Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characteristics of Wines and Dynamics of Malolactic Fermentation
2.2. Ethyl Fatty Acid Esters
2.3. Diacetyl and Its Metabolic Products
2.4. Sensory Evaluation
3. Materials and Methods
3.1. Microorganisms
3.2. Grape Variety
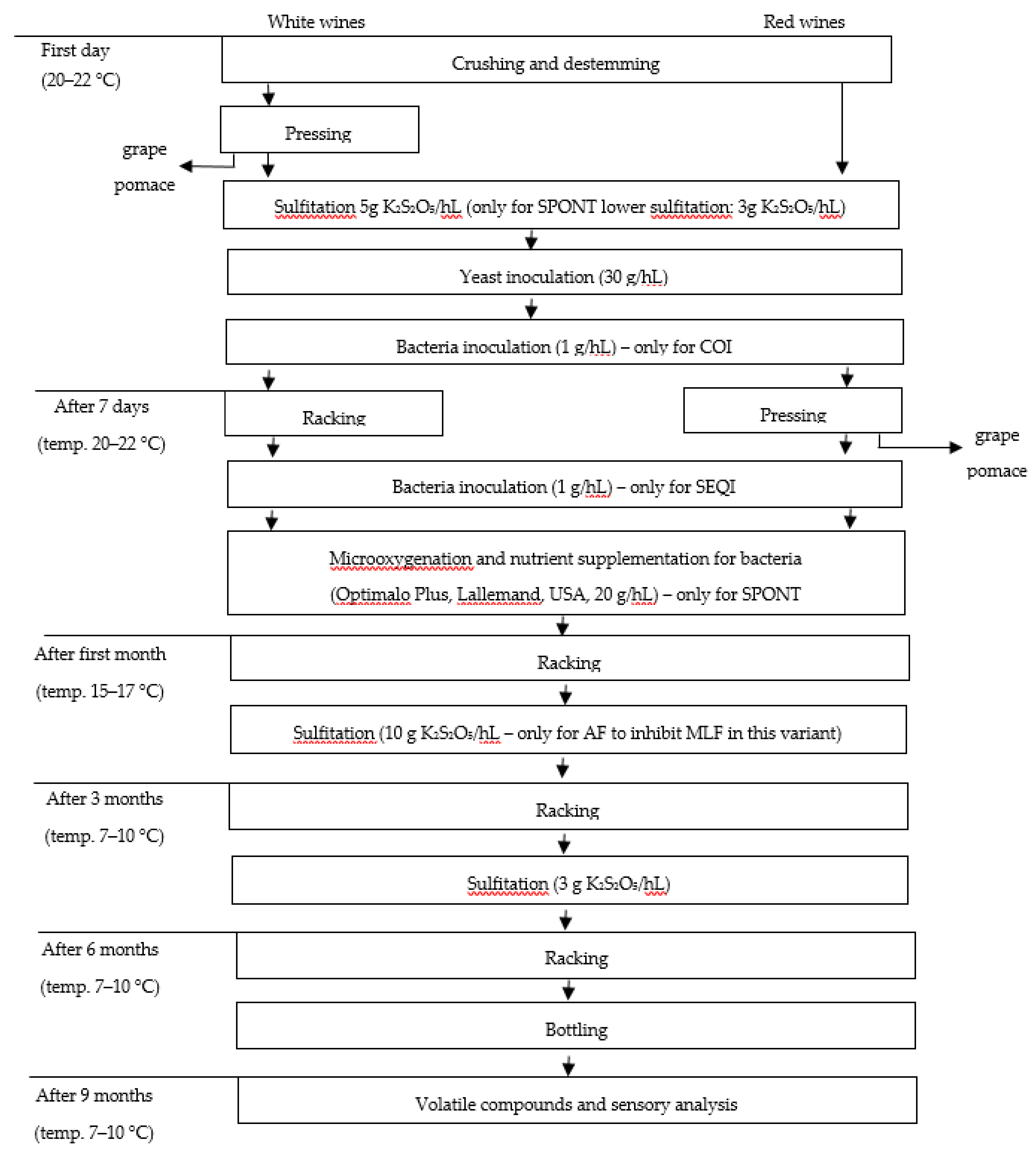
3.3. Parameters of the Vinification Process
3.4. Analysis of Volatile Compounds
3.5. Chemical Analysis of Must and Wine
3.6. Sensory Evaluation
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Davis, C.R.; Wibowo, D.; Eschenbruch, R.; Lee, T.H.; Fleet, G.H. Practical implications of malolactic fermentation: A review. Am. J. Enol. Vitic. 1985, 36, 290–301. [Google Scholar]
- Davis, C.R.; Wibowo, D.J.; Lee, T.H.; Fleet, G.H. Growth and metabolism of lactic acid bacteria during and after malolactic fermentation of wines at different pH. Appl. Environ. Microbiol. 1986, 51, 539–545. [Google Scholar] [PubMed]
- Wibowo, D.; Eschenbruch, R.; Davis, C.R.; Fleet, G.H.; Lee, T.H. Occurrence and growth of lactic acid bacteria in wine: A review. Am. J. Enol. Vitic. 1985, 36, 302–313. [Google Scholar]
- Henick-Kling, T. Malolactic fermentation. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Reading, UK, 1993; pp. 289–327. ISBN 0-415-27850-3. [Google Scholar]
- Versari, A.; Parpinello, G.P.; Cattaneo, M. Leuconostoc oenos and malolactic fermentation in wine: A review. J. Ind. Microbiol. Biotechnol. 1999, 23, 447–455. [Google Scholar] [CrossRef]
- Liu, S.Q. A review: Malolactic fermentation in wine—Beyond deacidification. J. Appl. Microbiol. 2002, 92, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Bartowsky, E.J.; Henschke, P.A. The ‘buttery’ attribute of wine—diacetyl—desirability, spoilage and beyond. Int. J. Food Microbiol. 2004, 96, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Antalick, G.; Perello, M.C.; de Revel, G. Characterization of fruity aroma modifications in red wines during malolactic fermentation. J. Agric. Food Chem. 2012, 60, 12371–12383. [Google Scholar] [CrossRef] [PubMed]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Krieger-Weber, S.; du Toit, M.; Rauhut, D. Impact of different malolactic fermentation inoculation scenarios on Riesling wine aroma. World J. Microbiol. Biotechnol. 