Amine-Functionalized Sugarcane Bagasse: A Renewable Catalyst for Efficient Continuous Flow Knoevenagel Condensation Reaction at Room Temperature
Abstract
:1. Introduction
2. Results and Discussion
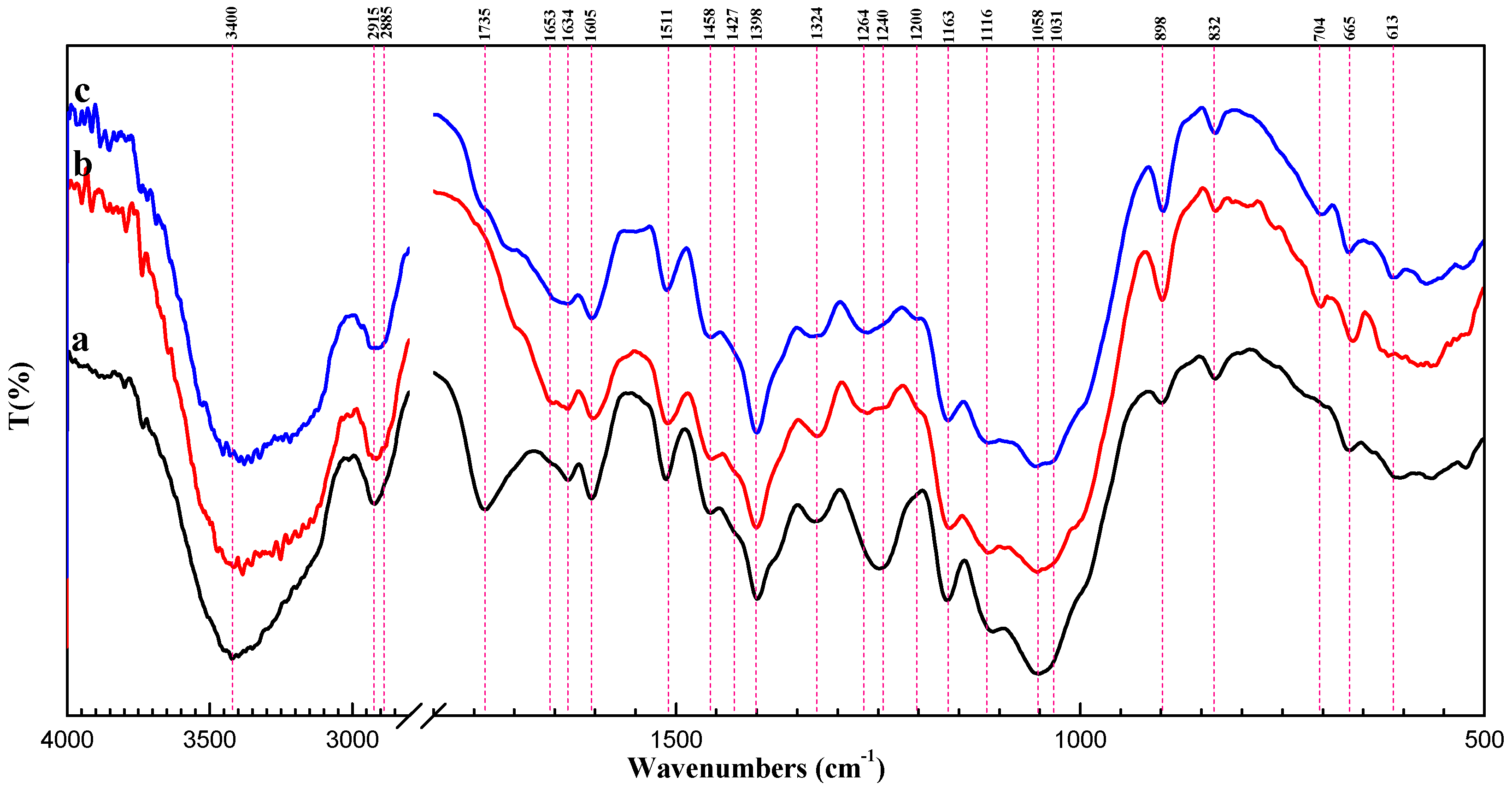
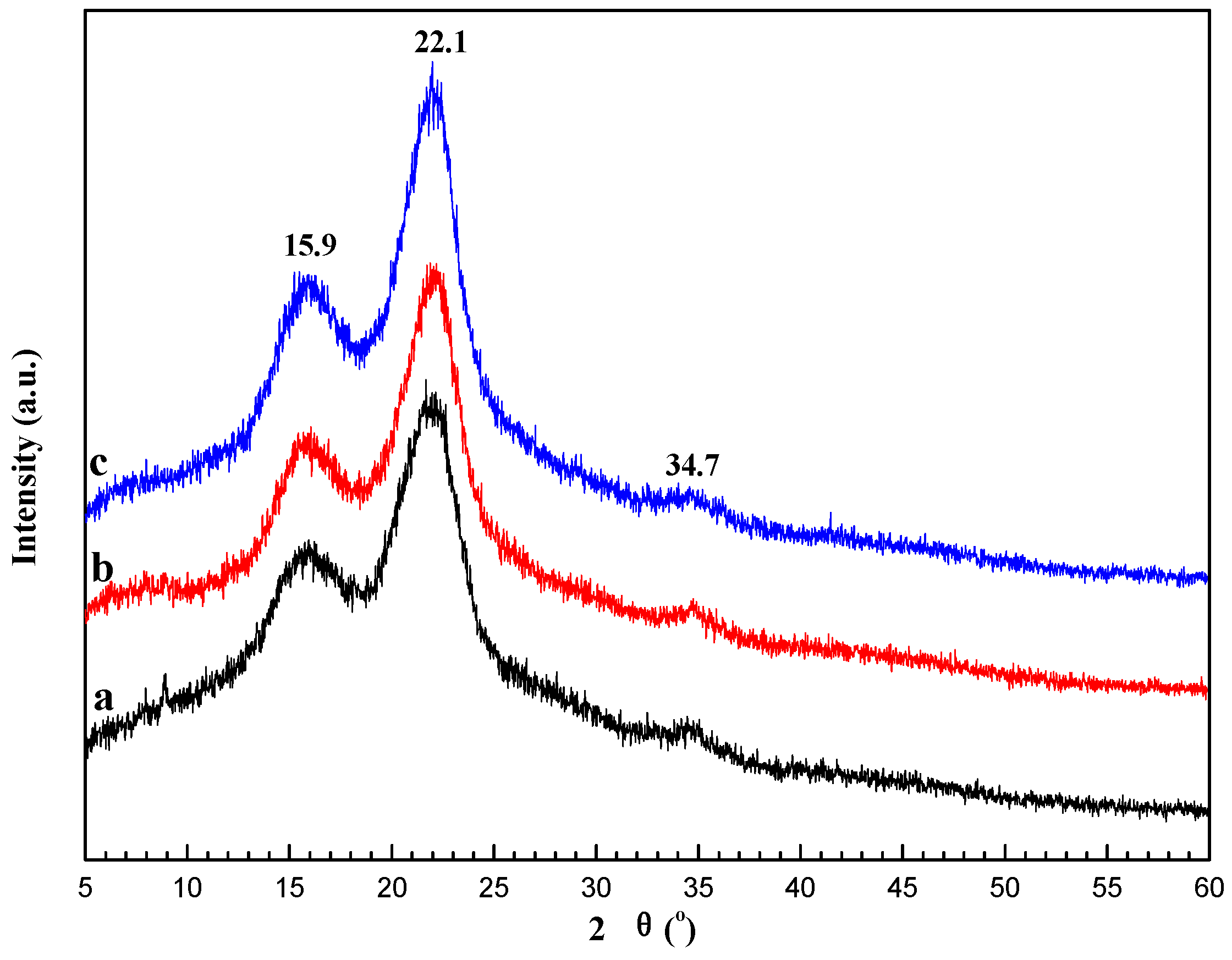
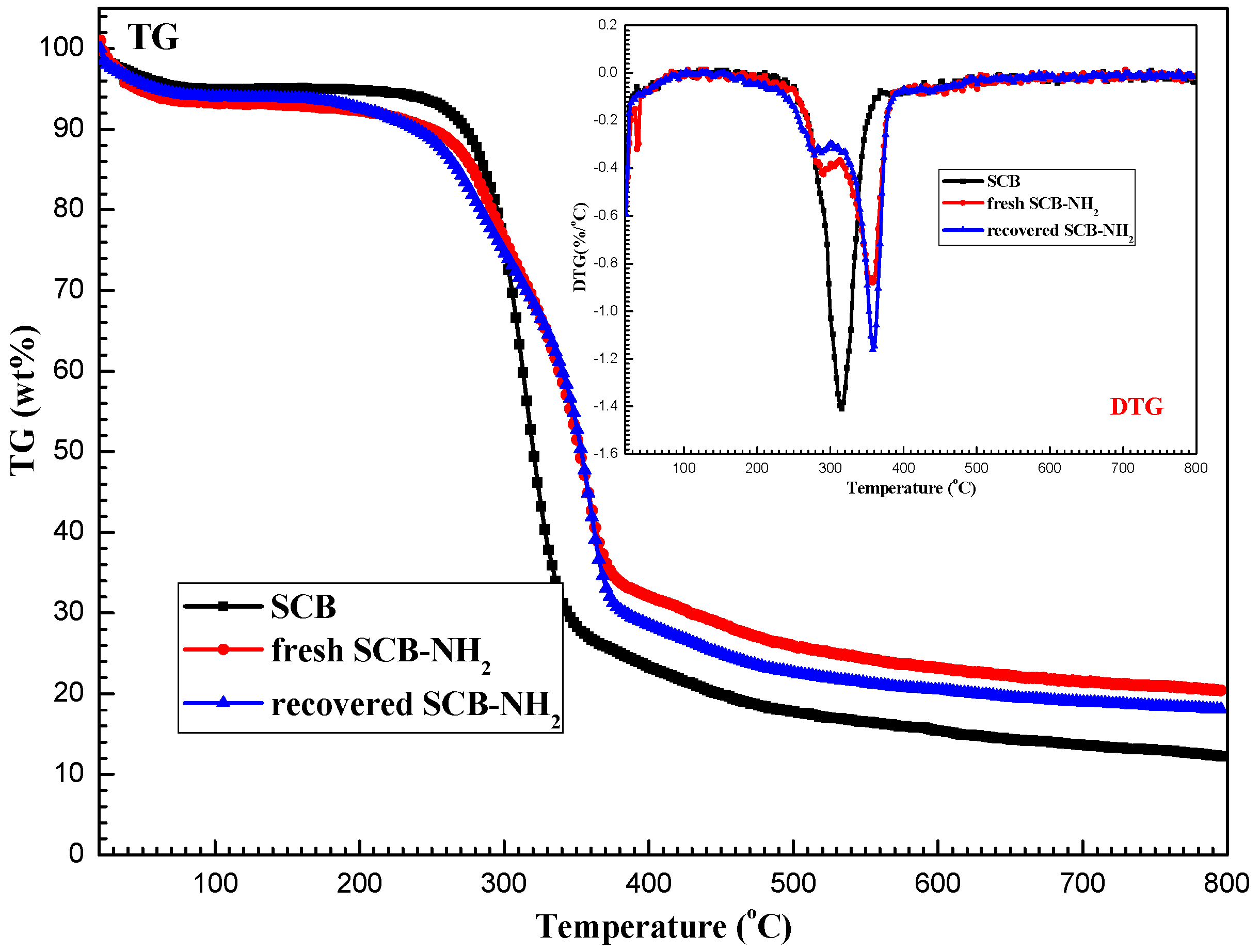
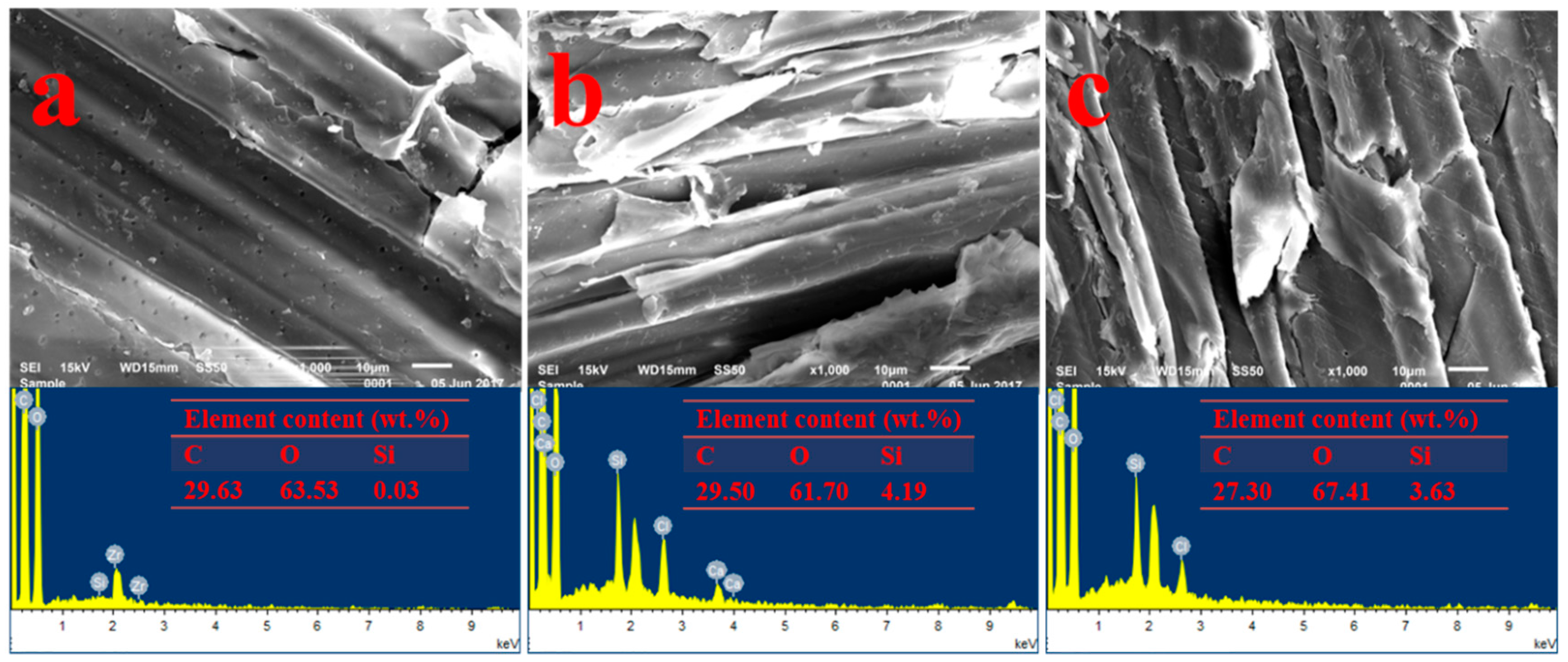
2.1. Characterization of SCB-NH2
2.2. Catalytic Performance in Batch Reaction
2.3. Continuous Flow Knoevenagel Condensation Reaction
3. Materials and Methods
3.1. Preparation and Characterization of SCB-NH2
3.2. Typical Batch Reaction
3.3. Continuous Flow Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, M.B.; March, J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6th ed.; Wiley: Hoboken, NJ, USA, 1968; pp. 1064–1065. ISBN 9780471720911. [Google Scholar]
- Voskressensky, L.G.; Festa, A.A.; Varlamov, A.V. Domino reactions based on Knoevenagel condensation in the synthesis of heterocyclic compounds. Recent advances. Tetrahedron 2014, 70, 551–572. [Google Scholar] [CrossRef]
- Jones, G. The Knoevenagel condensation. In Organic Reactions; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 15, pp. 204–599. [Google Scholar]
- List, B. Emil Knoevenagel and the roots of aminocatalysis. Angew. Chem. Int. Ed. 2010, 49, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The hitchhiker’s guide to flow chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, L.; Lanari, D.; Marrocchi, A.; Strappaveccia, G. Flow approaches towards sustainability. Green Chem. 2014, 16, 3680–3704. [Google Scholar] [CrossRef]
