A Novel Class of Tyrosyl-DNA Phosphodiesterase 1 Inhibitors That Contains the Octahydro-2H-chromen-4-ol Scaffold
Abstract
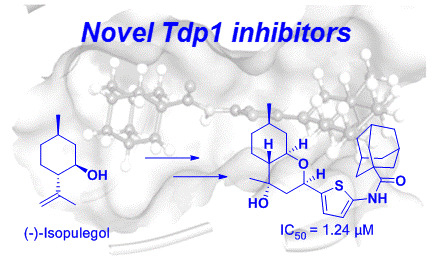
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Octahydro-2H-chromen-4-ol Derivatives
2.2. Tdp1 Inhibition and Binding Assays
2.3. Molecular Modelling
3. Materials and Methods
3.1. Chemistry
3.1.1. General Materials and Methods
3.1.2. Chemical Syntheses
3.2. Tdp1 Assay
3.3. Binding Studies
3.4. Molecular Modelling Methods
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ledesma, F.C.; El Khamisy, S.F.; Zuma, M.C.; Osborn, K.; Caldecott, K.W. A Human 5′-Tyrosyl DNA Phosphodiesterase That Repairs Topoisomerase-Mediated DNA Damage. Nature 2009, 461, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Interthal, H.; Pouliot, J.J.; Champoux, J.J. The Tyrosyl-DNA Phosphodiesterase Tdp1 Is a Member of the Phospholipase D Superfamily. Proc. Natl. Acad. Sci. USA 2001, 98, 12009–12014. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Burgin, A.B.; Huizenga, B.N.; Robertson, C.A.; Yao, K.C.; Nash, H.A.; Nash, H.A. A Eukaryotic Enzyme That Can Disjoin Dead-End Covalent Complexes between DNA and Type I Topoisomerases. Proc. Natl. Acad. Sci. USA 1996, 93, 11534–11539. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Elsayed, M.S.A.; Plescia, C.B.; Ravji, A.; Redon, C.E.; Kiselev, E.; Marchand, C.; Zeleznik, O.; Agama, K.; Pommier, Y.; et al. Synthesis and Biological Evaluation of the First Triple Inhibitors of Human Topoisomerase 1, Tyrosyl–DNA Phosphodiesterase 1 (Tdp1), and Tyrosyl–DNA Phosphodiesterase 2 (Tdp2). J. Med. Chem. 2017, 60, 3275–3288. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.-W.; Feng, Y.; Tran, T.D.; Shimizu, Y.; Pfeifer, T.; Vu, H.T.; Quinn, R.J. Achyrodimer F, a Tyrosyl-DNA Phosphodiesterase I Inhibitor from an Australian Fungus of the Family Cortinariaceae. Bioorg. Med. Chem. Lett. 2017, 27, 4007–4010. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, T.; Zakharenko, A.; Odarchenko, T.; Arabshahi, H.J.; Sannikova, V.; Zakharova, O.; Korchagina, D.; Reynisson, J.; Volcho, K.; Salakhutdinov, N.; et al. New inhibitors of tyrosyl-DNA phosphodiesterase I (Tdp 1) combining 7-hydroxycoumarin and monoterpenoid moieties. Bioorg. Med. Chem. 2016, 24, 5573–5581. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.; Luzina, O.; Koval, O.; Nilov, D.; Gushchina, I.; Dyrkheeva, N.; Švedas, V.; Salakhutdinov, N.; Lavrik, O. Tyrosyl-DNA Phosphodiesterase 1 Inhibitors: Usnic Acid Enamines Enhance the Cytotoxic Effect of Camptothecin. J. Nat. Prod. 2016, 79, 2961–2967. