1,4-β-d-Glucomannan from Dendrobium officinale Activates NF-кB via TLR4 to Regulate the Immune Response
Abstract
:1. Introduction
2. Results
2.1. Carbohydrate and Protein Contents of Crude and Pure O-Acetylated Glucomannan
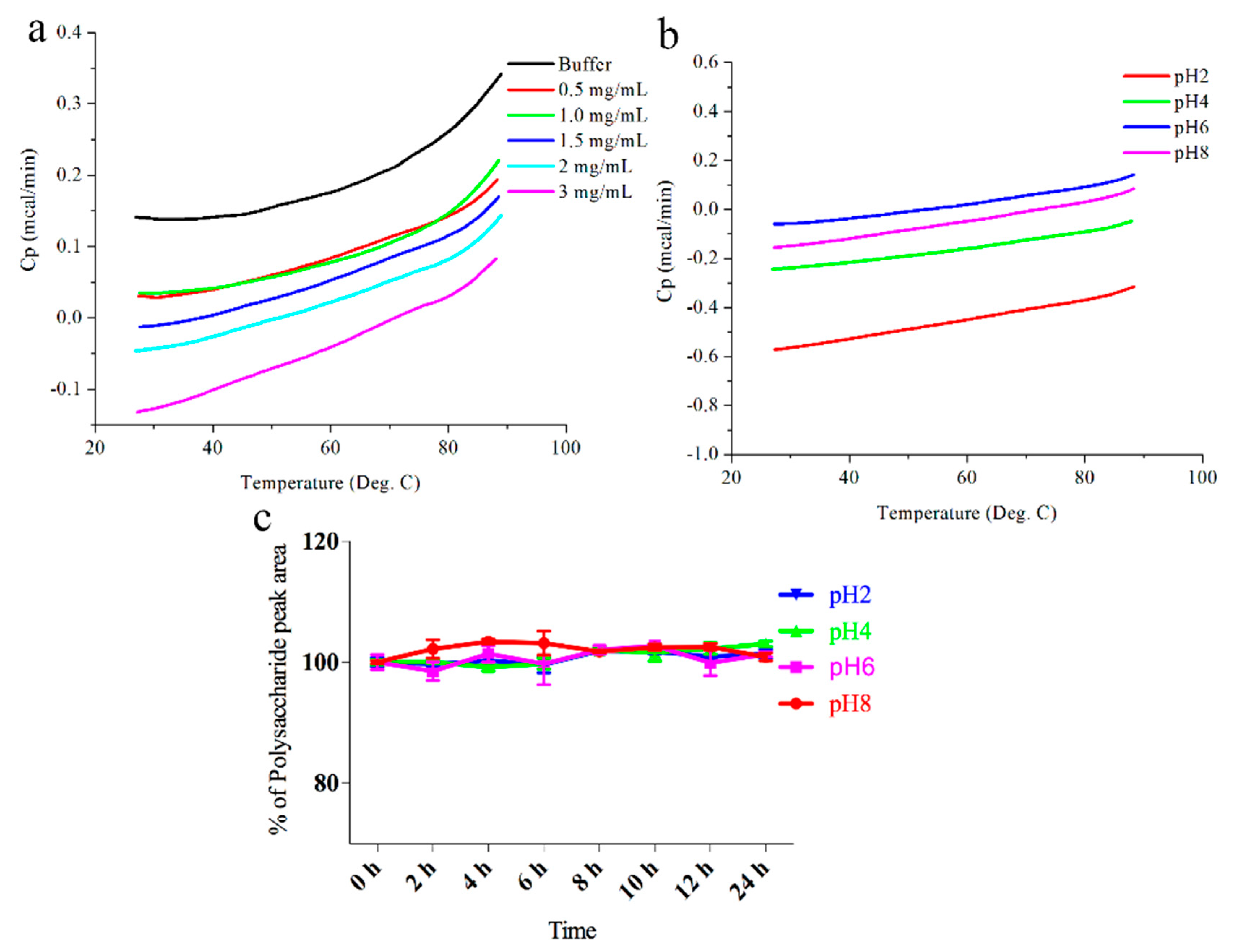
2.2. Stability of DOP-1-1 under Different pH Conditions
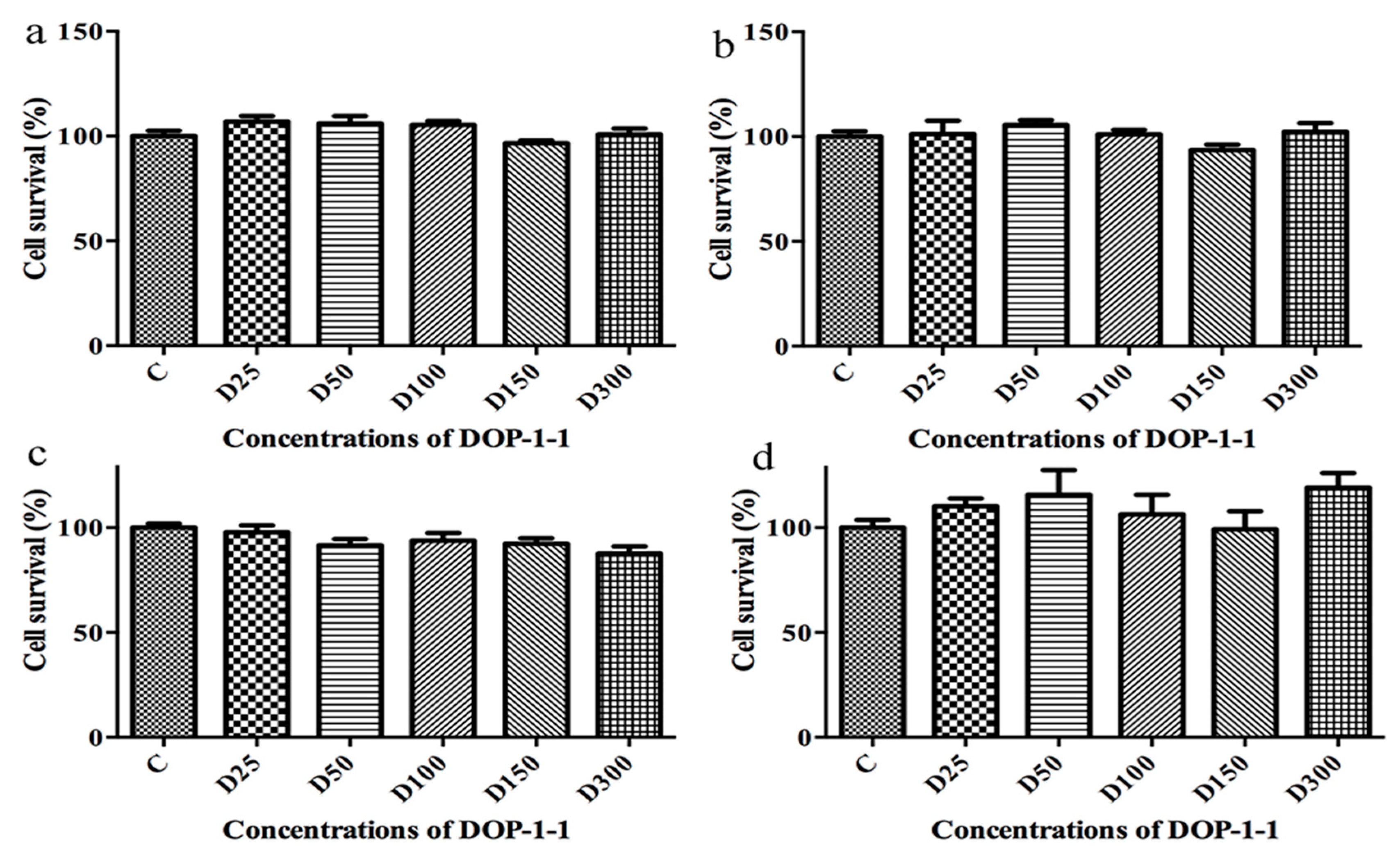
2.3. Toxicity of DOP-1-1 to THP-1 Monocytes
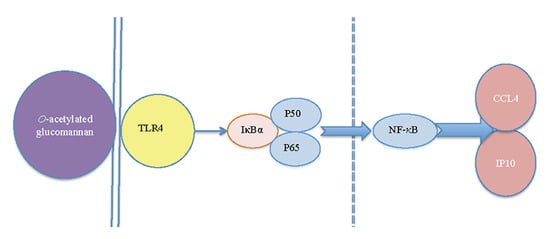
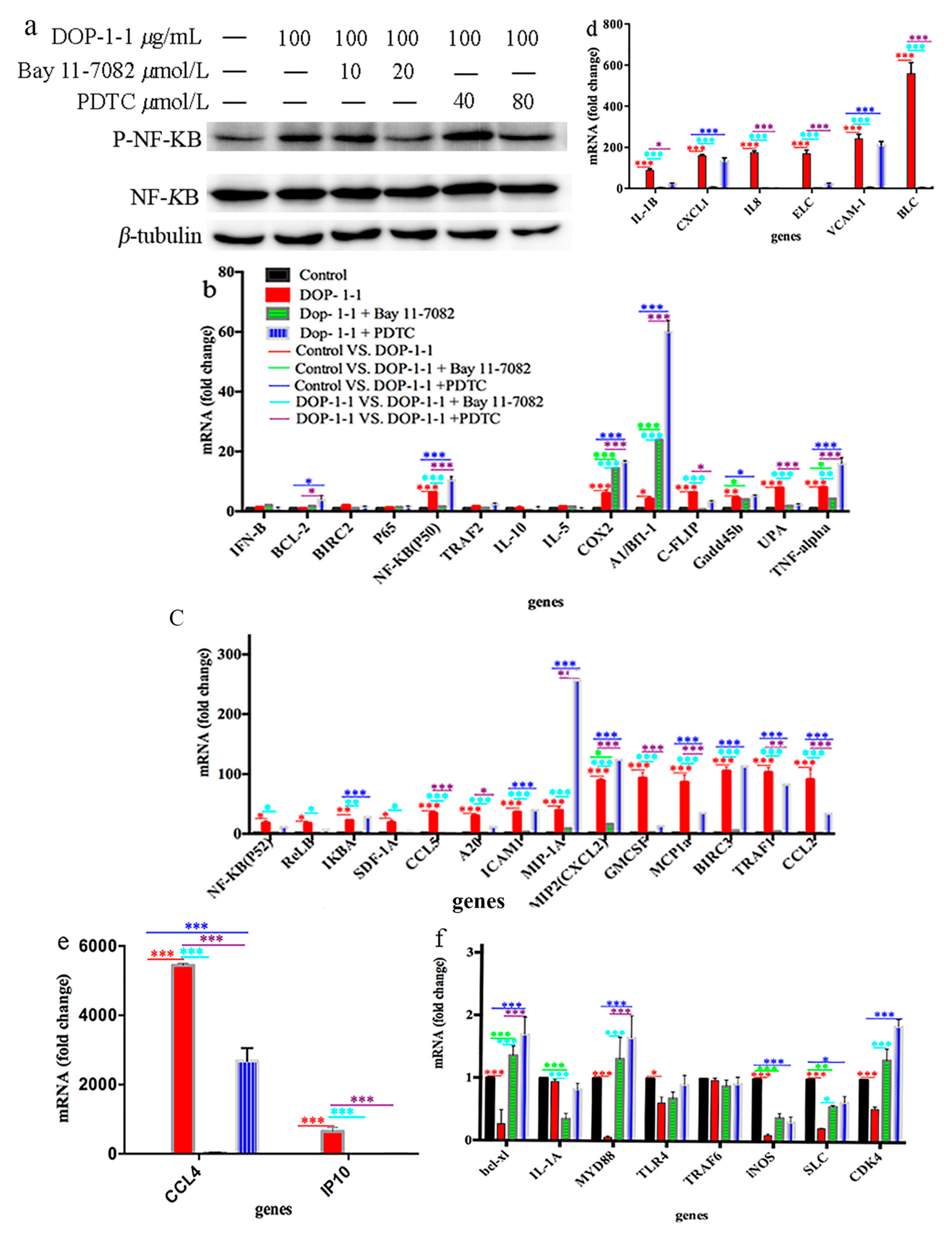
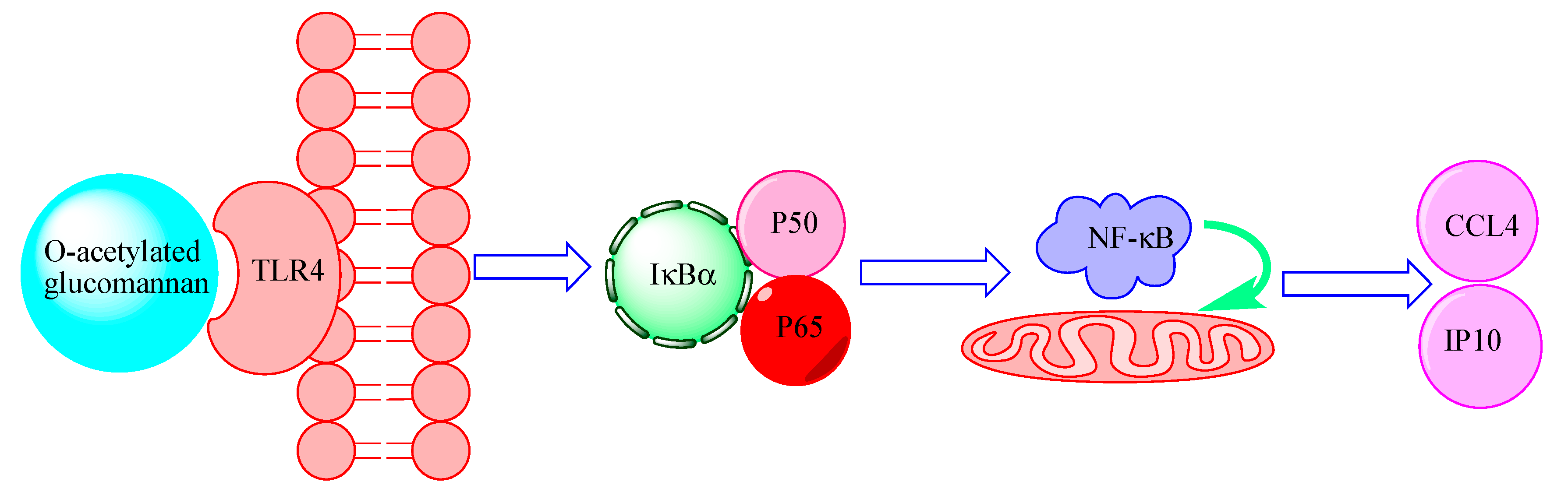
2.4. Effects of DOP-1-1 on NF-κB Signaling
2.5. TLR4 Is a Pattern-Recongnition Receptor for DOP-1-1
2.6. Expression of the Genes Associated with Nf-Κb Signaling
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Carbohydrate and Protein Contents of Crude and Pure O-Acetylated Glucomannan
4.3. Thermal Stability Analysis of O-Acetylated Glucomannan
4.4. Cell Culture
4.5. MTT Assay
4.6. Kinetic Evaluation of the Interactions between DOP-1-1 and THP-1 Cells
4.7. Western Blotting
4.8. Immunofluorescent Staining
4.9. Total Rna Extraction and Real-Time Reverse Transcription-Polymerase Chain Reaction (Rt-Pcr)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, X.J.; Nie, S.P.; Cai, H.; Zhang, G.Y.; Cui, S.W.; Xie, M.Y.; Phillips, G.O. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan): Part IV. Immunomodulatory activity in vivo. J. Funct. Foods 2015, 15, 525–532. [Google Scholar] [CrossRef]
- Lin, X.; Shaw, P.-C.; Sze, S.C.-W.; Tong, Y.; Zhang, Y. Dendrobium officinale polysaccharides ameliorate the abnormality of aquaporin 5, pro-inflammatory cytokines and inhibit apoptosis in the experimental Sjögren’s syndrome mice. Int. Immunopharmacol. 2011, 11, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-H.; Li, X.-F.; Wang, M.-N.; Zha, X.-Q.; Yang, X.-F.; Liu, Z.-J.; Luo, Y.-B.; Luo, J.-P. Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int. J. Biol. Macromol. 