Main Allelochemicals from the Rhizosphere Soil of Saussurea lappa (Decne.) Sch. Bip. and Their Effects on Plants’ Antioxidase Systems
Abstract
:1. Introduction
2. Results and Discussion
2.1. Allelochemicals from the Rhizosphere Soil of S. lappa
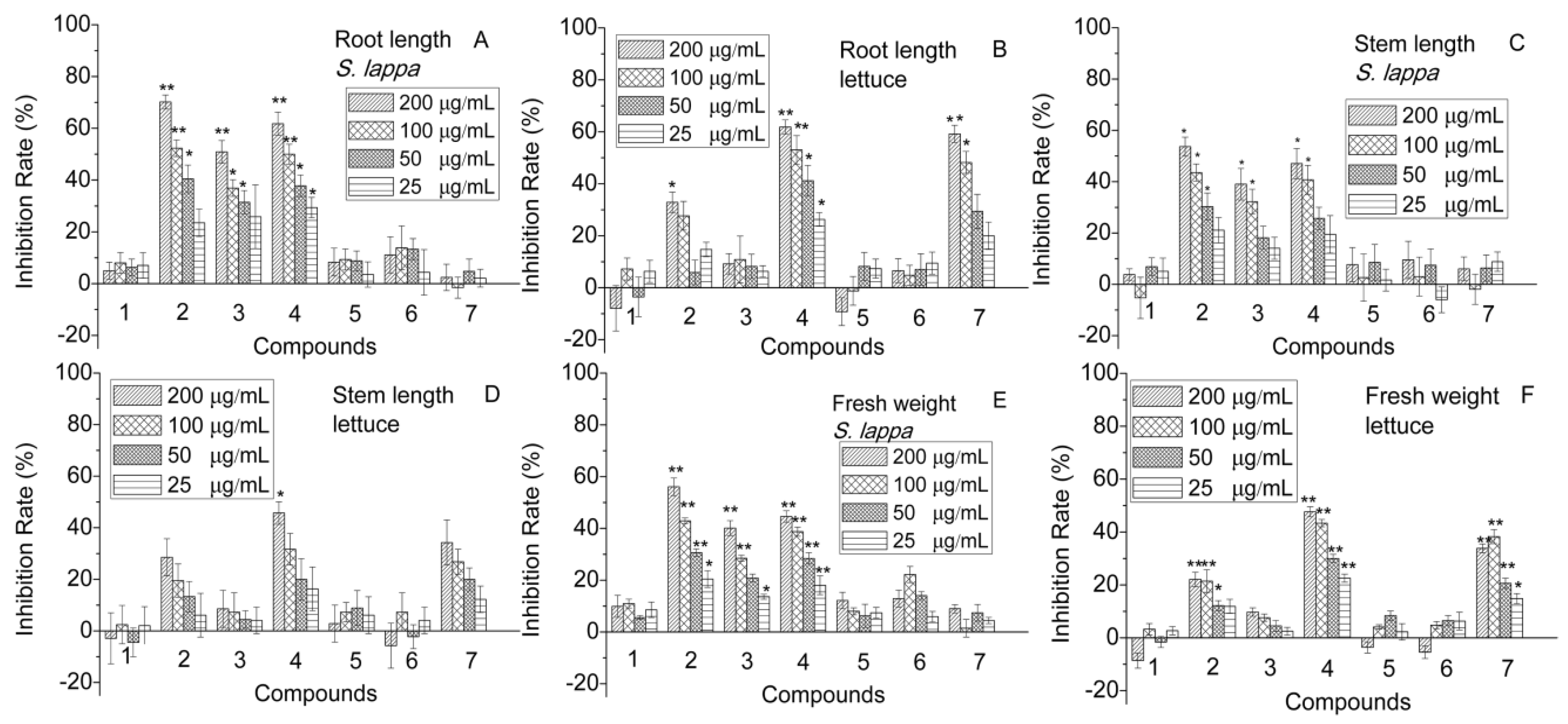
2.2. Activity of the Purified Compounds on the Seedling Growth of S. lappa and Lettuce
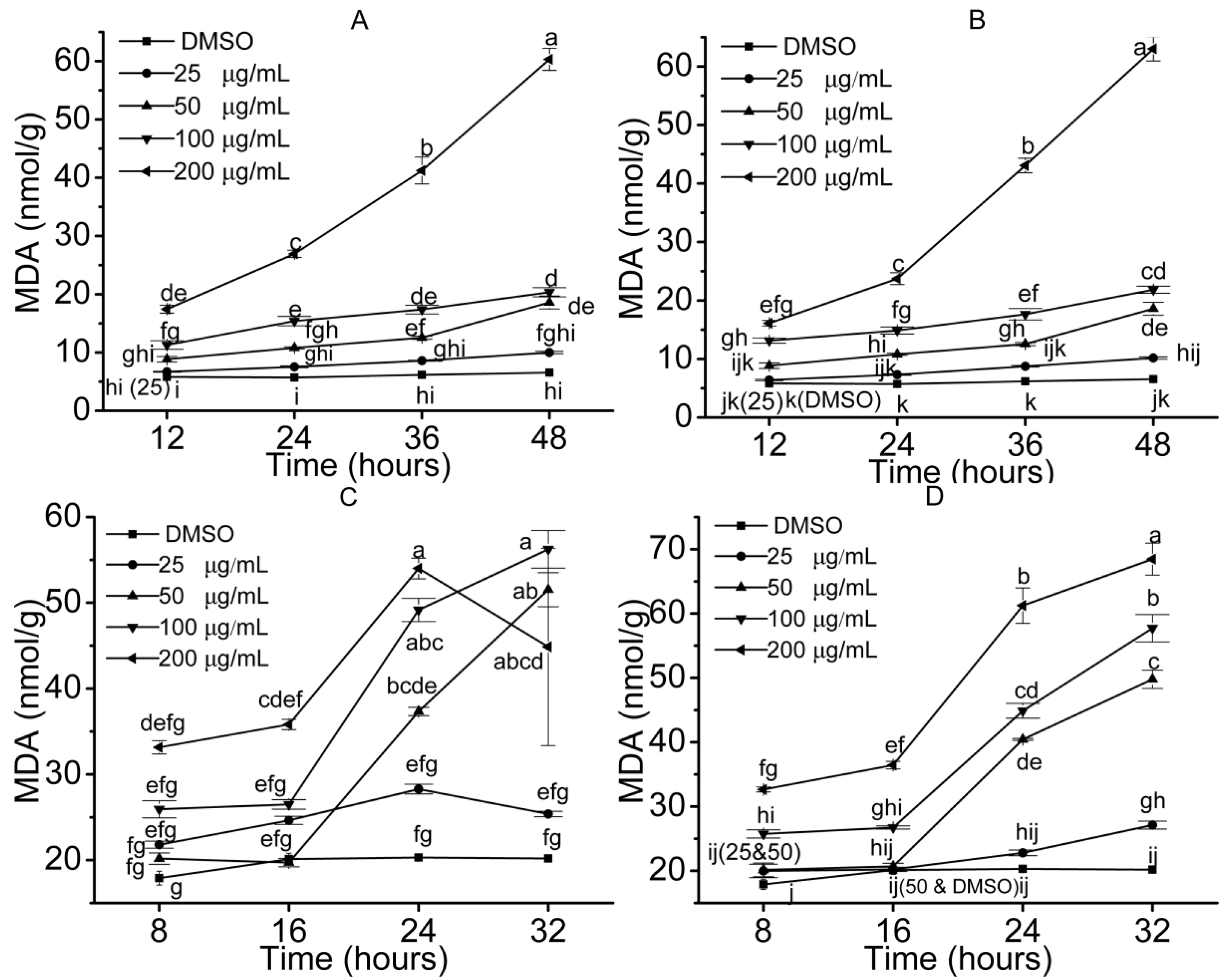
2.3. Stress Effects of the Isolated Compounds on the Protection Enzyme and ROS Accumulation in Treated Seedlings
2.4. Confirmation and Possible Release Ways of Allelochemicals
3. Materials and Methods
3.1. General Information
3.2. Soil and Plant Samples
3.3. The Isolation and Identification of the Allelochemicals
3.4. Activities of the Purified Compounds on the Growth of Seedlings
3.5. Stress Effects of Allelochemicals on Antioxidant Enzymes in Treated Seedlings
3.6. Effects of Allelochemicals on the Accumulation of ROS in the Treated Seedlings
3.7. Statistical Analysis
3.8. Analysis for the Possible Release Ways of Allelochemicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, M.; Zhang, J.; Li, Y.; Han, X.; Gao, K.; Fang, J. Bioassay-guided isolation of dehydrocostus lactone from Saussurea lappa: A new targeted cytosolic thioredoxin reductase anticancer agent. Arch. Biochem. Biophys. 2016, 607, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Yeasmin, R.; Motoki, S.; Yamamoto, S.; Nishihara, E. Allelochemicals inhibit the growth of subsequently replanted asparagus (Asparagus officinalis L.). Biol. Agric. Hortic. 2013, 29, 165–172. [Google Scholar] [CrossRef]
- Ren, X.; He, X.; Zhang, Z.; Yan, Z.; Jin, H.; Li, X.; Qin, B. Isolation, Identification, and Autotoxicity Effect of Allelochemicals from Rhizosphere Soils of Flue-Cured Tobacco. J. Agric. Food Chem. 2015, 63, 8975–8980. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Wang, S.-L.; Wang, P.; Kong, C.-H. Autoinhibition and soil allelochemical (cyclic dipeptide) levels in replanted Chinese fir (Cunninghamia lanceolata) plantations. Plant Soil 2014, 374, 793–801. [Google Scholar] [CrossRef]
