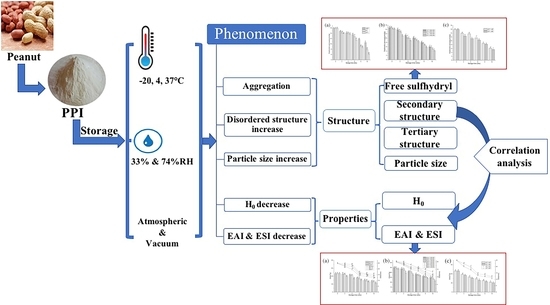
The Molecular Properties of Peanut Protein: Impact of Temperature, Relative Humidity and Vacuum Packaging during Storage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterzation of PPI
2.1.1. Free Sulfhydryl Content
2.1.2. Secondary Structure Content Determination
2.1.3. Fluorescence Spectroscopy
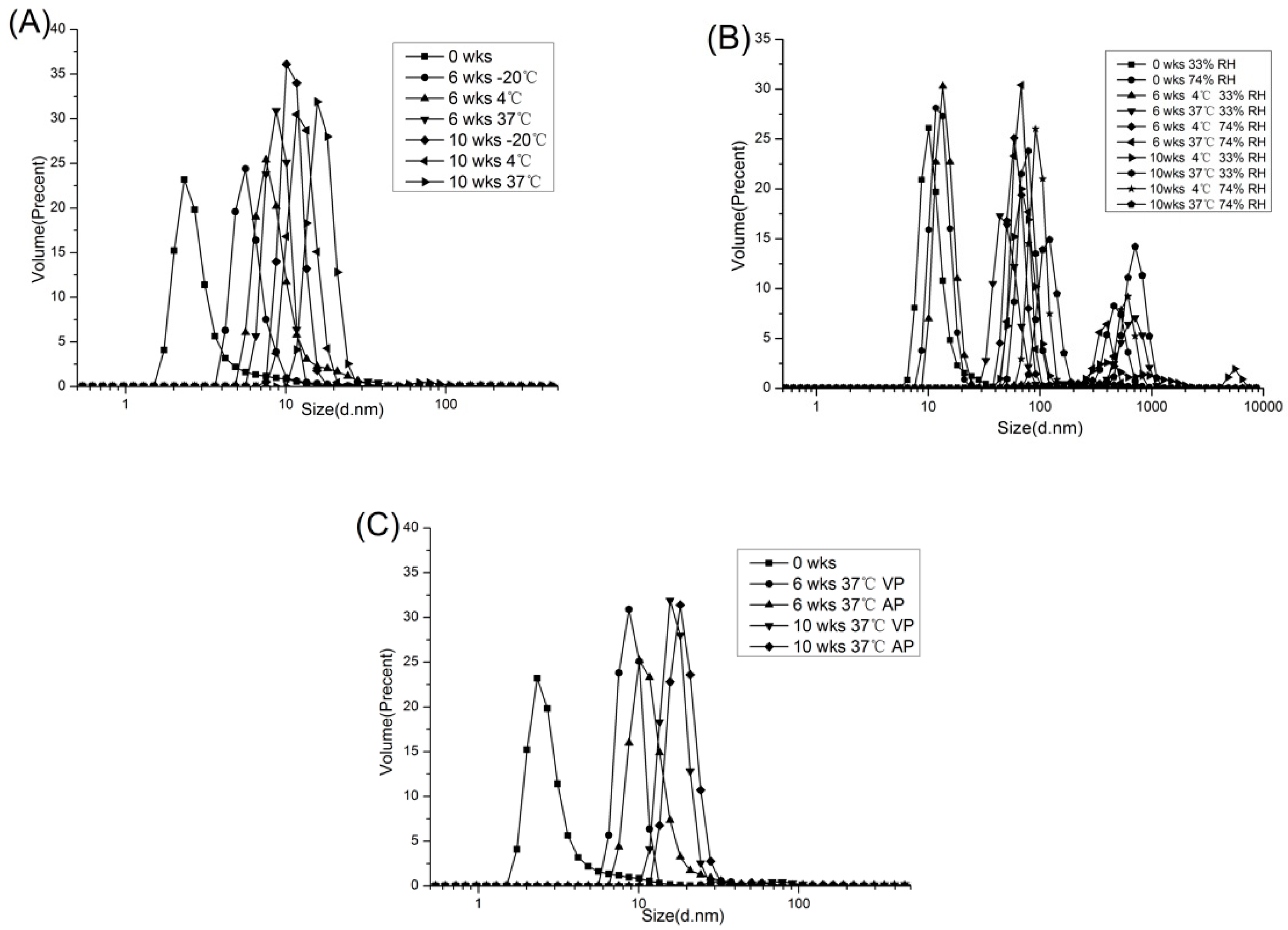
2.1.4. Distribution of Particle Size
2.2. Molecular Properties of PPI
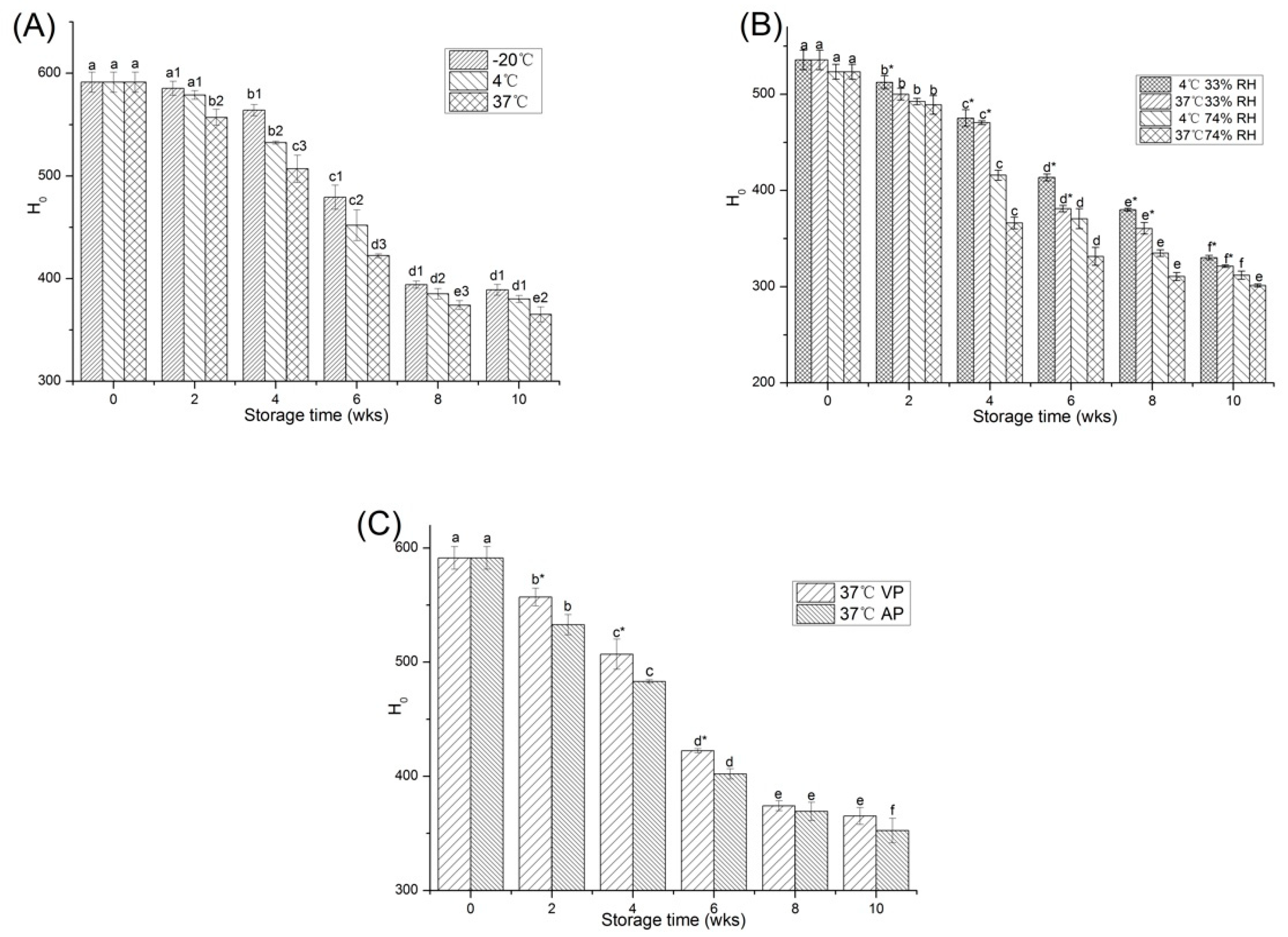
2.2.1. Surface Hydrophobicity
2.2.2. Emulsifying Properties
2.3. Correlation Analysis
3. Materials and Methods
3.1. Materials
3.2. Storage of PPI
3.3. Structural Characterization
3.3.1. Determination of Free Sulfhydryl Content
3.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.3. Fluorescence spectroscopy
3.3.4. Dynamic Light Scattering (DLS) Measurement
3.3.5. Determination of Surface Hydrophobicity (H0)
3.3.6. Determination of Emulsifying Properties
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gong, K.J.; Shi, A.M.; Liu, H.Z.; Liu, L.; Hu, H.; Adhikari, B. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016, 170, 33–40. [Google Scholar] [CrossRef]
- He, X.H.; Liu, H.Z.; Liu, L.; Zhao, G.L.; Wang, Q.; Chen, Q.L. Effects of high pressure on the physicochemical and functional properties of peanut protein isolates. Food Hydrocolloid. 2014, 36, 123–129. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Peanut protein concentrate: Production and functional properties as affected by processing. Food Chem. 2007, 103, 121–129. [Google Scholar] [CrossRef]
- Shih, M.C.; Hwang, T.S.; Chou, H.Y. Physicochemical and functional property changes in soy protein isolates stored under high relative humidity and temperature. J. Food Sci. Technol. 2016, 53, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Huang, M.; Wang, J.; Xu, Q.; Hammad, H.H.M.; Ma, M. A study of storage impact on ovalbumin structure of chicken egg. J. Food Eng. 2018, 219, 1–7. [Google Scholar] [CrossRef]
- Wang, P.; Han, T.; Wu, F.; Yang, N.; Chen, F.; Jin, Z. Effect of frozen storage on the foaming properties of wheat gliadin. Food Chem. 2014, 164, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kehrberg, N.L.; Johnson, J.M. Storage stability of dried sweet cheese whey. J. Food Sci. 2010, 40, 644–646. [Google Scholar] [CrossRef]