2012, 28, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Tristezza, M.; di Feo, L.; Tufariello, M.; Grieco, F.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. Simultaneous inoculation of yeasts and lactic acid bacteria: Effects on fermentation dynamics and chemical composition of Negroamaro wine. Food Sci. Technol. 2016, 66, 406–412. [Google Scholar] [CrossRef]
- Abrahamse, C.E.; Bartowsky, E.J. Timing of malolactic fermentation inoculation in Shiraz grape must and wine: Influence on chemical composition. World J. Microbiol. Biotechnol. 2012, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo Canas, P.M.; Perez Martin, F.; Garcia Romero, E.; Sesena Prieto, S.; Palop Herreros, M.L. Influence of inoculation time of an autochthonous selected malolactic bacterium on volatile and sensory profile of Tempranillo and Merlot wines. Int. J. Food Microbiol. 2012, 156, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lasik-Kurdyś, M.; Gumienna, M.; Nowak, J. Influence of malolactic bacteria inoculation scenarios on the efficiency of the vinification process and the quality of grape wine from the Central European region. Eur. Food Res. Technol. 2017, 243, 2163–2173. [Google Scholar] [CrossRef]
- Maicas, S.; Gil, J.V.; Pardo, I.; Ferrer, S. Improvement of volatile composition of wines by controlled addition of malolactic bacteria. Food Rech. Int. 1999, 32, 491–496. [Google Scholar] [CrossRef]
- Pozo-Bayon, M.A.; Alegria, E.G.; Polo, M.C.; Tenorio, C.; Martin-Alvarez, P.J.; Calvo de la Banda, M.T.; Ruiz-Larrea, F.; Moreno-Arribas, M.V. Wine volatile and amino acid composition after malolactic fermentation: Effect of Oenococcus oeni and Lactobacillus plantarum Starter Cultures. J. Agric. Food Chem. 2005, 53, 8729–8735. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M.; Moio, L. Changes in the concentration of yeast-derived volatile compounds of red wine during malolactic fermentation with four commercial starter cultures of Oenococcus oeni. J. Agric. Food Chem. 2005, 53, 10134–10139. [Google Scholar] [CrossRef] [PubMed]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Rauhut, D.; du Toit, M. Influence of pH and ethanol on malolactic fermentation and volatile aroma compound composition in white wines. Food Sci. Technol. 2011, 44, 2077–2086. [Google Scholar] [CrossRef]