- Asao, T.; Hasegawa, K.; Sueda, Y.; Tomita, K.; Taniguchi, K.; Hosoki, T.; Pramanik, M.H.R.; Matsui, Y. Autotoxicity of root exudates from taro. Sci. Hortic. 2003, 97, 389–396. [Google Scholar] [CrossRef]
- Bais, H.P.; Park, S.W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pratley, J.; Lemerle, D.; An, M.; Liu, D.L. Autotoxicity of wheat (Triticum aestivum L.) as determined by laboratory bioassays. Plant Soil 2007, 296, 85–93. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Xu, Y.; Mei, X.; Jiang, B.; Liao, J.; Yin, Z.; Zheng, J.; Zhao, Z.; Fan, L.; et al. Autotoxic Ginsenosides in the Rhizosphere Contribute to the Replant Failure of Panax notoginseng. PLoS ONE 2015, 10, e0118555. [Google Scholar] [CrossRef] [PubMed]
- Rasher, D.B.; Hay, M.E. Seaweed allelopathy degrades the resilience and function of coral reefs. Commun. Integr. Biol. 2010, 3, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Murrell, C.; Gerber, E.; Krebs, C.; Parepa, M.; Schaffner, U.; Bossdorf, O. Invasive knotweed affects native plants through allelopathy. Am. J. Bot. 2011, 98, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Kong, C.-H.; Li, Y.-H.; Wang, P.; Xu, X.-H. Crabgrass (Digitaria sanguinalis) Allelochemicals That Interfere with Crop Growth and the Soil Microbial Community. J. Agric. Food Chem. 2013, 61, 5310–5317. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Robinson, R.W. Allelopathy and resource competition: The effects of Phragmites australis invasion in plant communities. Bot. Stud. 2017, 58, 29. [Google Scholar] [CrossRef] [PubMed]
- Jilani, G.; Mahmood, S.; Chaudhry, A.N.; Hassan, I.; Akram, M. Allelochemicals: Sources, toxicity and microbial transformation in soil—A review. Ann. Microbiol. 2008, 58, 351–357. [Google Scholar] [CrossRef]