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.L.; Khomenko, T.M.; Zhukova, S.V.; Koval, O.A.; Zakharova, O.D.; Anarbaev, R.O.; Lebedeva, N.A.; Korchagina, D.V.; Komarova, N.I.; Vasiliev, V.G.; et al. Synthesis and Biological Evaluation of Novel Tyrosyl-DNA Phosphodiesterase 1 Inhibitors with a Benzopentathiepine Moiety. Bioorg. Med. Chem. 2015, 23, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, K.Y.; Suslov, E.V.; Zakharenko, A.L.; Zakharova, O.D.; Rogachev, A.D.; Korchagina, D.V.; Zafar, A.; Reynisson, J.; Nefedov, A.A.; Volcho, K.P.; et al. Aminoadamantanes Containing Monoterpene-Derived Fragments as Potent Tyrosyl-DNA Phosphodiesterase 1 Inhibitors. Bioorg. Chem. 2018, 76, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Antony, S.; Marchand, C.; Stephen, A.G.; Thibaut, L.; Agama, K.K.; Fisher, R.J.; Pommier, Y. Novel High-Throughput Electrochemiluminescent Assay for Identification of Human Tyrosyl-DNA Phosphodiesterase (Tdp1) Inhibitors and Characterization of Furamidine (NSC 305831) as an Inhibitor of Tdp1. Nucleic Acids Res. 2007, 35, 4474–4484. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.N.; Pommier, Y.; Marchand, C. Tyrosyl-DNA Phosphodiesterase 1 (Tdp1) Inhibitors. Expert Opin. Ther. Pat. 2011, 21, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Salomatina, O.V.; Popadyuk, I.I.; Zakharenko, A.L.; Zakharova, O.D.; Fadeev, D.S.; Komarova, N.I.; Reynisson, J.; Arabshahi, H.J.; Chand, R.; Volcho, K.P.; et al. Novel Semisynthetic Derivatives of Bile Acids as Effective Tyrosyl-DNA Phosphodiesterase 1 Inhibitors. Molecules 2018, 23, 679. [Google Scholar] [CrossRef] [PubMed]
- Arabshahi, H.J.; van Rensburg, M.; Pilkington, L.I.; Jeon, C.Y.; Song, M.; Gridel, L.-M.; Leung, E.; Barker, D.; Vuica-Ross, M.; Volcho, K.P.; et al. A Synthesis, in Silico, in Vitro and in Vivo Study of Thieno [2,3-b]Pyridine Anticancer Analogues. Medchemcomm 2015, 6, 1987–1997. [Google Scholar] [CrossRef]
- Yasumoto, T.; Murata, M. Marine Toxins. Chem. Rev. 1993, 93, 1897–1909. [Google Scholar] [CrossRef]
- Perron, F.; Albizati, K.F. Chemistry of Spiroketals. Chem. Rev. 1989, 89, 1617–1661. [Google Scholar] [CrossRef]
- Nazimova, E.V.; Shtro, A.A.; Anikin, V.B.; Patrusheva, O.S.; Il′ina, I.V.; Korchagina, D.V.; Zarubaev, V.V.; Volcho, K.P.; Salakhutdinov, N.F. Influenza Antiviral Activity of Br-Containing [2R,4R(S),4aR,7R,8aR]-4,7-Dimethyl-2-(Thiophen-2-YL)Octahydro-2H-Chromen-4-Ols Prepared from (–)-Isopulegol. Chem. Nat. Compd. 2017, 53, 260–264. [Google Scholar] [CrossRef]
- Nazimova, E.; Pavlova, A.; Mikhalchenko, O.; Il’ina, I.; Korchagina, D.; Tolstikova, T.; Volcho, K.; Salakhutdinov, N. Discovery of Highly Potent Analgesic Activity of Isopulegol-Derived (2R,4aR,7R,8aR)-4,7-Dimethyl-2-(Thiophen-2-Yl)Octahydro-2H-Chromen-4-Ol. Med. Chem. Res. 2016, 25, 1369–1383. [Google Scholar] [CrossRef]
- Baishya, G.; Sarmah, B.; Hazarika, N. An Environmentally Benign Synthesis of Octahydro-2H-Chromen-4-Ols via Modified Montmorillonite K10 Catalyzed Prins Cyclization Reaction. Synlett 2013, 24, 1137–1141. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Ganesh, A.V.; Narayana Kumar, G.G.K.S. Sc(OTf)3-Catalyzed One-Pot Ene-Prins Cyclization: A Novel Synthesis of Octahydro-2H-Chromen-4-Ols. Tetrahedron Lett. 2010, 51, 2963–2966. [Google Scholar] [CrossRef]