2014, 64, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.S.; Jia, M.; Chen, L.; Zhu, B.; Dong, H.X.; Si, J.P.; Peng, W.; Han, T. Cytotoxic and Antifungal Constituents Isolated from the Metabolites of Endophytic Fungus DO14 from Dendrobium officinale. Molecules 2016, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, F.; Wang, Y.; Li, X.; Wang, A.; Wang, C.; Guo, S. Discrimination of the rare medicinal plant Dendrobium officinale based on naringenin, bibenzyl, and polysaccharides. Sci. Chin. Life Sci. 2012, 55, 1092–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timothy, R.; Rudd, R.J.N.; Edwin, A. Yates, Selective Detection of Protein Secondary Structural Changes in Solution Protein-Polysaccharide Complexes Using Vibrational Circular Dichroism (VCD). J. Am. Chem. Soc. 2008, 130, 2138–2139. [Google Scholar]
- Rajendran, A.; Endo, M.; Sugiyama, H. State-of-the-art high-speed atomic force microscopy for investigation of single-molecular dynamics of proteins. Chem. Rev. 2014, 114, 1493–1520. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.T.; Velazquez-Campoy, A.; Vaz, W.L.; Cardoso, R.M.; Valerio, J.; Moreno, M.J. Kinetics and thermodynamics of chlorpromazine interaction with lipid bilayers: Effect of charge and cholesterol. J. Am. Chem. Soc. 2012, 134, 4184–4195. [Google Scholar] [CrossRef] [PubMed]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; Depalatis, L.; Raab, H.; Hazenbos, W.L.; Morisaki, J.H. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Michalet, X.; Weiss, S.; Jäger, M. Single-Molecule Fluorescence Studies of Protein Folding and Conformational Dynamics. Chem. Rev. 2006, 106, 1785–1813. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, R.; Kolusheva, S. Carbohydrate Biosensors. Chem. Rev. 2004, 104, 5987–6016. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, S.; Berti, F.; Mancuso, G.; Cozzi, R.; Tortoli, M.; Volpini, G.; Telford, J.L.; Beninati, C.; Maione, D.; Wack, A. Toll-like receptor 2 dependent immunogenicity of glycoconjugate vaccines containing chemically derived zwitterionic polysaccharides. Proc. Natl. Acad. Sci. USA 2009, 106, 17481–17486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaharada, Y.; Kelly, S.; Nielsen, M.W.; Hjuler, C.T.; Gysel, K.; Muszyński, A.; Carlson, R.W.; Thygesen, M.B.; Sandal, N.; Asmussen, M.H. Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 2015, 523, 308–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, S.F.; Liang, C.H.; Ho, M.Y.; Hsu, T.L.; Tsai, T.I.; Hsieh, Y.S.; Tsai, C.M.; Li, S.T.; Cheng, Y.Y.; Tsao, S.M. Immunization of fucose-containing polysaccharides from Reishi mushroom induces antibodies to tumor-associated Globo H-series epitopes. Proc. Natl. Acad. Sci. USA 2013, 110, 13809–13814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Chen, Y.; Zhang, Y.; Zhang, L.; Lu, X.; Chen, Z. Mannan-binding lectin directly interacts with Toll-like receptor 4 and suppresses lipopolysaccharide-induced inflammatory cytokine secretion from THP-1 cells. Cell. Mol. Immunol. 2011, 8, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simone, V.D.; Franzè, E.; Ronchetti, G.; Colantoni, A.; Fantini, M.C.; Fusco, D.D.; Sica, G.S.; Sileri, P.; Macdonald, T.T.; Pallone, F. Th17-type cytokines, IL-6 and TNF-|[alpha]| synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2014, 34, 3493–3503. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.S.; Shenderov, K.; Huang, N.N.; Kabat, J.; Abu-Asab, M.; Fitzgerald, K.A.; Sher, A.; Kehrl, J.H. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012, 13, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, X.; Zheng, H.L.; Hu, D.H.; Zhang, Y.T.; Guan, Q.H.; Liu, L.F.; Ding, Q.L.; Li, Y.M. Clematichinenoside inhibits VCAM-1 and ICAM-1 expression in TNF-α-treated endothelial cells via NADPH oxidase-dependent IκB kinase/NF-κB pathway. Free Radical Biol. Med. 2015, 78, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, S.; Sansonetti, A.; Durand, L.; Chomienne, C.; Robert-Lézénés, J.; Smadja-Joffe, F. CD44-ligation induces, through ERK1/2 pathway, synthesis of cytokines TNF-alpha and IL-6 required for differentiation of THP-1 monoblastic leukemia cells. Leukemia 2010, 24, 1372–1375. [Google Scholar] [CrossRef] [PubMed]
- Furler, R.L.; Uittenbogaart, C.H. Signaling through the P38 and ERK pathways: A common link between HIV replication and the immune response. Immunol. Res. 2010, 48, 99–109. [Google Scholar] [CrossRef] [PubMed]
- He, T.B.; Huang, Y.P.; Yang, L.; Liu, T.T.; Gong, W.Y.; Wang, X.J.; Sheng, J.; Hu, A.M. Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 83, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Liu, X.; Guo, H.; Zhang, H.; Zhu, J.; Ren, F. Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale (Tiepishihu) in vitro. J. Funct. Foods 2012, 4, 294–301. [Google Scholar] [CrossRef]
- Hua, Y.F.; Zhang, M.; Fu, C.X.; Chen, Z.H.; Chan, G.Y.S. Structural characterization of a 2-O-acetylglucomannan from Dendrobium officinale stem. Carbohydr. Res. 2004, 339, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Cui, S.W.; Nie, S.; Phillips, G.O.; Goff, H.D.; Wang, Q. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part II. Fine structures of O-acetylated residues. Carbohydr. Polym. 2015, 117, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, T.; Dawn, A.; Tsuchiya, Y.; Shinkai, S. Thermo- and solvent-responsive polymer complex created from supramolecular complexation between a helix-forming polysaccharide and a cationic polythiophene. J. Am. Chem. Soc. 2010, 132, 13928–13935. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bing, D.; Feng, Z.; Shuang, Y.; Bao, Y. A study on immunomodulatory mechanism of Polysaccharopeptide mediated by TLR4 signaling pathway. BMC Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.Z.; Hao, R.; Zha, X.Q.; Pan, L.H.; Liu, J.; Luo, J.P. Polysaccharide of Dendrobium huoshanense activates macrophages via toll-like receptor 4-mediated signaling pathways. Carbohydr. Polym. 2016, 146, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Gohlke, U.; Engström, O.; Hamark, C.; Scheidt, T.; Kunstmann, S.; Heinemann, U.; Widmalm, G.; Santer, M.; Barbirz, S. Bacteriophage tailspikes and bacterial O-antigens as a model system to study weak-affinity protein-polysaccharide interactions. J. Am. Chem. Soc. 2016, 138, 9109–9118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, M.; Zimmermann, A.G.; Roberts, R.A.; Zhang, L.; Swanson, K.V.; Wen, H.; Davis, B.K.; Allen, I.C.; Holl, E.K.; Ye, Z. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat. Immunol. 2012, 13, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, H.; Patel, A.K.; Sodhi, C.P.; Hackam, D.J.; Hackam, A.S. Novel Role for the Innate Immune Receptor Toll-Like Receptor 4 (TLR4) in the Regulation of the Wnt Signaling Pathway and Photoreceptor Apoptosis. PLoS ONE 2012, 7, e36560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Laver, T.; Hong, S.W.; Twitty, G.B.; Devos, A.; Devos, M.; Benveniste, E.N.; Nozell, S.E. An NF-κB p65-cIAP2 link is necessary for mediating resistance to TNF-α induced cell death in gliomas. J. Neurooncol. 2011, 102, 367–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corstjens, P.L.; Fat, E.M.; Dood, C.J.D.; Schipb, J.J.P.; Frankenb, K.L.; Chegouc, N.N.; Sutherlandd, J.S.; Howee, R.; Mihrete, A.; Kassaf, D.; et al. Multi-center evaluation of a user-friendly lateral flow assay to determine IP-10 and CCL4 levels in blood of TB and non-TB cases in Africa. Clin. Biochem. 