- Zakrys-Waliwander, P.I.; O’Sullivan, M.G.; O’Neill, E.E. The effects of high oxygen modified atmosphere packaging on protein oxidation of bovine m. longissimus dorsi, muscle during chilled storage. Food Chem. 2012, 131, 527–532. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, C.; Kong, X.; Hua, Y. Oxidative modification of soy protein by peroxyl radicals. Food Chem. 2009, 116, 295–301. [Google Scholar] [CrossRef]
- Davies, M.J. The oxidative environment and protein damage. BBA-Proteins Proteom. 2005, 1703, 93–109. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, H.; Li, L.; Yang, X.; Hao, S.; Chen, S. The effects of modified atmosphere packaging and enzyme inhibitors on protein oxidation of tilapia muscle during iced storage. LWT-Food Sci. Technol. 2017, 87, 186–193. [Google Scholar] [CrossRef]
- Zaritzky, N. Physical–chemical principles in freezing. CRC Press. 2012, 155, 3–38. [Google Scholar]
- Guo, F.; Xiong, Y.L.; Qin, F.; Jian, H.; Huang, X.; Chen, J. Examination of the causes of instability of soy protein isolate during storage through probing of the heat-Induced aggregation. J. Am. Oil Chem. Soc. 2015, 92, 1075–1084. [Google Scholar] [CrossRef]
- Sun, B.Y.; Shi, Y.G.; Su, C.; Liu, H.H. Effect of high temperature and humidity in storage on component and oil absorption of soy protein isolate. Adv. Mater. Res. 2010, 113, 416–419. [Google Scholar] [CrossRef]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Jongberg, S.; Andersen, M.L.; Skibsted, L.H. Thiol oxidation and protein cross-link formation during chill storage of pork patties added essential oil of oregano, rosemary, or garlic. Meat Sci. 2013, 95, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lu, Y.; Han, J.; Chen, Q.; Kong, B. Structure-modification by moderate oxidation in hydroxyl radical-generating systems promote the emulsifying properties of soy protein isolate. Food Struct. 2015, 6, 21–28. [Google Scholar] [CrossRef]