- Aponteguzman, J.; Shenje, R.; Huang, Y.; Woodham, W.; Saunders, S.R.; Mostaghimi, S.; Flack, K.M.; Pollet, P.L.; Eckert, C.A.; Liotta, C.L. A tandem, bicatalytic continuous flow cyclopropanation-homo-nazarov-type cyclization. Ind. Eng. Chem. Res. 2015, 54, 9550–9558. [Google Scholar] [CrossRef]
- Zhao, D.; Ding, K. Recent advances in asymmetric catalysis in flow. ACS Catal. 2013, 3, 928–944. [Google Scholar] [CrossRef]
- Hessel, V.; Kralisch, D.; Kockmann, N.; Noël, T.; Wang, Q. Novel process windows for enabling, accelerating, and uplifting flow chemistry. ChemSusChem 2013, 6, 746–789. [Google Scholar] [CrossRef] [PubMed]
- Wiles, C.; Watts, P. Continuous process technology: A tool for sustainable production. Green Chem. 2014, 16, 55–62. [Google Scholar] [CrossRef]
- Newman, S.G.; Jensen, K.F. The role of flow in green chemistry and engineering. Green Chem. 2013, 15, 1456–1472. [Google Scholar] [CrossRef]
- Christiaens, S.; Vantyghem, X.; Radoiu, M.; Vanden Eynde, J.J. Batch and continuous flow preparation of Hantzsch 1,4-dihydropyridines under microwave heating and simultaneous real-time monitoring by Raman spectroscopy. An exploratory study. Molecules 2014, 19, 9986–9998. [Google Scholar] [CrossRef] [PubMed]
- Wiles, C.; Watts, P.; Haswell, S.J. An investigation into the use of silica-supported bases within EOF-based flow reactors. Tetrahedron 2004, 60, 8421–8427. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Zhang, X.; Yeung, K.L. Continuous flow ZIF-8/NaA composite membrane microreactor for efficient Knoevenagel condensation. Catal. Commun. 2015, 68, 93–96. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Koga, H.; Qi, Z.D.; Saito, T.; Isogai, A. Nanofibrillar chitin aerogels as renewable base catalysts. Biomacromolecules 2014, 15, 4314–4319. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.Z.; Shi, S.; Wang, Y. Polystyrene-immobilized DABCO as a highly efficient and recyclable organocatalyst for the Knoevenagel condensation reaction. RSC Adv. 2013, 3, 23075–23079. [Google Scholar] [CrossRef]