- Izquierdo Canas, P.M.; Garcia Romero, E.; Perez Martin, F.; Sesena Prieto, S.; Heras Manso, J.M.; Palop Herreros, M.L. Bahaviour during malolactic fermentation of three strains of Oenococcus oeni used as direct inoculation and acclimatization cultures. S. Afr. J. Enol. Vitic. 2013, 34, 1–9. [Google Scholar] [CrossRef][Green Version]
- Izquierdo Canas, P.M.; Mena Morales, A.; Garcia Romero, E. Malolactic fermentation before and during wine aging in barrels. Food Sci. Technol. 2016, 66, 468–474. [Google Scholar] [CrossRef]
- Sauvaugeot, F.; Vivier, P. Effects of malolactic fermentation on sensory properties of four Burgundy wines. Am. J. Enol. Vitic. 1997, 48, 187–192. [Google Scholar]
- Gambaro, A.; Boido, E.; Zlotejablko, A.; Medina, K.; Lloret, A.; Dellacassa, E.; Carrau, F. Effect of malolactic fermentation on the aroma properties of Tannat wine. Aust. J. Grape Wine Res. 2001, 7, 27–32. [Google Scholar] [CrossRef]
- Du Plessis, H.W.; Steger, C.L.; Du Toit, M.; Lambrechts, M.G. The occurrence of malolactic fermentation in brandy base wine and its influence on brandy quality. J. Appl. Microbiol. 2002, 92, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Ruiz-Perez, P.; Izquierdo Canas, P.M.; Sesena Prieto, S.; Garcia Romero, E.; Palop Herreros, M.I.I. Malolactic fermentation and secondary metabolite production by Oenococcus oeni strains in low pH wines. J. Food Sci. 2012, 77, 579–585. [Google Scholar] [CrossRef]
- Valade, M.; Laurent, M. La maitrise de la fermentation malolactique en champagne. Biologgia. Oggi. 1992, 6, 35–41. [Google Scholar]
- Lloret, A.; Boido, E.; Lorenzo, D.; Medina, K.; Carrau, F.; Dellacassa, E. Aroma variation in Tannat wines: Effect of malolactic fermentation on ethyl lactate level and its enantiomeric distribution. Ital. J. Food Sci. 2002, 14, 175–180. [Google Scholar]
- Fleet, G.H. The microorganisms of winemaking—solation, enumeration and identification. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Reading, UK, 1993; pp. 55–77. ISBN 0-415-27850-3. [Google Scholar]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Herjavec, S.; Tupajic, P.; Majdak, A. Influence of malolactic fermentation on the quality of Riesling wine. Agric. Conspec. Sci. 2001, 66, 59–64. [Google Scholar]
