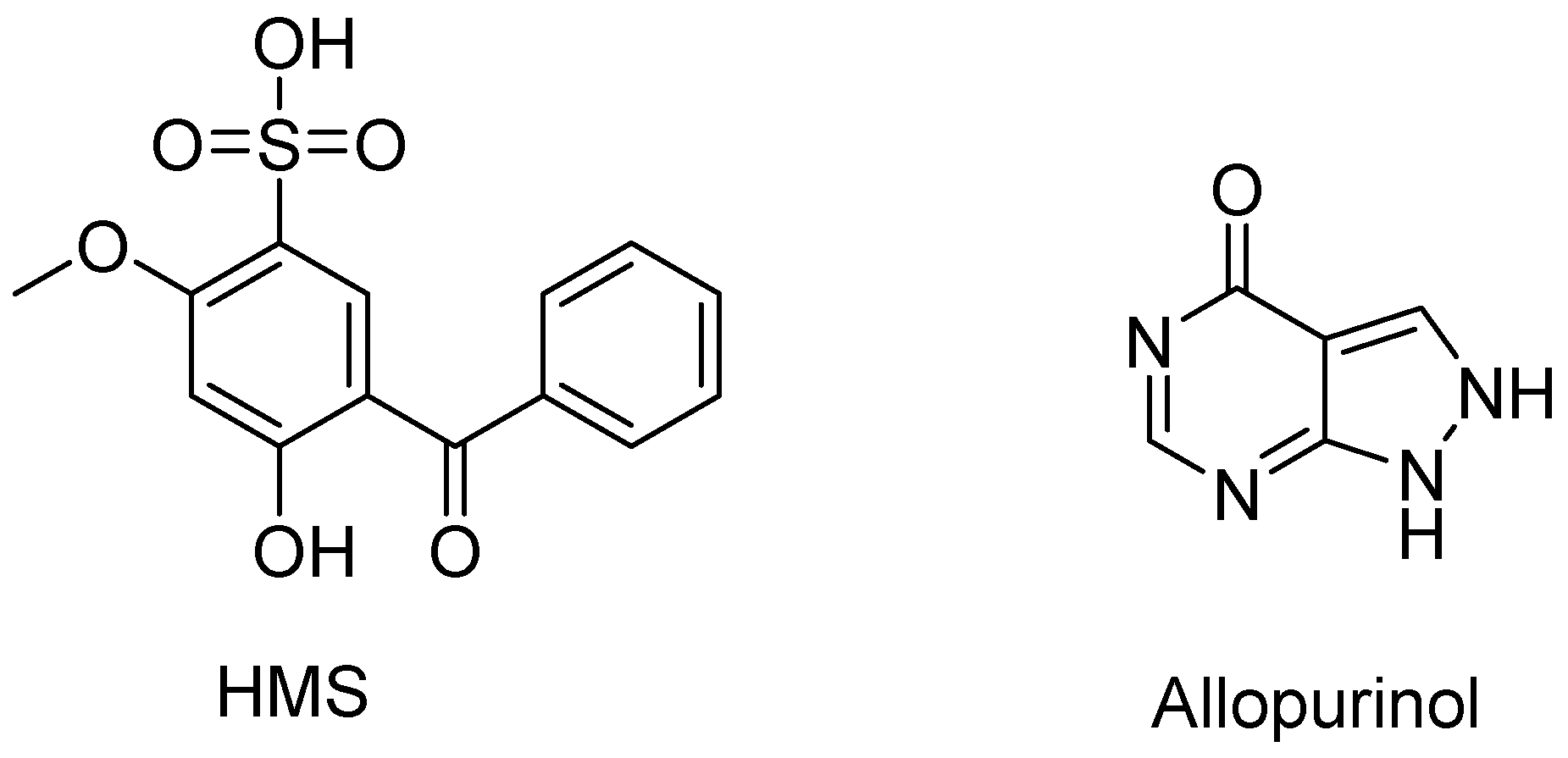
Anti-Hyperuricemic Effect of 2-Hydroxy-4-methoxy-benzophenone-5-sulfonic Acid in Hyperuricemic Mice through XOD
Abstract
:1. Introduction
2. Results
2.1. Hypouricemic of HMS In Vitro against XOD by Molecular Modeling and Enzymatic Activity Assays
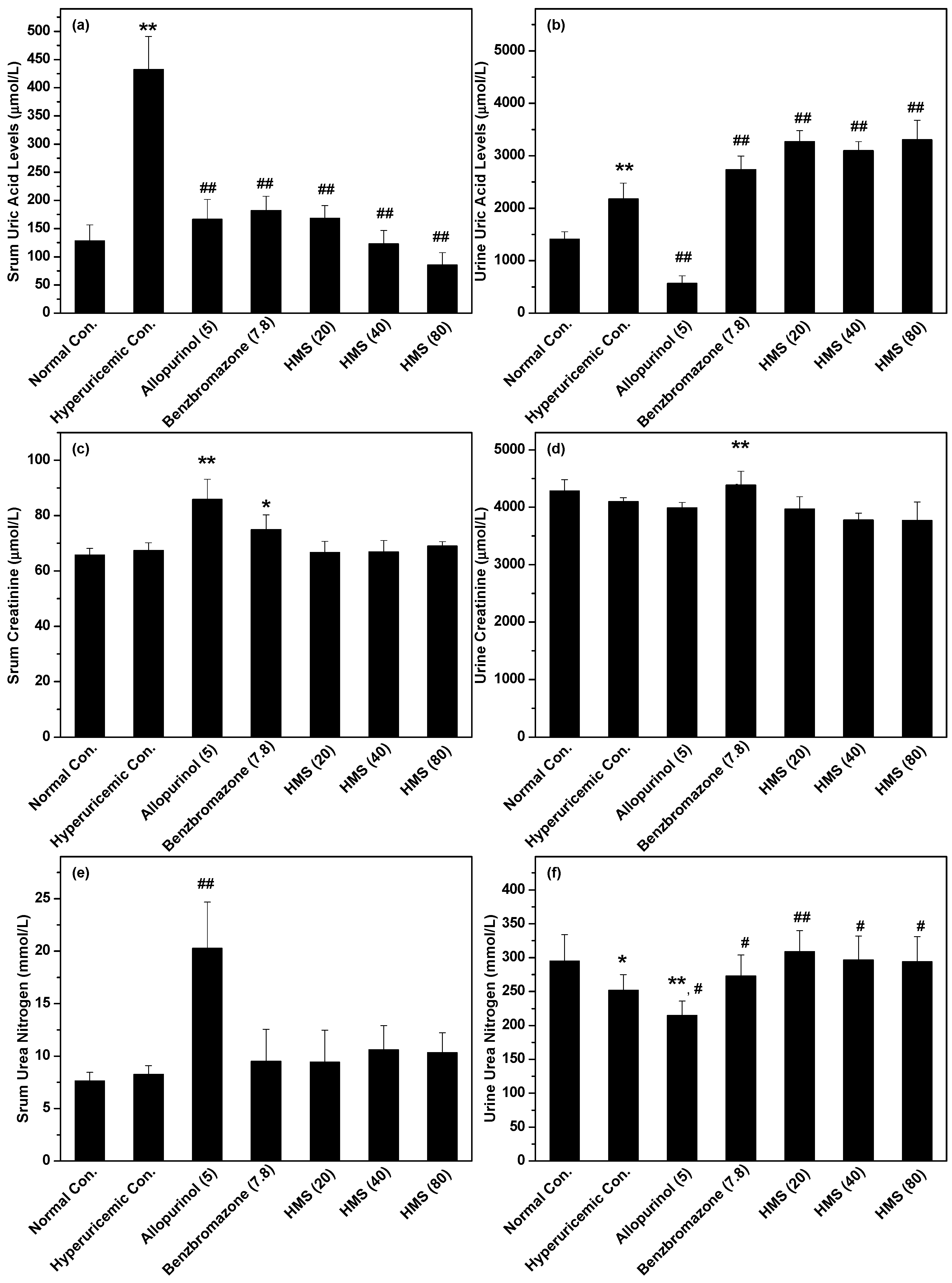
2.2. Hypouricemic Effect of HMS In Vivo on a Hyperuricemic Mouse Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Molecular Docking
4.3. XOD Inhibition
4.4. Animals
4.5. Drug Administration
4.6. Uric Acid, XOD, URAT1, OAT1, BUN and Creatinine Assays
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Xiao, M.; Wei, D.; Yin, J.; Wei, G.; Du, Y. Transformation mechanism of benzophenone-4 in free chlorine promoted chlorination disinfection. Water Res. 2013, 47, 6223–6233. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D.; Dorman, G.; Elliott, J.T.; Marecak, D.M.; Chaudhary, A. Benzophenone photoprobes for phosphoinositides, peptides and drugs. Photochem. Photobiol. 1997, 65, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, R.K.; Kayal, S.; Barak, A.; Orr-Ewing, A.J.; Umapathy, S. Intermolecular hydrogen bonding controlled intersystem crossing rates of benzophenone. J. Phys. Chem. Lett. 2018, 9, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- De Coster, S.; van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public. Health 2012, 2012, 713696. [Google Scholar] [CrossRef] [PubMed]
- Kurul, E.; Hekimoglu, S. Skin permeation of two different benzophenone derivatives from various vehicles. Int. J. Cosmetic Sci. 2001, 23, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Rzemieniec, J.; Lason, W.; Krzeptowski, W.; Kajta, M. Apoptosis induced by the UV filter benzophenone-3 in mouse neuronal cells is mediated via attenuation of eralpha/ppargamma and stimulation of erbeta/gpr30 signaling. Mol. Neurobiol. 2018, 55, 2362–2383. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Rzemieniec, J.; Lason, W.; Krzeptowski, W.; Kajta, M. Benzophenone-3 impairs autophagy, alters epigenetic status, and disrupts retinoid X receptor signaling in apoptotic neuronal cells. Mol. Neurobiol. 2018, 55, 5059–5074. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Bai, Y.; Ji, Q.; Huo, Y.; Liu, H.; Crittenden, J.C.; Qu, J. Combined genotoxicity of chlorinated products from tyrosine and benzophenone-4. J. Hazard. Mater. 