- Mikhalchenko, O.S.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F. A Practical Way to Synthesize Chiral Fluoro-Containing Polyhydro-2H -Chromenes from Monoterpenoids. Beilstein J. Org. Chem. 2016, 12, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Liu, X.; Qin, S.; Xie, M.; Lin, L.; Hu, C.; Feng, X. Completely OH-Selective FeCl 3 -Catalyzed Prins Cyclization: Highly Stereoselective Synthesis of 4-OH-Tetrahydropyrans. J. Am. Chem. Soc. 2012, 134, 17564–17573. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, M.N.; Panchenko, V.N.; Volcho, K.P.; Zakusin, S.V.; Krupskaya, V.V.; Gil, A.; Mikhalchenko, O.S.; Vicente, M.A. Effect of Acid Modification of Kaolin and Metakaolin on Brønsted Acidity and Catalytic Properties in the Synthesis of Octahydro-2H-Chromen-4-Ol from Vanillin and Isopulegol. J. Mol. Catal. A Chem. 2016, 414, 160–166. [Google Scholar] [CrossRef]
- Il’ina, I.; Pavlova, A.; Korchagina, D.; Ardashov, O.; Tolstikova, T.; Volcho, K.; Salakhutdinov, N. Synthesis and Analgesic Activity of Monoterpenoid-Derived Alkyl-Substituted Chiral Hexahydro-2H-Chromenes. Med. Chem. Res. 2017, 26, 1415–1426. [Google Scholar] [CrossRef]
- Sidorenko, A.Y.; Kravtsova, A.V.; Wärnå, J.; Aho, A.; Heinmaa, I.; Il’ina, I.V.; Ardashov, O.V.; Volcho, K.P.; Salakhutdinov, N.F.; Murzin, D.Y.; Agabekov, V.E. Preparation of Octahydro-2 H -Chromen-4-Ol with Analgesic Activity from Isopulegol and Thiophene-2-Carbaldehyde in the Presence of Acid-Modified Clays. Mol. Catal. 2018, 453, 139–148. [Google Scholar] [CrossRef]
- Patrusheva, O.S.; Volcho, K.P.; Salakhutdinov, N.F. Synthesis of oxygen-containing heterocyclic compounds based on monoterpenoids. Russ. Chem. Rev. 2018, 87, 771–796. [Google Scholar] [CrossRef]
- Jensen, P.W.; Falconi, M.; Kristoffersen, E.L.; Simonsen, A.T.; Cifuentes, J.B.; Marcussen, L.B.; Frøhlich, R.; Vagner, J.; Harmsen, C.; Juul, S.; et al. Real-Time Detection of TDP1 Activity Using a Fluorophore–quencher Coupled DNA-Biosensor. Biosens. Bioelectron. 2013, 48, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozhaitsev, E.S.; Zakharenko, A.L.; Suslov, E.V.; Korchagina, D.V.; Zakharova, O.D.; Vasil’eva, I.A.; Chepanova, A.A.; Black, E.; Patel, J.; Chand, R.; et al. Novel Inhibitors of DNA Repair Enzyme TDP1 Combining Monoterpenoid and Adamantane Fragments. Anticancer Agents Med. Chem. 2018, in press. [Google Scholar]
- Lebedeva, N.A.; Rechkunova, N.I.; Lavrik, O.I. AP-Site Cleavage Activity of Tyrosyl-DNA Phosphodiesterase 1. FEBS Lett. 2011, 585, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Liebeschuetz, J.W.; Cole, J.C.; Korb, O. Pose prediction and virtual screening performance of GOLD scoring functions in a standardized test. J. Comput. Aided Mol. Des. 2012, 26, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Logan, G.; Reynisson, J. Wine Compounds as a Source for HTS Screening Collections. A Feasibility Study. Mol. Inf. 2012, 31, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Zenei, T.; Hiroshi, T. Specific and Non-Specific Ligand Binding to Serum Albumin. Biochem. Pharmacol. 1985, 34, 1999–2005. [Google Scholar] [CrossRef]
- Davies, D.R.; Interthal, H.; Champoux, J.J.; Hol, W.G.J. Crystal Structure of a Human Tyrosyl-DNA Phosphodiesterase (Tdp1)-Tungstate Complex. J. Mol. Biol. 2003, 324, 917–932. [Google Scholar] [CrossRef]