- Nielsen, J.C.; Richelieu, M. Control of Flavor Development in Wine during and after Malolactic Fermentation by Oenococcus oeni. Appl. Environm. Microbiol. 1999, 65, 740–745. [Google Scholar]
- Ribereau Gayon, J.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Lactic acid bacteria. In Handbook of enology: The Microbiology of Wine and Vinifications, 2nd ed.; Ribereau-Gayon, J., Dubourdieu, D., Doneche, B., Lonvaud, A., Eds.; Whiley: London, UK, 2006; Volume 1, pp. 115–138. ISBN 0-470-01034-7. [Google Scholar]
- Costello, P. The chemistry of malolactic fermentation. In Malolactic fermentation in wine—understanding the science and the practice; Morenzoni, R., Ed.; Lallemand: Montreal, QC, Canada, 2006; pp. 4.1–4.11. [Google Scholar]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional date. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Lerm, E.; Engelbrecht, L.; du Toit, M. Malolactic fermentation: The ABC’s of MLF. S. Afr. J. Enol. Vitic. 2010, 31, 186–212. [Google Scholar] [CrossRef]
- Romano, P.; Brandolini, V.; Ansaloni, C.; Menziani, E. The production of 2,3-butanediol as a differentiating character in wine yeasts. World J Microbiol. Biotechnol. 1998, 14, 649–653. [Google Scholar] [CrossRef]
- Martineau, B.; Henick-Kling, T. Performance and diacetyl production of commercial strains of malolactic bacteria in wine. J. Appl. Bacteriol. 1995, 78, 526–536. [Google Scholar] [CrossRef]
- Massera, A.; Soria, A.; Catania, C.; Krieger, S.; Combina, M. Simultaneous inoculation of Malbec (Vitis vinifera) musts with yeast and bacteria: effects on fermentation performance, sensory and sanitary attributes of wines. Food Technol. Biotechnol. 2009, 47, 192–201. [Google Scholar]
- Munoz, V.; Beccaria, B.; Abreo, E. Simultaneous and successive inoculations of yeasts and lactic acid bacteria on the fermentation of an unsulfited Tannat grape must. Braz. J. Microbiol. 2014, 45, 59–66. [Google Scholar] [CrossRef] [PubMed]
- International Organisation of Vine and Wine. Compendium of International Methods of Analysis of Wines and Musts, 2018. Available online: http://www.oiv.int/public/medias/5772/compendium-2018-en-vol1.pdf (accessed on January 2018).
Sample Availability: Samples of the compounds are not available from the authors. |