2016, 49, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ge, M.; Lu, S.; Shi, J.; Li, X.; Wang, M.; Huang, J.; Shao, Y.; Huang, Z.; Zhang, J.; et al. Pro-inflammatory effects of the Th1 chemokine CXCL10 in acquired aplastic anaemia. Cytokine 2017, 94, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kodama, N.; Kakuno, T.; Nanba, H. Stimulation of the natural immune system in normal mice by polysaccharide from maitake mushroom. Mycoscience 2003, 44, 257–261. [Google Scholar] [CrossRef]
- Leyva, A.; Quintana, A.; Sánchez, M.; Rodríguez, E.N.; Cremata, J.; Sánchez, J.C. Rapid and sensitive anthrone-sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biologicals 2008, 36, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Krieg, R.C.; Dong, Y.; Schwamborn, K.; Knuechel, R. Protein quantification and its tolerance for different interfering reagents using the BCA-method with regard to 2D SDS PAGE. J. Biochem. Biophys. Methods 2005, 65, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; Mitchell, J.B.; Degraff, W.; Gamson, J.; Gazdar, A.F.; Johnson, B.E.; Glatstein, E.; Minna, J.D. Chemosensitivity testing of human lung cancer cell lines using the MTT assay. Br. J. Cancer 1988, 57, 540–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |


| Sample | Total Carbohydrate (% w/w) | Protein (% w/w) |
|---|---|---|
| Crude polysaccharide (DOP) | 76 | 0.002 |
| O-acetylated glucomannan (DOP-1-1) | 98.9 ± 0.74 | Not Detected |
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | AATCTGGCACCACACCTTCTAC | ATAGCACAGCCTGGATAGCAAC |
| iNOS | TACTCCACCAACAATGGCAA | ATAGCGGATGAGCTGAGCAT |
| MCP1 | AAGCAGAA GTGGGTTCAGGA | TAAAACAGGGTGTCTGGGGA |
| BIRC2(cIAP1) | GAATCTGGTTTCAGCTAGTCTGG | GGTGGGAGATAATGAATGTGCAA |
| IFNβ | GTCTCCTCCAAATTGCTCTC | ACAGGAGCTTCTGACACTGA |
| BCL-X-Long | CATGGCAGCAGTAAAGCAAG | TGCTGCATTGTTCCCATAGA |
| BIRC3(cIAP2) | AAGCTACCTCTCAGCCTACTTT | CCACTGTTTTCTGTACCCGGA |