- Jackson, M.; Mantsch, H.H. The use and misuse of ftir spectroscopy in the determination of protein structure. Crit. Crit. Rev. Biochem. 1995, 30, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Segat, A.; Misra, N.N.; Fabbro, A.; Buchini, F.; Lippe, G.; Cullen, P.J. Effects of ozone processing on chemical, structural and functional properties of whey protein isolate. Food Res. Int. 2014, 66, 365–372. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Z.; Han, D.; Li, Y.; Sun, X.; Wang, Z. Structural and functional properties changes of β-conglycinin exposed to hydroxyl radical-generating systems. Molecules 2017, 22, 1893. [Google Scholar] [CrossRef] [PubMed]
- Safonova, O.N.; Kholodova, E.A.; Golota, V.I. Ozone usage for adjustment of technological properties of wheat baking flour. In Proceedings of the 11th International Congress on Engineering and Food, Athens, Greece, 22–26 May 2011; pp. 1–6. [Google Scholar]
- Lefèvre, T.; Subirade, M. Structural and interaction properties of β-lactoglobulin as studied by FTIR spectroscopy. Int. J. Food Sci. Technol. 2010, 34, 419–428. [Google Scholar] [CrossRef]
- Nishanthi, M.; Chandrapala, J.; Vasiljevic, T. Properties of whey protein concentrate powders obtained by spray drying of sweet, salty and acid whey under varying storage conditions. J. Food Eng. 2017, 214, 137–146. [Google Scholar] [CrossRef]
- Keerati-U.-Rai, M.; Miriani, M.; Iametti, S.; Bonomi, F.; Corredig, M. Structural changes of soy proteins at the oil-water interface studied by fluorescence spectroscopy. Colloids Surf. B Biointerfaces 2012, 93, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Katekhong, W.; Charoenrein, S. Changes in physical and gelling properties of freeze-dried egg white as a result of temperature and relative humidity. J. Sci. Food Agric. 2016, 96, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhao, M.; Sun, W.; Ren, J.; Cui, C. Effect of oxidation on the emulsifying properties of soy protein isolate. Food Res. Int. 2013, 52, 26–32. [Google Scholar] [CrossRef]
- Martins, V.B.; Netto, F.M. Physicochemical and functional properties of soy protein isolate as a function of water activity and storage. Food Res. Int. 2006, 39, 145–153. [Google Scholar] [CrossRef]