| pH | Total Acidity (g/L) * | Volatile Acidity (g/L) ** | Ethanol (% v/v) | Residual Sugars (g/L) | |||
|---|---|---|---|---|---|---|---|
| After 1 Week *** | 3 Months After Bottling | ||||||
| Chardonnay | |||||||
| White wines | AF | 3.35 ± 0.02 a | 7.15 ± 0.12 c | 0.75 ± 0.03 b | 12.5 ± 0.12 a | 17.53 ± 0.51 b | 5.08 ± 0.17 c |
| COI | 3.65 ± 0.04 c | 5.75 ± 0.09 a | 0.69 ± 0.04 a | 12.4 ± 0.16 a | 11.12 ± 0.55 a | 2.05 ± 0.53 a | |
| SEQI | 3.49 ± 0.06 b | 6.34 ± 0.11 b | 0.70 ± 0.01 a | 12.5 ± 0.19 a | 19.23 ± 0.42 c | 2.52 ± 0.23 a | |
| SPONT | 3.38 ± 0.04 a | 7.03 ± 0.14 c | 0.85 ± 0.03 c | 12.4 ± 0.13 a | 18.92 ± 0.31 c | 4.52 ± 0.41 b | |
| Kerling | |||||||
| AF | 3.26 ± 0.02 a | 7.35 ± 0.12 c | 0.66 ± 0.05 c | 11.2 ± 0.11 a | 16.71 ± 0.26 c | 4.71 ± 0.09 d | |
| COI | 3.58 ± 0.02 d | 6.12 ± 0.09 a | 0.41 ± 0.06 a | 11.1 ± 0.08 a | 14.39 ± 0.74 b | 2.09 ± 0.12 a | |
| SEQI | 3.42 ± 0.05 c | 7.17 ± 0.14 b | 0.49 ± 0.03 b | 11.0 ± 0.06 a | 12.43 ± 0.41 a | 2.41 ± 0.11 b | |
| SPONT | 3.37 ± 0.07 b | 7.19 ± 0.11 b | 0.77 ± 0.06 d | 11.2 ±0.13 a | 17.38 ± 0.55 d | 4.05 ± 0.18 c | |
| Red wines | Pinot noir A | ||||||
| AF | 3.71 ± 0.05 a | 5.69 ± 0.07 b | 0.44 ± 0.04 b | 13.2 ± 0.12 a | 18.18 ± 0.42 d | 4.78 ± 0.15 b | |
| COI | 4.00 ± 0.09 d | 5.06 ± 0.09 a | 0.53 ± 0.07 c | 13.1 ± 0.16 a | 12.53 ± 0.75 a | 2.14 ± 0.06 c | |
| SEQI | 3.90 ± 0.06 c | 5.77 ± 0.11 c | 0.39 ± 0.06 a | 13.3 ± 0.22 a | 16.87 ± 0.39 b | 2.37 ± 0.08 a | |
| SPONT | 3.81 ± 0.03 b | 5.52 ± 0.09 b | 0.69 ± 0.03 d | 13.3 ± 0.18 a | 17.94 ± 0.46 c | 3.94 ± 0.11 d | |
| Pinot noir B | |||||||
| AF | 3.52 ± 0.04 a | 6.48 ± 0.16 b | 0.41 ± 0.04 a | 12.1 ± 0.11 a | 19.62 ± 0.38 d | 5.08 ± 0.09 d | |
| COI | 3.81 ± 0.05 c | 5.81 ± 0.09 a | 0.42 ± 0.03 a | 12.1 ± 0.09 a | 13.93 ± 0.25 a | 3.12 ± 0.13 a | |
| SEQI | 3.75 ± 0.02 b | 5.94 ± 0.07 a | 0.47 ± 0.09 a | 12.0 ± 0.13 a | 17.12 ± 0.74 b | 3.80 ± 0.11 b | |
| SPONT | 3.58 ± 0.08 a | 6.44 ± 0.11 b | 0.66 ± 0.05 b | 12.0 ± 0.17 a | 18.27 ± 0.71 c | 4.44 ± 0.16 c | |
| Rondo | |||||||
| AF | 3.30 ± 0.03 a | 6.92 ± 0.12 c | 0.49 ± 0.04 a | 12.2 ± 0.14 a | 16.48 ± 0.52 d | 4.45 ± 0.19 c | |
| COI | 3.66 ± 0.05 d | 5.37 ± 0.13 a | 0.59 ± 0.04 b | 12.2 ± 0.12 a | 12.69 ± 0.84 a | 3.24 ± 0.12 a | |
| SEQI | 3.53 ± 0.03 c | 6.52 ± 0.06 b | 0.56 ± 0.09 b | 12.0 ± 0.06 a | 14.36 ± 0.51 b | 4.00 ± 0.15 b | |