- Parida, K.M.; Rath, D. Amine functionalized MCM-41: An active and reusable catalyst for Knoevenagel condensation reaction. J. Mol. Catal. A Chem. 2009, 310, 93–100. [Google Scholar] [CrossRef]
- Martins, L.; Hölderich, W.; Hammer, P.; Cardoso, D. Preparation of different basic Si-MCM-41 catalysts and application in the Knoevenagel and Claisen-Schmidt condensation reactions. J. Catal. 2010, 271, 220–227. [Google Scholar] [CrossRef]
- Gascon, J.; Aktay, U.; Hernandez-Alonso, M.D.; Klink, G.P.M.V.; Kapteijn, F. Amino-based metal-organic frameworks as stable, highly active basic catalysts. J. Catal. 2009, 261, 75–87. [Google Scholar] [CrossRef]
- Nguyen, L.T.L.; Le, K.K.A.; Truong, H.X.; Phan, N.T.S. Metal-organic frameworks for catalysis: The Knoevenagel reaction using zeolite imidazolate framework ZIF-9 as an efficient heterogeneous catalyst. Catal. Sci. Technol. 2012, 2, 521–528. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, H.F.; Xi, F.G.; Gao, E.Q. Amino-functionalized Zr(IV) metal-organic framework as bifunctional acid-base catalyst for Knoevenagel condensation. J. Mol. Catal. A Chem. 2014, 390, 198–205. [Google Scholar] [CrossRef]
- Ren, Y.; Lu, J.; Jiang, O.; Cheng, X.; Chen, J. Amine-grafted on lanthanide metal-organic frameworks: Three solid base catalysts for Knoevenagel condensation reaction. Chin. J. Catal. 2015, 36, 1949–1956. [Google Scholar] [CrossRef]