| Bcl-2 | GAGGATTGTGGCCTCTTTG | ACAGTTCCACAAAGGCATCC |
| c-FLIP | AATTCAAGGCTCAGAAGCGA | GGCAGAAACTCTGCTGTTCC |
| gadd45β | ACAGTGGGGGTGTACGAGTC | TTGATGTCGTTGTCACAGCA |
| TRAF1 | AGAACCCGAGGAATGGCGA | TGAAGGAGCAGCCGACACC |
| TRAF2 | AGGTCTGCCCCAAGTTCCC | GCTGTTTCTCACCCTCTACCGT |
| A1/Bf1-1 | CAGGCTGGCTCAGGACTATC | TGTTCTGGCAGTGTCTACGG |
| IL8 | GTCCTTGTTCCACTGTGCCT | GCTTCCACATGTCCTCACAA |
| IL-1α | TGGCTCATTTTCCCTCAAAAGTTG | AGAAATCGTGAAATCCGAAGTCAAG |
| IL-1β | ACGAATCTCCGACCACCACT | CCATGGCCACAACAACTGAC |
| TNF-α | CCCCAGGGACCTCTCTCTAATC | GGTTTGCTACAACATGGGCTAC A |
| A20 | TCCTCAGGCTTTGTATTTGAGC | TGTGTATCGGTGCATGGTTTTA |
| IL-6 | GTGTGAAAGCAGCAAAGAG | CTCCAAAAGACCAGTGATG |
| IL-12 | TGGAGTGCCAGGAGGACAGT | TCTTGGGTGGGTCAGGTTTG |
| COX2 | TTCTCCTTGAAAGGACTTATGGGTAA | AGAACTTGCATTGATGGTGACTGTTT |
| MIP2(CXCL2) | TTCACAGTGTGTGGTCAACATTT | TCTGCTCTAACACAGAGGGAAAC |
| VCAM-1 | TGGGCTGTGAATCCCCATCT | GGGTCAGCGCGTGGAATTGGTC |
| uPA | ATCTGCCTGCCCTCGATGTATAA | TTTCAGCTGCTCCGGATAGAGATAG |
| BLC | ACTCTGCTAATGAGCCTGGAC | CCTTGGACTGGAGAGAGGCT |
| ELC(CCL19) | CCATCCCTGGGTACATCGTG | GCAGTCTCTGGATGATGCGT |
| SLC | GTCTCCCAGGGAGCATGAGA | GGGAGCCGTATCAGGTCCA |
| SDF-1α | ACTAAAACCTTGTGAGAGATGA | GGGTCTAAATGCTGCAAACCT |
| BAFF | GGCAGGTACTACGACCATCTC | TGGGCCTTTTCTCACAGAAGT |
| ICAM1 | CCCATGAAACCGAACACAC | ACTCTGTTCAGTGTGGCACC |
| MIP-1α | ATGCAGGTCTCCACTGCTG | TCAGGCACTCAGCTCCAG |
| CXCL1 | AGGGAATTCACCCCAAGAAC | CACCAGTGAGCTTCCTCCTC |
| CCL2 | CTTCTGTGCCTGCTGCTCAT | CGGAGTTTGGGTTTGCTTGTC |
| IP10 | GGGAGCAAAATCGATGCAGTGCT | GCAGCCTCTGTGTGGTCCATCC |
| IFNα | CCTGATGAAGGAGGACTCCATT | AAAAAGGTGAGCTGGCATACG |
| CCL4(MIP1β) | GCTAGTAGCTGCCTTCTGCTCTCC | CAGTTCCAGCTGATACACG TACTCC |
| CCL5 | CTGCTGCTTTGCCTACATTGC | GTTCAGGTTCAAGGACTCTCCATC |
| CXCL10 | AAGCAGTTAGCAAGGAAAGGTC | TTGAAGCAGGGTCAGAACATC |
| IL-2 | GAACTAAAGGGATCTGAAACAACATTC | TGTTGAGATGATGCTTTGACAAAA |