- Liu, X.; Tian, F.; Tian, Y.; Wu, Y.; Dong, F.; Xu, J.; Zheng, Y. Isolation and Identification of Potential Allelochemicals from Aerial Parts of Avena fatua L. and Their Allelopathic Effect on Wheat. J. Agric. Food Chem. 2016, 64, 3492–3500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Skoneczny, D.; Weidenhamer, J.D.; Mwendwa, J.M.; Weston, P.A.; Gurr, G.M.; Callaway, R.M.; Weston, L.A. Identification and localization of bioactive naphthoquinones in the roots and rhizosphere of Paterson’s curse (Echium plantagineum), a noxious invader. J. Exp. Bot. 2016, 67, 3777–3788. [Google Scholar] [CrossRef] [PubMed]
- Traversa, A.; Loffredo, E.; Gattullo, C.E.; Senesi, N. Water-extractable organic matter of different composts: A comparative study of properties and allelochemical effects on horticultural plants. Geoderma 2010, 156, 287–292. [Google Scholar] [CrossRef]
- Broch Mignoni, D.S.; Simes, K.; Braga, M.R. Potential allelopathic effects of the tropical legume Sesbania virgata on the alien Leucaena leucocephala related to seed carbohydrate metabolism. Biol. Invasions 2018, 20, 165–180. [Google Scholar] [CrossRef]
- Cerkowniak, M.; Bogus, M.I.; Wloka, E.; Stepnowski, P.; Golebiowski, M. Application of headspace solid-phase microextraction followed by gas chromatography coupled with mass spectrometry to determine esters of carboxylic acids and other volatile compounds in Dermestes maculatus and Dermestes ater lipids. Biomed. Chromatogr. 2018, 32, e4051. [Google Scholar] [CrossRef] [PubMed]
- Vrankovic, J.; Zivic, M.; Radojevic, A.; Peric-Mataruga, V.; Todorovic, D.; Markovic, Z.; Zivic, I. Evaluation of oxidative stress biomarkers in the freshwater gammarid Gammarus dulensis exposed to trout farm outputs. Ecotoxicol. Environ. Saf. 2018, 163, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant. Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Il Jung, H.; Kuk, Y.I.; Back, K.; Burgos, N.R. Resistance pattern and antioxidant enzyme profiles of protoporphyrinogen oxidase (PROTOX) inhibitor-resistant transgenic rice. Pest. Biochem. Physiol. 2008, 91, 53–65. [Google Scholar] [CrossRef]