- Li, G.; Xiao, J.; Zhang, W. Efficient and reusable amine-functionalized polyacrylonitrile fiber catalysts for Knoevenagel condensation in water. Green Chem. 2012, 14, 2234–2242. [Google Scholar] [CrossRef]
- Xue, B.; Zhu, J.; Liu, N.; Li, Y. Facile functionalization of graphene oxide with ethylenediamine as a solid base catalyst for Knoevenagel condensation reaction. Catal. Commun. 2015, 64, 105–109. [Google Scholar] [CrossRef]
- Rana, S.; Jonnalagadda, S.B. Synthesis and characterization of amine functionalized graphene oxide and scope as catalyst for Knoevenagel condensation reaction. Catal. Commun. 2017, 92, 31–34. [Google Scholar] [CrossRef]
- Huh, S.; Chen, H.T.; Wiench, J.W.; Pruski, M.; Lin, V.S. Cooperative catalysis by general acid and base bifunctionalized mesoporous silica nanospheres. Angew. Chem. 2005, 117, 1860–1864. [Google Scholar] [CrossRef]
- Rostami, A.; Atashkar, B.; Gholami, H. Novel magnetic nanoparticles Fe3O4-immobilized domino Knoevenagel condensation, Michael addition, and cyclization catalyst. Catal. Commun. 2013, 37, 69–74. [Google Scholar] [CrossRef]
- Varadwaj, G.B.; Rana, S.; Parida, K.M. Amine functionalized K10 montmorillonite: A solid acid-base catalyst for the Knoevenagel condensation reaction. Dalton Trans. 2013, 42, 5122–5129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Sun, X.; Lou, F.; An, L.; Zhao, P. Facile one-pot synthesis of amine-functionalized mesoporous silica nanospheres for water-medium Knoevenagel reaction under microwave irradiation. Catal. Lett. 2015, 145, 1072–1079. [Google Scholar] [CrossRef]
- Tadesse, H.; Luque, R. Advances on biomass pretreatment using ionic liquids: An overview. Energy Environ. Sci. 2011, 4, 3913–3929. [Google Scholar] [CrossRef]