- Liu, W.R.; Langer, R.; Klibanov, A.M. Moisture-induced aggregation of lyophilized proteins in the solid state. Biotechnol. Bioeng. 1991, 37, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, C.; Ullah, N.; Cao, J.; Lan, Y.; Ge, W.; Hackman, R.M.; Li, Z.; Chen, L. Susceptibility of whey protein isolate to oxidation and changes in physicochemical, structural, and digestibility characteristics. J. Dairy Sci. 2015, 98, 7602–7613. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, C.; Hua, Y. Structural modification of soy protein by the lipid peroxidation product malondialdehyde. J. Sci. Food Agric. 2010, 43, 133–140. [Google Scholar] [CrossRef]
- Graaf, L.A.D. Denaturation of proteins from a non-food perspective. J. Biotechnol. 2000, 79, 299–306. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L.; Chen, J. Role of β-conglycinin and glycinin subunits in the pH-shifting-induced structural and physicochemical changes of soy protein isolate. J. Food Sci. 2011, 76, 293. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Tu, Z.C.; Xiao, H.; Wang, H.; Huang, X.Q.; Liu, G.X. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food Bioprod. Process. 2014, 92, 30–37. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Agyare, K.K.; Addo, K. Hydrolyzed wheat gluten suppresses transglutaminase-mediated gelation but improves emulsification of pork myofibrillar protein. Meat Sci. 2008, 80, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tian, Z.G.; Chen, L.Y. Effects of deamidation on structure and functional properties of barley hordein. J. Agric. Food Chem. 2010, 58, 11448–11455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Zhou, C.; Atungulu, G.G.; Xu, K.; Ma, H. Surface topography, nano-mechanics and secondary structure of wheat gluten pretreated by alternate dual-frequency ultrasound and the correlation to enzymolysis. Ultrason.-Sonochem. 2016, 31, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.; Toma, S.J.; Nakai, S. Determination of sh- and ss-groups in some food proteins using ellman’s reagent. J. Food Sci. 1974, 39, 49–51. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wang, Z.J.; Handa, C.L.; Xu, J. Effects of ultrasound pre-treatment on the structure of β-conglycinin and glycincin and the antioxidant activity of their hydrolysates. Food Chem. 2017, 218, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hua, Y.; Qiu, A. Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Res. Int. 2006, 39, 240–249. [Google Scholar] [CrossRef]