- Kanoun-Boule, M.; Vicente, J.A.F.; Nabais, C.; Prasad, M.N.V.; Freitas, H. Ecophysiological tolerance of duckweeds exposed to copper. Aquat. Toxicol. 2009, 91, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radic, S.; Babic, M.; Skobic, D.; Roje, V.; Pevalek-Kozlina, B. Ecotoxicological effects of aluminum and zinc on growth and antioxidants in Lemna minor L. Ecotoxicol. Environ. Saf. 2010, 73, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Moon, H.W.; Oh, Y.; Kim, K.; Kim, D.D.; Lim, C.J. Defensive Properties of Ginsenoside Re against UV-B-Induced Oxidative Stress through Up-Regulating Glutathione and Superoxide Dismutase in HaCaT Keratinocytes. Iran. J. Pharm. Res. 2018, 17, 249–260. [Google Scholar] [PubMed]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Yan, X.U. Analysis of Chemical Constituents of Styela canopus. J. Anhui Agric. Sci. 2011, 10, 083. [Google Scholar]
- Zhang, T.; Ma, L.; Wu, F.; Chen, R. Chemical constituents from a portion of ethanolicextract of Saussurea lappa roots. Chin. J. Chin. Mater. Med. 2012, 37, 1232–1236. [Google Scholar]
- Park, H.W.; Lee, J.H.; Choi, S.-U.; Baek, N.-I.; Kim, S.-H.; Yang, J.H.; Kim, D.K. Cytotoxic Germacranolide Sesquiterpenes from the Bark of Magnolia kobus. Arch. Pharm. Res. 2010, 33, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Adegawa, S.; Miyase, T.; Ueno, A. Sesquiterpene lactones from Diaspananthus-uniflorus (SCH, BIP) kitam. Chem. Pharm. Bull. 1987, 35, 1479–1485. [Google Scholar] [CrossRef]
- Yan, R.Y.; Chen, R.Y. Studies on chemical constituents of Suregada glomerulata II. China J. Chin. Mater. Med. 2007, 32, 1653. [Google Scholar]
- Vasconcelos, J.M.J.; Silva, A.M.S.; Cavaleiro, J.A.S. Chromones and flavanones from Artemisia campestris subsp. maritima. Phytochemistry 1998, 49, 1421–1424. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liang, Y.Z.; Li, X.N. Chemical Composition and Antimicrobial Activity of the Essential Oils from Clove. Nat. Prod. Res. Dev. 2006, 18, 381–385. [Google Scholar]
- Zhang, Z.H.; Dai, Z.; Hu, X.R.; Lin, R.C. Isolation and Structure Elucidation of Chemical Constituents from Pinellia ternata. Chin. Tradit. Herbal Drugs 2013, 36, 1620–1622. [Google Scholar]