- Ma, H.; Wang, F.; Yu, Y.; Wang, L.; Li, X. Autocatalytic production of 5-hydroxymethylfurfural from fructose-based carbohydrates in a biphasic system and its purification. Ind. Eng. Chem. Res. 2015, 54, 2657–2666. [Google Scholar] [CrossRef]
- Teng, J.; Ma, H.; Wang, F.; Wang, L.; Li, X. Catalytic fractionation of raw biomass to biochemicals and organosolv lignin in a methyl isobutyl ketone/H2O biphasic system. ACS Sustain. Chem. Eng. 2016, 4, 2020–2026. [Google Scholar] [CrossRef]
- Teng, J.; Ma, H.; Wang, F.; Wang, L.; Li, X. A facile and eco-effective catalytic system for synthesis of 5-hydroxymethylfurfural from gucose. Bioresources 2016, 11, 2152–2165. [Google Scholar] [CrossRef]
- Fushimi, C.; Katayama, S.; Tasaka, K.; Suzuki, M.; Tsutsumi, A. Elucidation of the interaction among cellulose, xylan, and lignin in steam gasification of woody biomass. AIChE J. 2009, 55, 529–537. [Google Scholar] [CrossRef]
- Liu, R.; Yu, H.; Huang, Y. Structure and morphology of cellulose in wheat straw. Cellulose 2005, 12, 25–34. [Google Scholar] [CrossRef]
- Yuan, T.Q.; Xu, F.; He, J.; Sun, R.C. Structural and physico-chemical characterization of hemicelluloses from ultrasound-assisted extractions of partially delignified fast-growing poplar wood through organic solvent and alkaline solutions. Biotechnol. Adv. 2010, 28, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chen, S.; Chen, S.; Zhuang, L.; Ma, N.; Xu, T.; Li, Q.; Hou, X. Preparation and characterization of amine-functionalized sugarcane bagasse for CO2 capture. J. Environ. Manag. 2016, 168, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, D.M.; Johnson, J.R.; Koros, W.J. Aminosilane-functionalized cellulosic polymer for increased carbon dioxide sorption. Ind. Eng. Chem. Res. 2012, 51, 503–514. [Google Scholar] [CrossRef]