| SPONT | 3.47 ± 0.07 b | 6.88 ± 0.12 c | 0.69 ± 0.07 c | 12.1 ± 0.26 a | 15.73 ± 0.73 c | 5.03 ± 0.13 d | |
| Ethyl Lactate | Ethyl Propanoate | Ethyl Hexanoate | Ethyl Octanoate | Diethyl Succinate | ||
|---|---|---|---|---|---|---|
| White wine | Chardonnay | |||||
| AF | 8.54 ± 0.31 d | 0.31 ± 0.03 b | 0.83 ± 0.07 a | 1.17 ± 0.09 a | 0.68 ± 0.01 d | |
| COI | 134.97 ± 3.93 a | 0.36 ± 0.05 a | 0.86 ± 0.04 a | 1.11 ± 0.09 a | 2.63 ± 0.02 a | |
| SEQ | 115.63 ± 5.59 b | 0.42 ± 0.04 a | 0.79 ± 0.09 a | 1.14 ± 0.04 a | 1.42 ± 0.06 b | |
| SPONT | 72.43 ± 2.64 c | 0.39 ± 0.05 a | 0.81 ± 0.05 a | 1.12 ± 0.06 a | 0.89 ± 0.03 c | |
| Kerling | ||||||
| AF | 8.62 ± 0.68 d | 0.14 ± 0.04 b | 0.53 ± 0.03 a | 0.88 ± 0.05 a | 0.32 ± 0.05 d | |
| COI | 132.57 ± 4.04 a | 0.27 ± 0.07 a | 0.57 ± 0.05 a | 0.93 ± 0.04 a | 1.14 ± 0.03 a | |
| SEQI | 111.66 ± 5.84 b | 0.23 ± 0.03 a | 0.53 ± 0.04 a | 0.89 ± 0.06 a | 0.55 ± 0.05 b | |
| SPONT | 71.04 ± 3.61 c | 0.21 ± 0.04 a | 0.55 ± 0.04 a | 0.85 ± 0.08 a | 0.49 ± 0.05 c | |
| Red wine | Pinot noir A | |||||
| AF | 14.44 ± 0.86 d | 0.51 ± 0.03 b | 0.74 ± 0.06 a | 1.23 ± 0.04 b | 0.54 ± 0.03 d | |
| COI | 151.25 ± 4.23 a | 0.73 ± 0.05 a | 0.77 ± 0.07 a | 1.31 ± 0.03 a | 2.07 ± 0.09 a | |
| SEQI | 97.32 ± 6.59 b | 0.77 ± 0.03 a | 0.74 ± 0.05 a | 1.26 ± 0.02 b | 1.32 ± 0.07 b | |
| SPONT | 64.97 ± 4.44 c | 0.74 ± 0.03 a | 0.71 ± 0.05 a | 1.22 ± 0.05 b | 0.76 ± 0.06 c | |
| Pinot noir B | ||||||
| AF | 11.43 ±0.41 d | 0.39 ± 0.03 b | 0.66 ± 0.08 a | 1.08 ± 0.06 a | 0.47 ± 0.03 d | |
| COI | 173.76 ± 5.72 a | 0.62 ± 0.03 a | 0.71 ± 0.04 a | 1.14 ± 0.08 a | 1.96 ± 0.07 a | |
| SEQI | 91.26 ± 3.66 b | 0.60 ± 0.04 a | 0.68 ± 0.05 a | 1.09 ± 0.05 a | 0.88 ± 0.05 b | |
| SPONT | 65.33 ± 3.43 c | 0.61 ± 0.03 a | 0.65 ± 0.06 a | 1.06 ± 0.09 a | 0.61 ± 0.07 c | |
| Rondo | ||||||
| AF | 9.01 ± 0.56 d | 0.27 ± 0.03 b | 0.64 ± 0.03 a | 0.94 ± 0.05 a | 0.51 ± 0.06 d | |
| COI | 137.41 ± 6.92 a | 0.34 ± 0.04 a | 0.69 ± 0.04 a | 0.99 ± 0.04 a | 1.88 ± 0.11 a | |
| SEQI | 84.78 ± 4.47 b | 0.38 ± 0.02 a | 0.66 ± 0.03 a | 0.93 ± 0.07 a | 1.12 ± 0.08 b | |
| SPONT | 41.64 ± 4.33 c | 0.35 ± 0.03 a | 0.62 ± 0.07 a | 0.93 ± 0.08 a | 0.74 ± 0.06 c |