- Shuo, L.I.; Hong, H.L. Study on the Chemical Constituents of Saussurea lappa. Chin. J. Nat. Med. 2004, 2, 62–64. [Google Scholar]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd Allah, E.F. Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Baziramakenga, R.; Leroux, G.D.; Simard, R.R. Effects of benzoic and cinnamic-acids on membrane-permeability of soybean roots. J. Chem. Ecol. 1995, 21, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Bazdar, M. Effects of Prangos ferulacea aqueous and hydroalcoholic extracts obtained from different organs on the regeneration of Trifolium resupinatum. Acta Physiol. Plant 2018, 40, 50. [Google Scholar] [CrossRef]
- Yao, S.K.; Li, F.L.; Li, F.; Feng, X.; Peng, L.N.; Dong, J.M.; Feng, Z.; Teng, C.H.; Zhang, J.F.; Zhao, Q.Q.; et al. Allelopathic effects of Iva xanthiifolia on the germination and seedling growth of Raphanus sativus, Brassica campestris, B-pekinensis, B-juncea and B-oleracea and identification of allelochemicals. Allelopath. J. 2018, 44, 201–218. [Google Scholar] [CrossRef]
- Jang, S.J.; Kim, K.R.; Yun, Y.B.; Kim, S.S.; Kuk, Y.I. Inhibitory effects of Italian ryegrass (Lolium multiflorum Lam.) seedlings of rice (Oryza sativa L.). Allelopath. J. 2018, 44, 219–231. [Google Scholar] [CrossRef]
- Yakefu, Z.; Huannixi, W.; Ye, C.; Zheng, T.; Chen, S.; Peng, X.; Tian, Z.; Wang, J.; Yang, Y.; Ma, Z.; et al. Inhibitory effects of extracts from Cinnamomum camphora fallen leaves on algae. Water Sci. Technol. 2018, 77, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tominaga, T.; Ohno, O.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Plant growth inhibitory activity and active substances with allelopathic potential of cogongrass (Imperata cylindrica) rhizome. Weed Biol. Manag. 2018, 18, 92–98. [Google Scholar] [CrossRef]
- Politycka, B. Peroxidase activity and lipid peroxidation in roots of cucumber seedlings influenced by derivatives of cinnamic and benzoic acids. Acta Physiol. Plant 1996, 18, 365–370. [Google Scholar]
- Zhou, X.G.; Wang, Z.L.; Pan, D.D.; Wu, F.Z. Effects of vanillin on cucumber (Cucumis sativus L.) seedling rhizosphere Bacillus and Pseudomonas spp. community structures. Allelopath. J. 2018, 43, 255–264. [Google Scholar] [CrossRef]