- Ribeiro, M.A.; Oikawa, H.; Mori, M.N.; Napolitano, C.M.; Duarte, C.L. Degradation mechanism of polysaccharides on irradiated sugarcane bagasse. Radiat. Phys. Chem. 2013, 84, 115–118. [Google Scholar] [CrossRef]
- Fonsecamaldonado, R.; Ribeiro, L.F.; Furtado, G.P.; Arruda, L.M.; Meleiro, L.P.; Alponti, J.S.; Botelhomachado, C.; Vieira, D.S.; Bonneil, E.; Furriel, R.D.P.M. Synergistic action of co-expressed xylanase/laccase mixtures against milled sugar cane bagasse. Process Biochem. 2014, 49, 1152–1161. [Google Scholar] [CrossRef]
- Rocha, G.J.D.M.; Nascimento, V.M.; Gonçalves, A.R.; Silva, V.F.N.; Martín, C. Influence of mixed sugarcane bagasse samples evaluated by elemental and physical-chemical composition. Ind. Crop. Prod. 2015, 64, 52–58. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.; Negulescu, I.I.; Moore, M.A.; Collier, B.J. Evaluating efficiency of alkaline treatment for waste bagasse. J. Macromol. Sci. B 2005, 44, 397–411. [Google Scholar] [CrossRef]
- Chen, J.H.; Xing, H.T.; Guo, H.X.; Li, G.P.; Weng, W.; Hu, S.R. Preparation, characterization and adsorption properties of a novel 3-aminopropyltriethoxysilane functionalized sodium alginate porous membrane adsorbent for Cr(III) ions. J. Hazard. Mater. 2013, 248–249, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Ying, A.; Ni, Y.; Xu, S.; Liu, S.; Yang, J.; Li, R. Novel DABCO-based ionic liquids: Green and efficient catalysts with dual catalytic roles for aqueous Knoevenagel condensation. Ind. Eng. Chem. Res. 2014, 53, 5678–5682. [Google Scholar] [CrossRef]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A. Green chemistry tools to influence a medicinal chemistry and research chemistry-based organisation. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Henderson, R.K.; Jiménezgonzález, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide—Embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Zhao, X.L.; Yang, K.F.; Zhang, Y.P.; Zhu, J.; Xu, L.W. Sevelamer as an efficient and reusable heterogeneous catalyst for the Knoevenagel reaction in water. Chin. Chem. Lett. 2014, 25, 1141–1144. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Tian, M.; Chu, G.; Quan, C. Fabrication of amino-functionalized Fe3O4@Cu3(BTC)2 for heterogeneous Knoevenagel condensation. Chin. J. Catal. 2016, 37, 420–427. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, H.; Luo, J.; Wei, Y. A magnetic nanoparticle supported dual acidic ionic liquid: A “quasi-homogeneous” catalyst for the one-pot synthesis of benzoxanthenes. Green Chem. 2012, 14, 202–208. [Google Scholar] [CrossRef]
- Koga, H.; Kitaoka, T.; Isogai, A. In situ modification of cellulose paper with amino groups for catalytic applications. J. Mater. Chem. 2011, 21, 9356–9361. [Google Scholar] [CrossRef]
- Wu, Y.; Zhen, L.; Xia, C. Silica-gel-supported dual acidic ionic liquids as efficient catalysts for the synthesis of polyoxymethylene dimethyl ethers. Ind. Eng. Chem. Res. 2016, 55, 1859–1865. [Google Scholar] [CrossRef]
- Koga, H.; Kitaoka, T.; Isogai, A. Chemically-modified cellulose paper as a microstructured catalytic reactor. Molecules 2015, 20, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Samples | Element Content (wt. %) | ||
|---|---|---|---|
| C | H | N | |
| SCB | 41.52 ± 0.22 | 7.97 ± 0.07 | 0.19 ± 0.02 |
| Fresh SCB-NH2 | 41.24 ± 0.30 | 8.00 ± 0.05 | 1.98 ± 0.01 |