2017, 322, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Frikeche, J.; Couteau, C.; Roussakis, C.; Coiffard, L.J. Research on the immunosuppressive activity of ingredients contained in sunscreens. Arch. Dermatol. Res. 2015, 307, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In vitro and in vivo estrogenicity of uv screens. Environ. Health Persp. 2001, 109, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-Q.; Du, Y.; Zhang, Z.-Y.; Jiang, W.-J.; Guo, Y.-M.; Lu, X.-W.; Zhang, Y.-M.; Sun, L.-W. Acute toxicity and ecological risk assessment of benzophenone and N,N-diethyl-3 methylbenzamide in personal care products. Int. J. Env. Res. Pub. He. 2016, 13, 925. [Google Scholar] [CrossRef] [PubMed]
- Molins-Delgado, D.; Díaz-Cruz, M.S.; Barceló, D. Ecological risk assessment associated to the removal of endocrine-disrupting parabens and benzophenone-4 in wastewater treatment. J. Hazard. Mater. 2016, 310, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Feng, B.M.; Zhao, Y.Q.; Sun, Y.; Liu, B.; Liu, F.; Chen, G.; Bai, J.; Hua, H.M.; Wang, H.F.; et al. Polyketide butenolide, diphenyl ether, and benzophenone derivatives from the fungus aspergillus flavipes PJ03-11. Bioorg. Med. Chem. Lett. 2016, 26, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Anandhi Senthilkumar, H.; Figueroa, M.; Wu, S.-B.; Fata, J.E.; Kennelly, E.J.; Long, C. UPLC-QTOFMSE-guided dereplication of the endangered chinese species garcinia paucinervis to identify additional benzophenone derivatives. J. Nat. Prod. 2016, 79, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Lin, X.; Han, L.; Ma, J.; Ma, Q.; Zhong, J.; Liu, Y.; Sun, T.; Wang, J.; Huang, X. New metabolites and bioactive chlorinated benzophenone derivatives produced by a marine-derived fungus pestalotiopsis heterocornis. Mar. Drugs 2017, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, S.; Chattopadhyay, S.K. The potential health benefit of polyisoprenylated benzophenones from garcinia and related genera: Ethnobotanical and therapeutic importance. Fitoterapia 2013, 89, 86–125. [Google Scholar] [CrossRef] [PubMed]
- Thirusangu, P.; Vigneshwaran, V.; Ranganatha, V.L.; Vijay Avin, B.R.; Khanum, S.A.; Mahmood, R.; Jayashree, K.; Prabhakar, B.T. A tumoural angiogenic gateway blocker, benzophenone-1b represses the hif-1α nuclear translocation and its target gene activation against neoplastic progression. Biochem. Pharmacol. 