| IL-4 | TGCTTCCCCCTCTGTTCTTCCT | GGCAGCGAGTGTCCTTCTCATG |
| IL-5 | AGCCATGAGGATGCTTCTGC | AAGCAGTGCCAAGGTCTCTT |
| IL-10 | GCCTAACATGCTTCGAGATC | CTCATGGCTTTGTAGATGCC |
| CDK4 | AGTTCGTGAGGTGGCTTTA | GGGTGCCTTGTCCAGATA |
| GMCSF | TCAGGATGGTCATCTTGGAG | TCTTCTGCCATGCCTGTATC |
| IκBα | CTCCGAGACTTTCGAGGAAATAC | GCCATTGTAGTTGGTAGCCTTCA |
| NF-κB(p50) | CCTGGATGACTCTTGGGAAA | TCAGCCAGCTGTTTCATGTC |
| NF-κB(p52) | GAACAGCCTTGCATCTAGCC | TTTTCAGCAT GGATGTCAGC |
| p65(RelA) | TCTGCTTCCAGGTGACAGTG | GCCAGAGTTTCGGTTCACTC |
| c-Rel | CGAACCCAATTTATGACAAC | TTTTGTTTCTTTGCTTTATTGC |
| RelB | CTGCTTCCAGGCCTCATATC | CGCAGCTCTGATGTGTTTGT |
| TLR4 | AGAAGCAGTGAGGATGATGCC | TTCTGTGTGGTTTAGGGCCA |
| MYD88 | GAAGCCTAGAGGCCATTCTG | GGCTTGTGATCTCAGGTGAA |
| TRAF6 | CACGTGGATACCAACTGCTC | TGTGTGCATCTCCTGTCTTG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-P.; He, T.-B.; Cuan, X.-D.; Wang, X.-J.; Hu, J.-M.; Sheng, J. 1,4-β-d-Glucomannan from Dendrobium officinale Activates NF-кB via TLR4 to Regulate the Immune Response. Molecules 2018, 23, 2658. https://doi.org/10.3390/molecules23102658
Huang Y-P, He T-B, Cuan X-D, Wang X-J, Hu J-M, Sheng J. 1,4-β-d-Glucomannan from Dendrobium officinale Activates NF-кB via TLR4 to Regulate the Immune Response. Molecules. 2018; 23(10):2658. https://doi.org/10.3390/molecules23102658
Chicago/Turabian StyleHuang, Yan-Ping, Tao-Bin He, Xian-Dan Cuan, Xuan-Jun Wang, Jiang-Miao Hu, and Jun Sheng. 2018. "1,4-β-d-Glucomannan from Dendrobium officinale Activates NF-кB via TLR4 to Regulate the Immune Response" Molecules 23, no. 10: 2658. https://doi.org/10.3390/molecules23102658
APA StyleHuang, Y.-P., He, T.-B., Cuan, X.-D., Wang, X.-J., Hu, J.-M., & Sheng, J. (2018). 1,4-β-d-Glucomannan from Dendrobium officinale Activates NF-кB via TLR4 to Regulate the Immune Response. Molecules, 23(10), 2658. https://doi.org/10.3390/molecules23102658