- QikProp. QikProp; Schrödinger Press, L.L.C.: New York, NY, USA, 2017. [Google Scholar]
- Ioakimidis, L.; Thoukydidis, L.; Naeem, S.; Mirza, A.; Reynisson, J. Benchmarking the Reliability of QikProp. Correlation between Experimental and Predicted Values. QSAR Comb. Sci. 2008, 27, 445–456. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| Compound | IC50, µM | KD, μM | ||||
|---|---|---|---|---|---|---|
| R | (4S) | (4R) | (4S) | (4R) | ||
| 8 |  | NO2- | >15 | >15 | 30.6 ± 9.3 | 23.3 ± 7.4 |
| 9 | NH2- | >15 | >15 | n.d. | n.d. | |
| 10 |  | CH3- | 5.8 ± 3.0 | 2.9 ± 0.8 | n.d. | n.d. |
| 11 | CF3- | 1.4 ± 0.3 | 4.0 ± 0.4 | 2.0 ± 1.2 | 19.8 ± 2.4 | |
| 12 |  | 5.0 ± 1.5 | 3.3 ± 0.2 | 24.5 ± 5.7 | n.d. | |
| 13 |  | 1.24 ± 0.02 | 2.8 ± 0.6 | 17.9 ± 3.4 | 12.4 ± 7.5 | |
| Fur * | 1.2 ± 0.3 | n.d. | ||||
| Compound | GS | CS | ChemPLP | ASP | IC50 (μM) |
|---|---|---|---|---|---|
| (4R)-8 | 40.1 | 26.0 | 47.9 | 27.4 | >15 |
| (4S)-8 | 41.0 | 25.3 | 45.0 | 26.6 | >15 |
| (4R)-9 | 41.5 | 25.0 | 41.1 | 27.2 | >15 |
| (4S)-9 | 42.2 | 25.5 | 45.5 | 29.0 | >15 |
| (4R)-10 | 42.3 | 25.9 | 50.7 | 28.9 | 2.9 ± 0.8 |
| (4S)-10 | 44.0 | 25.2 | 49.4 | 28.4 | 5.8 ± 3.0 |
| (4R)-11 | 39.6 | 23.8 | 51.8 | 34.4 | 4.0 ± 0.4 |
| (4S)-11 | 44.0 | 25.2 | 50.6 | 31.3 | 1.4 ± 0.3 |
| (4R)-12 | 55.8 | 30.3 | 57.2 | 29.1 | 3.3 ± 0.2 |
| (4S)-12 | 56.0 | 30.8 | 56.4 | 29.8 | 5.0 ± 1.5 |
| (4R)-13 | 51.5 | 32.3 | 57.1 | 30.5 | 2.8 ± 0.6 |
| (4S)-13 | 52.5 | 31.4 | 56.1 | 30.4 | 1.24 ± 0.02 |
| (4R)-14 | 60.9 | 30.9 | 60.4 | 31.3 | - |
| (4S)-14 | 62.5 | 29.6 | 61.3 | 32.8 | - |
| (4R)-15 | 50.2 | 30.5 | 64.8 | 32.6 | - |
| (4S)-15 | 53.6 | 28.2 | 59.6 | 28.5 | - |
| (4R)-16 | 48.7 | 23.2 | 51.6 | 25.9 | - |
| (4S)-16 | 48.8 | 21.5 | 48.5 | 27.5 | - |
| (4R)-17 | 49.2 | 21.9 | 49.3 | 25.1 | - |
| (4S)-17 | 51.2 | 22.2 | 47.9 | 27.7 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li-Zhulanov, N.S.; Zakharenko, A.L.; Chepanova, A.A.; Patel, J.; Zafar, A.; Volcho, K.P.; Salakhutdinov, N.F.; Reynisson, J.; Leung, I.K.H.; Lavrik, O.I. A Novel Class of Tyrosyl-DNA Phosphodiesterase 1 Inhibitors That Contains the Octahydro-2H-chromen-4-ol Scaffold. Molecules 2018, 23, 2468. https://doi.org/10.3390/molecules23102468
Li-Zhulanov NS, Zakharenko AL, Chepanova AA, Patel J, Zafar A, Volcho KP, Salakhutdinov NF, Reynisson J, Leung IKH, Lavrik OI. A Novel Class of Tyrosyl-DNA Phosphodiesterase 1 Inhibitors That Contains the Octahydro-2H-chromen-4-ol Scaffold. Molecules. 2018; 23(10):2468. https://doi.org/10.3390/molecules23102468
Chicago/Turabian StyleLi-Zhulanov, Nikolai S., Alexandra L. Zakharenko, Arina A. Chepanova, Jinal Patel, Ayesha Zafar, Konstantin P. Volcho, Nariman F. Salakhutdinov, Jóhannes Reynisson, Ivanhoe K. H. Leung, and Olga I. Lavrik. 2018. "A Novel Class of Tyrosyl-DNA Phosphodiesterase 1 Inhibitors That Contains the Octahydro-2H-chromen-4-ol Scaffold" Molecules 23, no. 10: 2468. https://doi.org/10.3390/molecules23102468