| Recovered SCB-NH2 | 41.74 ± 0.24 | 7.98 ± 0.06 | 1.82 ± 0.01 |
 | |||
| Entry | Catalyst Dogase (g) | Time (min) | Yield (%) b |
| 1 | — | 10 ± 0.5 (h) | 89.2 ± 2.4 |
| 2 | 0.05 | 58 ± 5 | 92.9 ± 3.1 |
| 3 | 0.10 | 33 ± 3 | 94.3 ± 2.8 |
| 4 | 0.15 | 15 ± 2 | 95.1 ± 2.6 |
| 5 | 0.20 | 12 ± 2 | 94.1 ± 3.2 |
| 6 | 0.25 | 10 ± 1 | 93.8 ± 2.9 |
| 7 | 0.30 | 9 ± 1 | 94.3 ± 2.1 |
| 8 c | 0.15 | 4 ± 0.2 (h) | 94.9 ± 2.6 |
| 9 d | 0.15 | 4 ± 0.2 (h) | 87.9 ± 2.7 |
| 10 e | — | 28 ± 1.5 (h) | 85.2 ± 2.8 |
 | |||
| Entry | Solvent | Time (min) | Yield (%) b |
| 1 | — | 10 ± 1 | 95.2 ± 1.9 |
| 2 c | THF | >24 (h) | 51.5 ± 2.1 |
| 3 c | Acetone | >24 (h) | 54.3 ± 3.2 |
| 4 | MeOH | 66 ± 3 | 93.3 ± 2.7 |
| 5 | EtOH | 57 ± 3 | 94.1 ± 2.5 |
| 6 | 98% EtOH | 25 ± 2 | 94.3 ± 2.8 |
| 7 | 95% EtOH | 15 ± 2 | 95.1 ± 2.6 |
| 8 | 90% EtOH | 10 ± 1 | 94.1 ± 2.3 |
| 9 | 80% EtOH | 9 ± 1 | 93.8 ± 3.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Teng, J.; Wang, S.; Ma, H. Amine-Functionalized Sugarcane Bagasse: A Renewable Catalyst for Efficient Continuous Flow Knoevenagel Condensation Reaction at Room Temperature. Molecules 2018, 23, 43. https://doi.org/10.3390/molecules23010043
Qiao Y, Teng J, Wang S, Ma H. Amine-Functionalized Sugarcane Bagasse: A Renewable Catalyst for Efficient Continuous Flow Knoevenagel Condensation Reaction at Room Temperature. Molecules. 2018; 23(1):43. https://doi.org/10.3390/molecules23010043
Chicago/Turabian StyleQiao, Yanhui, Junjiang Teng, Shuangfei Wang, and Hao Ma. 2018. "Amine-Functionalized Sugarcane Bagasse: A Renewable Catalyst for Efficient Continuous Flow Knoevenagel Condensation Reaction at Room Temperature" Molecules 23, no. 1: 43. https://doi.org/10.3390/molecules23010043





