure in Living Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 14579–14584. [Google Scholar] [CrossRef] [PubMed]
- Collie, G.W.; Parkinson, G.N.; Neidle, S.; Rosu, F.; De Pauw, E.; Gabelica, V. Electrospray Mass Spectrometry of Telomeric RNA (TERRA) Reveals the Formation of Stable Multimeric G-Quadruplex Structures. J. Am. Chem. Soc. 2010, 132, 9328–9334. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. Chemistry in Human Telomere Biology: Structure, Function and Targeting of Telomere DNA/RNA. Chem. Soc. Rev. 2011, 40, 2719–2740. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ishizuka, T.; Kimura, T.; Komiyama, M.A. U-Tetrad Stabilizes Human Telomeric RNA G-Quadruplex Structure. J. Am. Chem. Soc. 2010, 132, 7231–7233. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.D.; Ishizuka, T.; Zhu, X.Q.; Li, Y.; Sugiyama, H.; Xu, Y. Unusual Topological RNA Architecture with an Eight-Stranded Helical Fragment Containing A-, G-, and U-Tetrads. J. Am. Chem. Soc. 2017, 139, 2565–2568. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.D.; Ishizuka, T.; Xu, Y. Antiparallel RNA G-quadruplex Formed by Human Telomere RNA Containing 8-Bromoguanosine. Sci. Rep. 2017, 7, 6695. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.L.; Ishizuka, T.; Sakamoto, T.; Fujimoto, K.; Uechi, T.; Kenmochi, N.; Xu, Y. Characterization of Human Telomere RNA G-Quadruplex Structures in vitro and in Living Cells using 19F NMR Spectroscopy. Nucleic Acids Res. 2017, 45, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.L.; Ishizuka, T.; Iwanami, A.; Oyoshi, T.; Xu, Y. A Simple and Sensitive 19F NMR Approach for Studying the Interaction of RNA G-Quadruplex with Ligand Molecule and Protein. ChemistrySelect 2017, 2, 4170–4175. [Google Scholar] [CrossRef]
- Xu, Y.; Ishizuka, T.; Yang, J.; Ito, K.; Katada, H.; Komiyama, M.; Hayashi, T. Oligonucleotide Models of Telomeric DNA and RNA Form a Hybrid G-quadruplex Structure as a Potential Component of Telomeres. J. Biol. Chem. 2012, 287, 41787–41796. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.L.; Centore, R.C.; O’Sullivan, R.J.; Rai, R.; Tse, A.; Songyang, Z.; Chang, S.; Karlseder, J.; Zou, L. TERRA and HnRNPA1 Orchestrate an RPA-to-POT1 Switch on Telomeric Single-Stranded DNA. Nature 2011, 471, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ishizuka, T.; Bao, H.L.; Wada, K.; Takeda, Y.; Iida, K.; Nagasawa, K.; Yang, D.; Xu, Y. Structure-Dependent Binding of hnRNP A1 to Telomere RNA. J. Am. Chem. Soc. 2017, 139, 7533–7539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wu, L.; Ren, J.; Xu, Y.; Qu, X. Targeting Human Telomeric Higher-Order DNA: Dimeric G-quadruplex Units Serve as Preferred Binding Site. J. Am. Chem. Soc. 2013, 135, 18786–18789. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Tateishi-Karimata, H.; Largy, E.; Korkut, D.N.; Bourdoncle, A.; Miyoshi, D.; Zhang, J.; Ju, H.; Wang, W.; Sugimoto, N.; et al. Unexpected Position-Dependent Effects of Ribose G-Quartets in G-Quadruplexes. J. Am. Chem. Soc. 2017, 139, 7768–7779. [Google Scholar]