| Ethyl Acetate | Isoamyl Acetate | 2-Phenethyl Acetate | Sum of Esters Total/Without Ethyl Lactate and Ethyl Acetate | ||
|---|---|---|---|---|---|
| White wine | Chardonnay | ||||
| AF | 63.43 ± 1.27 c | 0.56 ± 0.07 b | 0.23 ± 0.01 b | 75.75/3.78 | |
| COI | 51.67 ± 2.03 d | 0.42 ± 0.04 c | 0.69 ± 0.03 a | 192.71/6.07 | |
| SEQI | 68.44 ± 3.16 b | 0.63 ± 0.08 b | 0.62 ± 0.08 a | 189.09/5.02 | |
| SPONT | 88.41 ± 2.87 a | 0.89 ± 0.03 a | 0.73 ± 0.05 a | 165.67/4.83 | |
| Kerling | |||||
| AF | 72.66 ± 3.016 b | 0.66 ± 0.02 b | 0.33 ± 0.02 b | 84.14/2.86 | |
| COI | 58.12 ± 5.37 d | 0.49 ± 0.04 c | 0.49 ± 0.07 a | 194.58/3.89 | |
| SEQI | 63.27 ± 5.82 c | 0.51 ± 0.03 c | 0.52 ± 0.03 a | 178.16/3.23 | |
| SPONT | 110.26 ± 4.93 a | 0.94 ± 0.03 a | 0.55 ± 0.04 a | 184.89/3.59 | |
| Red wine | Pinot noir A | ||||
| AF | 77.42 ± 3.28 c | 0.33 ± 0.05 c | 0.45 ± 0.03 b | 95.66/3.8 | |
| COI | 71.33 ± 6.71 c | 0.28 ± 0.06 c | 0.92 ± 0.03 a | 228.66/6.08 | |
| SEQI | 81.58 ± 4.55 b | 0.44 ± 0.02 b | 0.88 ± 0.04 a | 184.31/5.41 | |
| SPONT | 94.11 ± 5.19 a | 0.76 ± 0.03 a | 0.94 ± 0.06 a | 164.21/5.13 | |
| Pinot noir B | |||||
| AF | 69.31 ± 6.07 c | 0.66 ± 0.09 b | 0.37 ± 0.03 b | 84.37/3.63 | |
| COI | 66.93 ± 4.31 c | 0.47 ± 0.03 c | 0.84 ± 0.07 a | 246.43/5.74 | |
| SEQI | 75.21 ± 4.89 b | 0.58 ± 0.09 b | 0.79 ± 0.09 a | 171.09/4.62 | |
| SPONT | 88.13 ± 5.26 a | 0.83 ± 0.04 a | 0.87 ± 0.07 a | 158.09/4.63 | |
| Rondo | |||||
| AF | 79.17 ± 4.11 c | 0.31 ± 0.02 c | 0.41 ± 0.02 b | 91.26/3.08 | |
| COI | 78.33 ± 4.85 c | 0.44 ± 0.07 b | 0.83 ± 0.05 a | 220.91/5.17 | |
| SEQI | 86.87 ± 3.17 b | 0.38 ± 0.06 b | 0.81 ± 0.09 a | 175.93/4.28 | |
| SPONT | 119.33 ± 4.69 a | 0.79 ± 0.03 a | 0.88 ± 0.05 a | 165.28/4.31 |
| 2,3-Butanedione (Diacetyl) | 3-Hydroxy-2-Butanone (Acetoin) | 2,3-Butanediol | ||
|---|---|---|---|---|
| White wines | Chardonnay | |||
| AF | 1.13 ± 0.22 d | 0.86 ± 0.06 d | 254.32 ± 11.34 d | |
| COI | 2.19 ± 0.15 c | 3.11 ± 0.39 c | 331.56 ± 15.33 c | |
| SEQI | 3.42 ± 0.19 b | 5.95 ± 0.26 b | 479.93 ± 9.14 b | |
| SPONT | 7.44 ± 0.08 a | 8.79 ± 0.42 a | 712.42 ± 23.65 a | |
| Kerling | ||||
| AF | 0.94 ± 0.24 d | 0.73 ± 0.04 d | 165.36 ± 12.75 d | |
| COI | 3.02 ± 0.16 c | 4.31 ± 0.22 c | 349.62 ± 16.83 c | |
| SEQI | 4.09 ± 0.08 b | 6.18 ± 0.42 b | 513.88 ± 8.31 b | |