- David, A.S.; Thapa-Magar, K.B.; Afkhami, M.E. Microbial mitigation-exacerbation continuum: A novel framework for microbiome effects on hosts in the face of stress. Ecology 2018, 99, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Cao, X. Enzymology bases for different sensitivity to GA3 of semidwarf genes in rice. J. Jiangsu Agric. Coll. 1994, 15, 10–13. [Google Scholar]
- Carvalho Andrade, M.; da Silva, A.A.; Carvalho, R.D.C.; Santiago, J.D.A.; Souza de Oliveira, A.M.; Francis, D.M.; Maluf, W.R. Quantitative trait loci associated with trichomes in the Solanum galapagense accession LA1401. Genet. Resour. Crop. Evol. 2018, 65, 1671–1685. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta 2000, 293, 157–166. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kobayashi, Y.; Devi, S.R.; Rikiishi, S.; Matsumoto, H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002, 128, 63–72. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–7 are not available from the authors. |





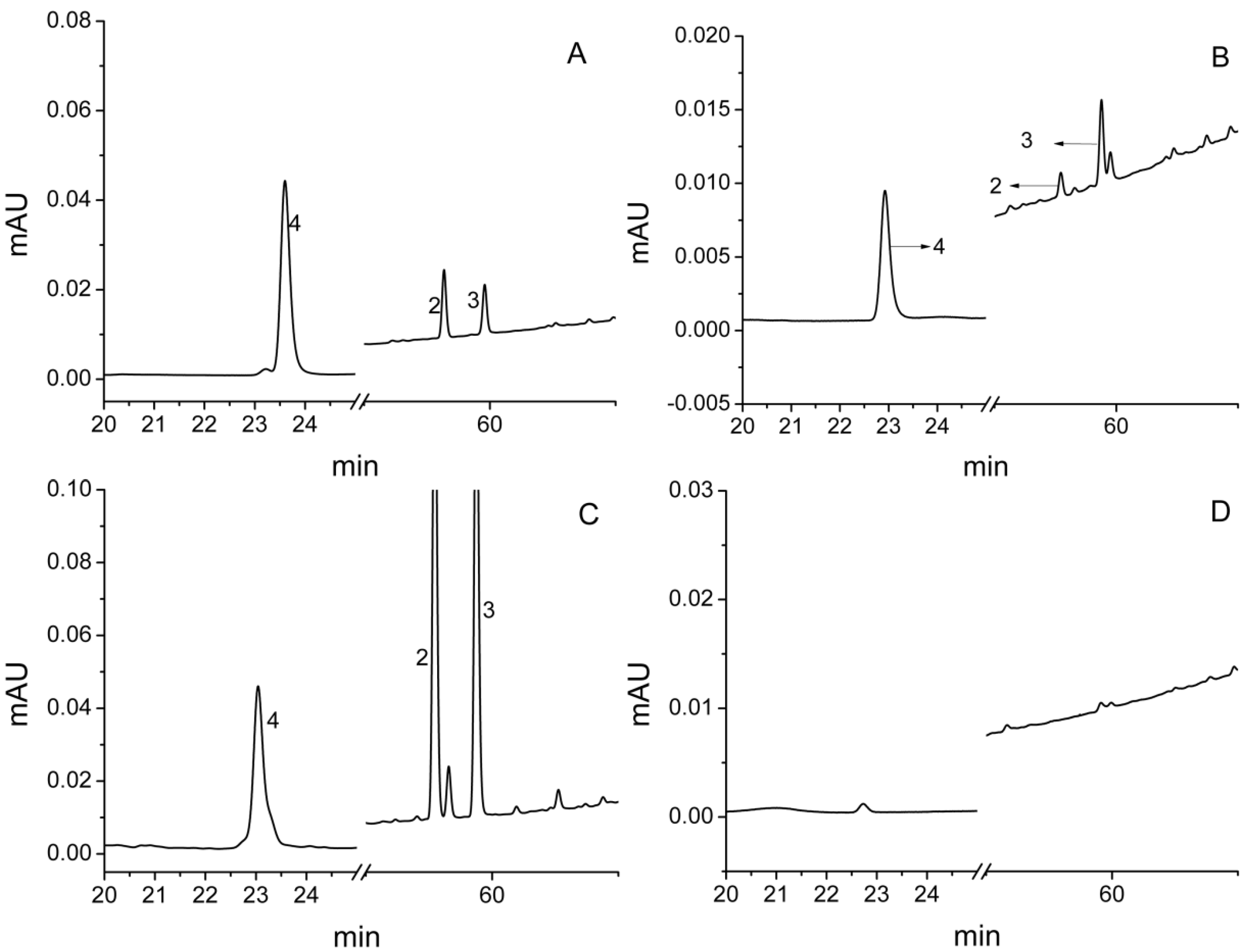
| Compound | Retention Time (RT, min) | Concentration (μg/g) |
|---|---|---|
| 2 | 58.18 | 0.14 |
| 3 | 59.80 | 0.76 |
| 4 | 23.60 | 0.27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xie, M.; Li, X.; Jin, H.; Yang, X.; Yan, Z.; Su, A.; Qin, B. Main Allelochemicals from the Rhizosphere Soil of Saussurea lappa (Decne.) Sch. Bip. and Their Effects on Plants’ Antioxidase Systems. Molecules 2018, 23, 2506. https://doi.org/10.3390/molecules23102506
Liu J, Xie M, Li X, Jin H, Yang X, Yan Z, Su A, Qin B. Main Allelochemicals from the Rhizosphere Soil of Saussurea lappa (Decne.) Sch. Bip. and Their Effects on Plants’ Antioxidase Systems. Molecules. 2018; 23(10):2506. https://doi.org/10.3390/molecules23102506
Chicago/Turabian StyleLiu, Jingkun, Min Xie, Xiuzhuang Li, Hui Jin, Xiaoyan Yang, Zhiqiang Yan, Anxiang Su, and Bo Qin. 2018. "Main Allelochemicals from the Rhizosphere Soil of Saussurea lappa (Decne.) Sch. Bip. and Their Effects on Plants’ Antioxidase Systems" Molecules 23, no. 10: 2506. https://doi.org/10.3390/molecules23102506