- Chang, S.K.C. Protein Analysis; Springer: New York, NY, USA, 2010; pp. 133–146. ISBN 9780817643409. [Google Scholar]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of protein: Evaluation of turbidmetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds peanut protein isolate and stored peanut protein isolate are available from the authors. |



| Temperature (°C) | Time (Weeks) | ||
|---|---|---|---|
| 0 | 6 | 10 | |
| α-Helix (%) | |||
| −20 °C | 14.76 ± 0.04 a | x 14.58 ± 0.07 b | x 14.35 ± 0.05 c |
| 4 °C | 14.76 ± 0.04 a | xy 14.43 ± 0.09 b | x 14.31 ± 0.07 b |
| 37 °C | 14.76 ± 0.04 a | y 14.39 ± 0.07 b | x 14.29 ± 0.03 c |
| 37 °C AP | 14.76 ± 0.04 a | * 14.24 ± 0.05 b | * 14.18 ± 0.07 b |
| β-Sheet (%) | |||
| −20 °C | 36.76 ± 0.07 a | x 36.41 ± 0.08 b | x 35.60 ± 0.08 c |
| 4 °C | 36.76 ± 0.07 a | y 36.11 ± 0.05 b | x 35.51 ± 0.06 c |
| 37 °C | 36.76 ± 0.07 a | z 35.95 ± 0.04 b | y 35.31 ± 0.04 c |
| 37 °C AP | 36.76 ± 0.07 a | * 35.62 ± 0.04 b | * 35.17 ± 0.03 c |
| β-Turn (%) | |||
| −20 °C | 34.23 ± 0.07 c | z 34.70 ± 0.10 b | y 35.37 ± 0.08 a |
| 4 °C | 34.23 ± 0.07 c | y 35.01 ± 0.09 b | xy 35.41 ± 0.10 a |
| 37 °C | 34.23 ± 0.07 c | x 35.16 ± 0.05 b | x 35.58 ± 0.08 a |
| 37 °C AP | 34.23 ± 0.07 c | * 35.43 ± 0.04 b | * 35.80 ± 0.07 a |
| Random Coil (%) | |||
| −20 °C | 14.25 ± 0.04 b | y 14.31 ± 0.03 b | y 14.68 ± 0.05 a |
| 4 °C | 14.25 ± 0.04 c | x 14.45 ± 0.06 b | x 14.77 ± 0.03 a |
| 37 °C | 14.25 ± 0.04 c | x 14.50 ± 0.03 b | x 14.82 ± 0.02 a |
| 37 °C AP | 14.25 ± 0.04 c | * 14.71 ± 0.05 b | 14.85 ± 0.04 a |
| Temperature (°C) | Time (Weeks) | ||
|---|---|---|---|
| 0 | 6 | 10 | |
| α-Helix (%) | |||
| 4 °C 33% RH | 14.69 ± 0.08 a | * 14.48 ± 0.07 b | * 14.33 ± 0.05 b |
| 37 °C 33% RH | 14.69 ± 0.08 a | 14.35 ± 0.08 b | * 14.27 ± 0.08 b |
| 4 °C 74% RH | 14.59 ± 0.10 a | 14.30 ± 0.04 b | 14.16 ± 0.07 b |
| 37 °C 74% RH | 14.59 ± 0.10 a | 14.19 ± 0.10 b | 14.10 ± 0.03 b |
| β-Sheet (%) | |||
| 4 °C 33% RH | 36.60 ± 0.09 a | *36.03 ± 0.08 b | * 35.35 ± 0.04 c |
| 37 °C 33% RH | 36.60 ± 0.09 a | *35.74 ± 0.04 b | * 35.19 ± 0.05 c |