| SPONT | 8.33 ± 0.22 a | 11.05 ± 0.98 a | 783.63 ± 10.63 a | |
| Red wines | Pinot noir A | |||
| AF | 1.71 ± 0.15 d | 1.62 ± 0.17 d | 288.97 ± 12.06 d | |
| COI | 3.80 ± 0.19 c | 5.11 ± 0.36 c | 361.35 ± 9.72 c | |
| SEQI | 5.24 ± 0.16 b | 6.94 ± 0.45 b | 647.55 ± 17.83 b | |
| SPONT | 9.22 ± 0.23 a | 12.39 ± 0.41 a | 806.37 ± 14.97 a | |
| Pinot noir B | ||||
| AF | 1.31 ± 0.14 d | 1.04 ± 0.12 c | 267.42 ± 8.53 d | |
| COI | 4.06 ± 0.09 c | 6.48 ± 0.48 b | 493.52 ± 11.84 c | |
| SEQI | 5.91 ± 0.11 b | 7.17 ± 0.56 b | 631.67 ± 17.59 b | |
| SPONT | 8.72 ± 0.15 a | 12.52 ± 0.46 a | 718.13 ± 13.77 a | |
| Rondo | ||||
| AF | 1.72 ± 0.05 d | 1.53 ± 0.14 d | 264.94 ± 6.48 d | |
| COI | 3.81 ± 0.09 c | 4.97 ± 0.32 c | 355.13 ± 15.83 c | |
| SEQI | 5.50 ± 0.13 b | 7.03 ± 0.26 b | 584.42 ± 18.28 b | |
| SPONT | 8.80 ± 0.07 a | 11.43 ± 0.31 a | 652.36 ± 15.75 a |
| Chardonnay | Kerling | Pinot Noir A | Pinot Noir B | Rondo | |
|---|---|---|---|---|---|
| °Bx | 22.5 ± 0.1 | 20.5 ± 0.1 | 24.0 ± 0.2 | 22.0 ± 0.1 | 22.0 ± 0.2 |
| Sugars: glucose + fructose (g/L) | 193.12 ± 2.11 | 171.33 ± 2.54 | 203.54 ± 3.05 | 185.47 ± 3.17 | 181.62 ± 2.84 |
| pH | 3.31 ± 0.03 | 3.19 ± 0.03 | 3.64 ± 0.05 | 3.41 ± 0.06 | 3.26 ± 0.07 |
| Total acidity (g/L) * | 11.64 ± 0.12 | 12.14 ± 0.14 | 9.38 ± 0.09 | 10.95 ± 0.23 | 11.52 ± 0.26 |
| Malic acid (g/L) | 8.14 ± 0.09 | 9.5 ± 0.05 | 6.54 ± 0.07 | 8.11 ± 0.14 | 7.83 ± 0.21 |
| Citric acid (g/L) | 0.33 ± 0.02 | 0.41 ± 0.02 | 0.28 ± 0.02 | 0.35 ± 0.02 | 0.31 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasik-Kurdyś, M.; Majcher, M.; Nowak, J. Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines. Molecules 2018, 23, 2549. https://doi.org/10.3390/molecules23102549
Lasik-Kurdyś M, Majcher M, Nowak J. Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines. Molecules. 2018; 23(10):2549. https://doi.org/10.3390/molecules23102549
Chicago/Turabian StyleLasik-Kurdyś, Małgorzata, Małgorzata Majcher, and Jacek Nowak. 2018. "Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines" Molecules 23, no. 10: 2549. https://doi.org/10.3390/molecules23102549
APA StyleLasik-Kurdyś, M., Majcher, M., & Nowak, J. (2018). Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines. Molecules, 23(10), 2549. https://doi.org/10.3390/molecules23102549