2017, 125, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Carballo, D.; Malak, S.; Bardenheuer, W.; Freistuehler, M.; Reusch, H.P. Cytotoxic activity of nemorosone in neuroblastoma cells. J. Cell. Mol. Med. 2008, 12, 2598–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciochina, R.; Grossman, R.B. Polycyclic polyprenylated acylphloroglucinols. Chem. Rev. 2006, 106, 3963–3986. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Rubio, O.; Frontana-Uribe, B.A.; Ramirez-Apan, T.; Cardenas, J. Polyisoprenylated benzophenones in cuban propolis; biological activity of nemorosone. Z. Naturforsch. C. 2002, 57, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghorbani, M.; Thirusangu, P.; Gurupadaswamy, H.D.; Girish, V.; Shamanth Neralagundi, H.G.; Prabhakar, B.T.; Khanum, S.A. Synthesis and antiproliferative activity of benzophenone tagged pyridine analogues towards activation of caspase activated DNase mediated nuclear fragmentation in Dalton’s lymphoma. Bioorg. Chem. 2016, 65, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.Y.; Tsai, H.J.; Chiang, H.C. Benzophenones as xanthine oxidase inhibitors. Anticancer Res. 1999, 19, 1131–1135. [Google Scholar] [PubMed]
- Ranganatha, V.L.; Begum, A.B.; Naveen, P.; Zameer, F.; Hegdekatte, R.; Khanum, S.A. Synthesis, xanthine oxidase inhibition, and antioxidant screening of benzophenone tagged thiazolidinone analogs. Arch. Pharm. 2014, 347, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T. Clinical practice. Gout. N. Engl. J. Med. 2011, 364, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.K.; Miner, J.N. Uric acid transporter inhibitors for gout. ADMET DMPK 2017, 5, 59–74. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Han, C.; Wu, D.; Xia, X.; Gu, J.; Guan, H.; Shan, Z.; Teng, W. Prevalence of hyperuricemia and gout in mainland china from 2000 to 2014: A systematic review and meta-analysis. Biomed. Res. Int. 2015, 2015, 762820. [Google Scholar] [CrossRef] [PubMed]
- Enroth, C.; Eger, B.T.; Okamoto, K.; Nishino, T.; Nishino, T.; Pai, E.F. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: Structure-based mechanism of conversion. PNAS 2000, 97, 10723–10728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smelcerovic, A.; Tomovic, K.; Smelcerovic, Z.; Petronijevic, Z.; Kocic, G.; Tomasic, T.; Jakopin, Z.; Anderluh, M. Xanthine oxidase inhibitors beyond allopurinol and febuxostat; an overview and selection of potential leads based on in silico calculated physico-chemical properties, predicted pharmacokinetics and toxicity. Eur. J. Med. Chem. 2017, 135, 491–516. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Endou, H. Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease. Clin. Exp. Nephrol. 2005, 9, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Doring, A.; Gieger, C.; Mehta, D.; Gohlke, H.; Prokisch, H.; Coassin, S.; Fischer, G.; Henke, K.; Klopp, N.; Kronenberg, F.; et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat. Genet. 2008, 40, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.K.; Law, S.M.; Brooks, C.L., 3rd. Flexible cdocker: Development and application of a pseudo-explicit structure-based docking method within charmm. J. Comput. Chem. 2016, 37, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Yong, T.; Chen, S.; Xie, Y.; Chen, D.; Zhou, X.; Li, D.; Li, M.; Su, L.; Zuo, D. Hypouricemic effect of 2,5-dihydroxyacetophenone, a computational screened bioactive compound from ganoderma applanatum, on hyperuricemic mice. Int. J. Mol. Sci. 2018, 19, 1394. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.J.; Coburn, H.; Douglass, R.; Babson, A.L. A simplified alkaline phosphotungstate assay for uric acid in serum. Clin. Chem. 1971, 17, 158–160. [Google Scholar] [PubMed]
- Slot, C. Plasma creatinine determination. A new and specific jaffe reaction method. Scand. J. Clin. Lab. Invest. 1965, 17, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Talke, H.; Schubert, G.E. Enzymatische harnstoffbestimmung in blut und serum im optischen test nach warburg. Klin. Wochenschr. 1965, 43, 174–175. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound HMS is available from the authors. |








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, T.; Li, D.; Li, M.; Liang, D.; Diao, X.; Deng, C.; Chen, S.; Xie, Y.; Chen, D.; Zuo, D. Anti-Hyperuricemic Effect of 2-Hydroxy-4-methoxy-benzophenone-5-sulfonic Acid in Hyperuricemic Mice through XOD. Molecules 2018, 23, 2671. https://doi.org/10.3390/molecules23102671
Yong T, Li D, Li M, Liang D, Diao X, Deng C, Chen S, Xie Y, Chen D, Zuo D. Anti-Hyperuricemic Effect of 2-Hydroxy-4-methoxy-benzophenone-5-sulfonic Acid in Hyperuricemic Mice through XOD. Molecules. 2018; 23(10):2671. https://doi.org/10.3390/molecules23102671
Chicago/Turabian StyleYong, Tianqiao, Dan Li, Muxia Li, Danling Liang, Xue Diao, Chenling Deng, Shaodan Chen, Yizhen Xie, Diling Chen, and Dan Zuo. 2018. "Anti-Hyperuricemic Effect of 2-Hydroxy-4-methoxy-benzophenone-5-sulfonic Acid in Hyperuricemic Mice through XOD" Molecules 23, no. 10: 2671. https://doi.org/10.3390/molecules23102671