| 4 °C 74% RH | 36.51 ± 0.08 a | 35.47 ± 0.08 b | 34.96 ± 0.05 c |
| 37 °C 74% RH | 36.51 ± 0.08 a | 35.26 ± 0.09 b | 34.47 ± 0.08 c |
| β-Turn (%) | |||
| 4 °C 33% RH | * 34.41 ± 0.09 a | * 35.13 ± 0.10 b | * 35.83 ± 0.06 c |
| 37 °C 33% RH | * 34.41 ± 0.09 a | * 35.50 ± 0.06 b | * 36.02 ± 0.05 c |
| 4 °C 74% RH | 34.55 ± 0.08 a | 35.74 ± 0.07 b | 36.33 ± 0.09 c |
| 37 °C 74% RH | 34.55 ± 0.08 a | 35.98 ± 0.08 b | 36.74 ± 0.07 c |
| Random Coil (%) | |||
| 4 °C 33% RH | 14.30 ± 0.08 a | * 14.36 ± 0.07 ab | 14.49 ± 0.04 b |
| 37 °C 33% RH | 14.30 ± 0.08 a | * 14.41 ± 0.06 ab | * 14.52 ± 0.07 b |
| 4 °C 74% RH | 14.35 ± 0.10 a | 14.49 ± 0.06 ab | 14.55 ± 0.04 b |
| 37 °C 74% RH | 14.35 ± 0.10 a | 14.57 ± 0.10 b | 14.69 ± 0.07 b |
| α-Helix | β-Sheet | β-Turn | Random | EAI | ESI | H0 | |
|---|---|---|---|---|---|---|---|
| α-Helix | 1 | ||||||
| β-Sheet | 0.986 | 1 | |||||
| β-Turn | −0.995 * | −0.997 * | 1 | ||||
| Random | −0.968 | −0.994 * | 0.983 | 1 | |||
| EAI | 0.993 | 0.989 | −0.996 * | −0.969 | 1 | ||
| ESI | 0.974 | 0.969 | −0.980 | −0.945 | 0.985 | 1 | |
| H0 | 0.997 * | 0.986 | −0.994 | −0.968 | 0.994 | 0.979 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Jin, H.; Li, Y.; Feng, H.; Liu, C.; Xu, J. The Molecular Properties of Peanut Protein: Impact of Temperature, Relative Humidity and Vacuum Packaging during Storage. Molecules 2018, 23, 2618. https://doi.org/10.3390/molecules23102618
Sun X, Jin H, Li Y, Feng H, Liu C, Xu J. The Molecular Properties of Peanut Protein: Impact of Temperature, Relative Humidity and Vacuum Packaging during Storage. Molecules. 2018; 23(10):2618. https://doi.org/10.3390/molecules23102618
Chicago/Turabian StyleSun, Xiaotong, Hua Jin, Yangyang Li, Haiying Feng, Chunhong Liu, and Jing Xu. 2018. "The Molecular Properties of Peanut Protein: Impact of Temperature, Relative Humidity and Vacuum Packaging during Storage" Molecules 23, no. 10: 2618. https://doi.org/10.3390/molecules23102618
APA StyleSun, X., Jin, H., Li, Y., Feng, H., Liu, C., & Xu, J. (2018). The Molecular Properties of Peanut Protein: Impact of Temperature, Relative Humidity and Vacuum Packaging during Storage. Molecules, 23(10), 2618. https://doi.org/10.3390/molecules23102618