- Xu, Y.; Noguchi, Y.; Sugiyama, H. The New Models of the Human Telomere d[AGGG(TTAGGG)3] in K+ Solution. Bioorg. Med. Chem. 2006, 14, 5584–5591. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sugiyama, H. Highly Efficient Photochemical 2'-Deoxyribonolactone Formation at the Diagonal Loop of a 5-Iodouracil-Containing Antiparallel G-Quartet. J. Am. Chem. Soc. 2004, 126, 6274–6279. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. Structure Function and Targeting of Human Telomere RNA. Methods 2012, 57, 100–105. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

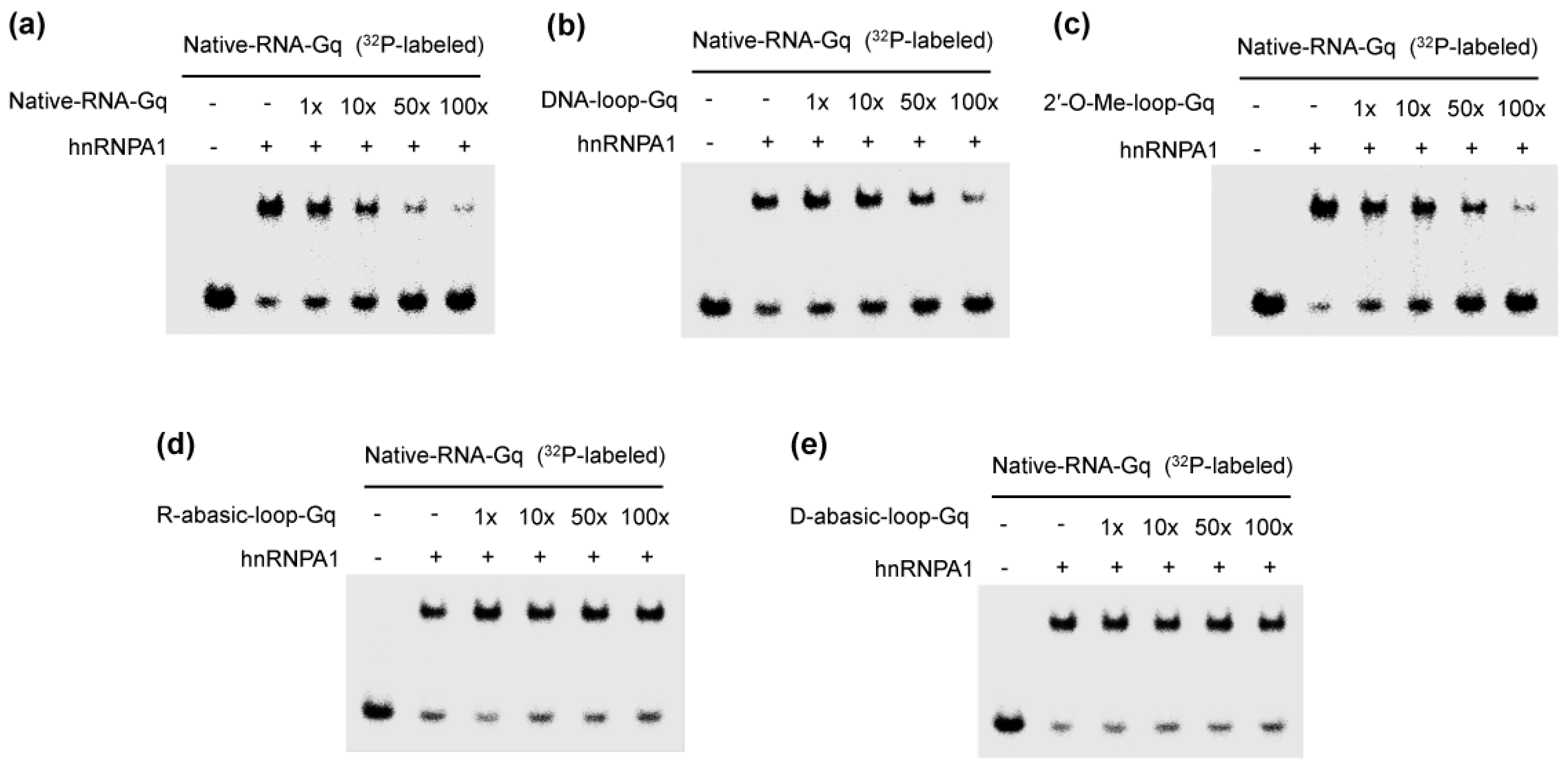
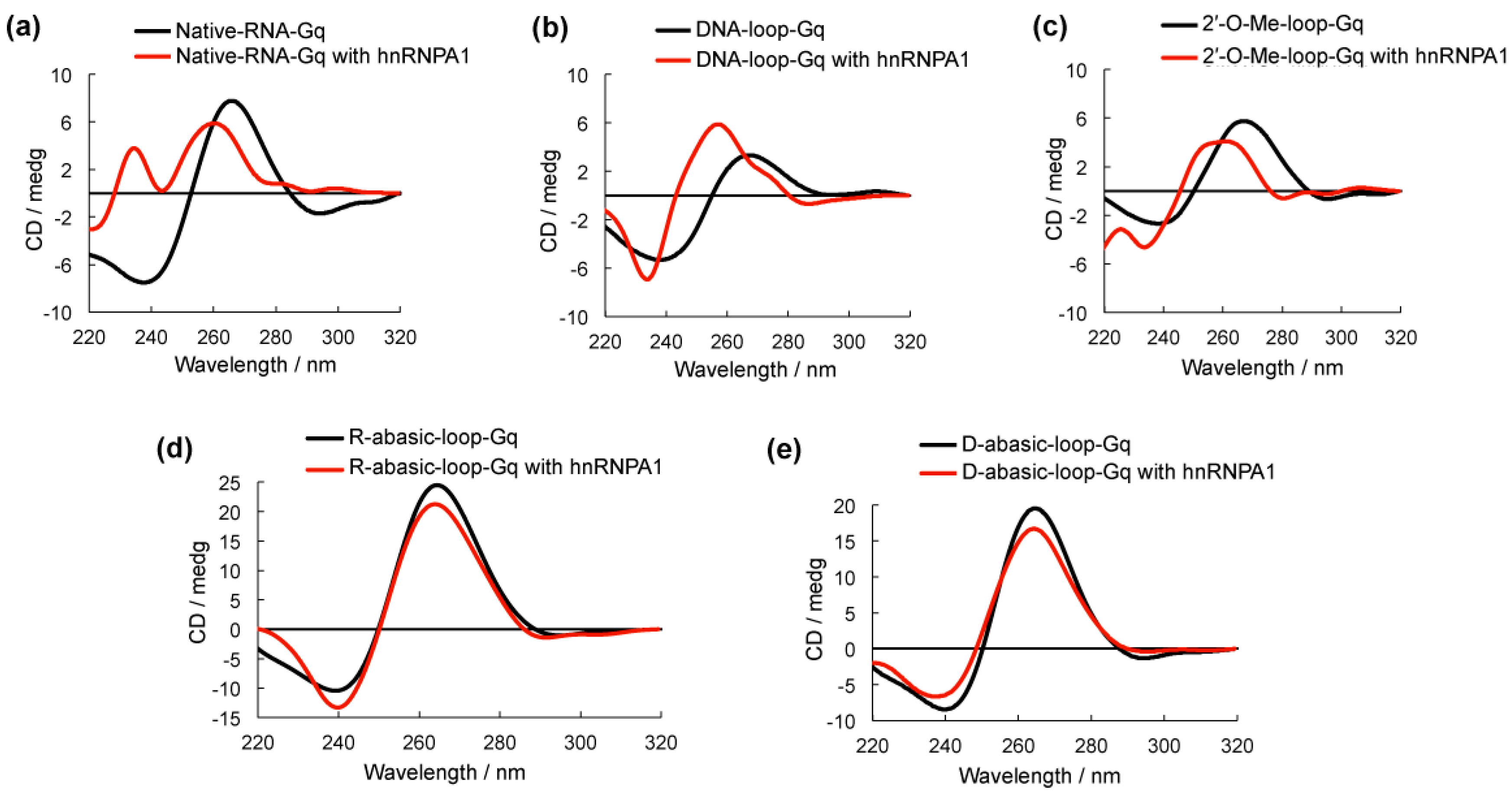
| Name | Sequence |
|---|---|
| Native-RNA-Gq | UUAGGG UUAGGG |
| DNA-loop-Gq | r(UUAGGG) d(TTA)r(GGG) |
| R-abasic-loop-Gq | r(UUAGGG)RRRr(GGG) |
| D-abasic-loop-Gq | r(UUAGGG)DDDr(GGG) |
| 2′-O-Me-loop-Gq | r(UUAGGG)UmUmAmr(GGG) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xu, Y. HnRNPA1 Specifically Recognizes the Base of Nucleotide at the Loop of RNA G-Quadruplex. Molecules 2018, 23, 237. https://doi.org/10.3390/molecules23010237
Liu X, Xu Y. HnRNPA1 Specifically Recognizes the Base of Nucleotide at the Loop of RNA G-Quadruplex. Molecules. 2018; 23(1):237. https://doi.org/10.3390/molecules23010237
Chicago/Turabian StyleLiu, Xiao, and Yan Xu. 2018. "HnRNPA1 Specifically Recognizes the Base of Nucleotide at the Loop of RNA G-Quadruplex" Molecules 23, no. 1: 237. https://doi.org/10.3390/molecules23010237





