A Near-Infrared Fluorescent Probe Based on a FRET Rhodamine Donor Linked to a Cyanine Acceptor for Sensitive Detection of Intracellular pH Alternations
Abstract
:1. Introduction
2. Results
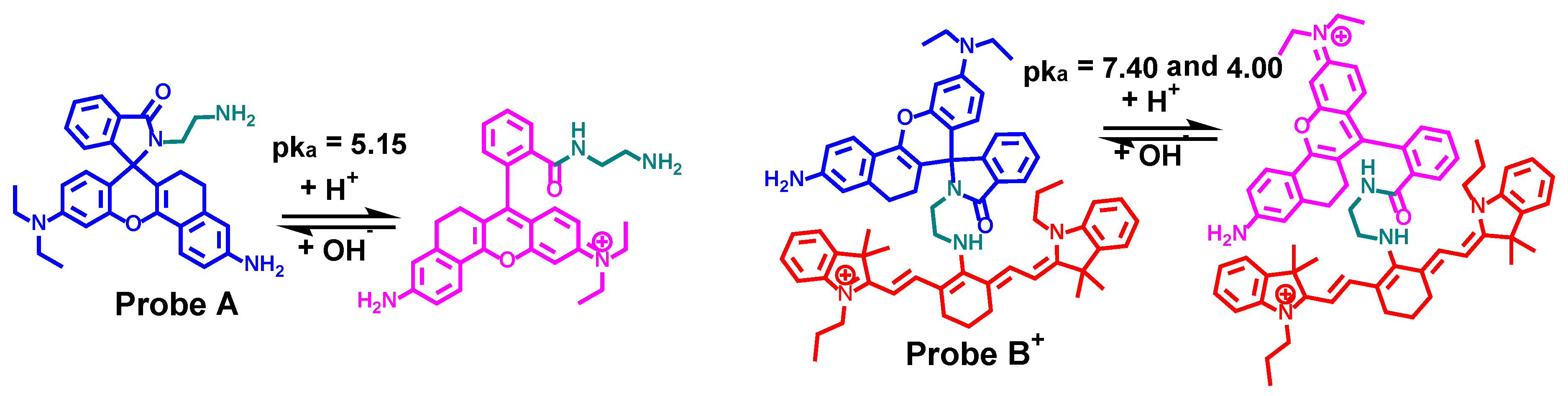
2.1. Synthesis of Fluorescent Probes
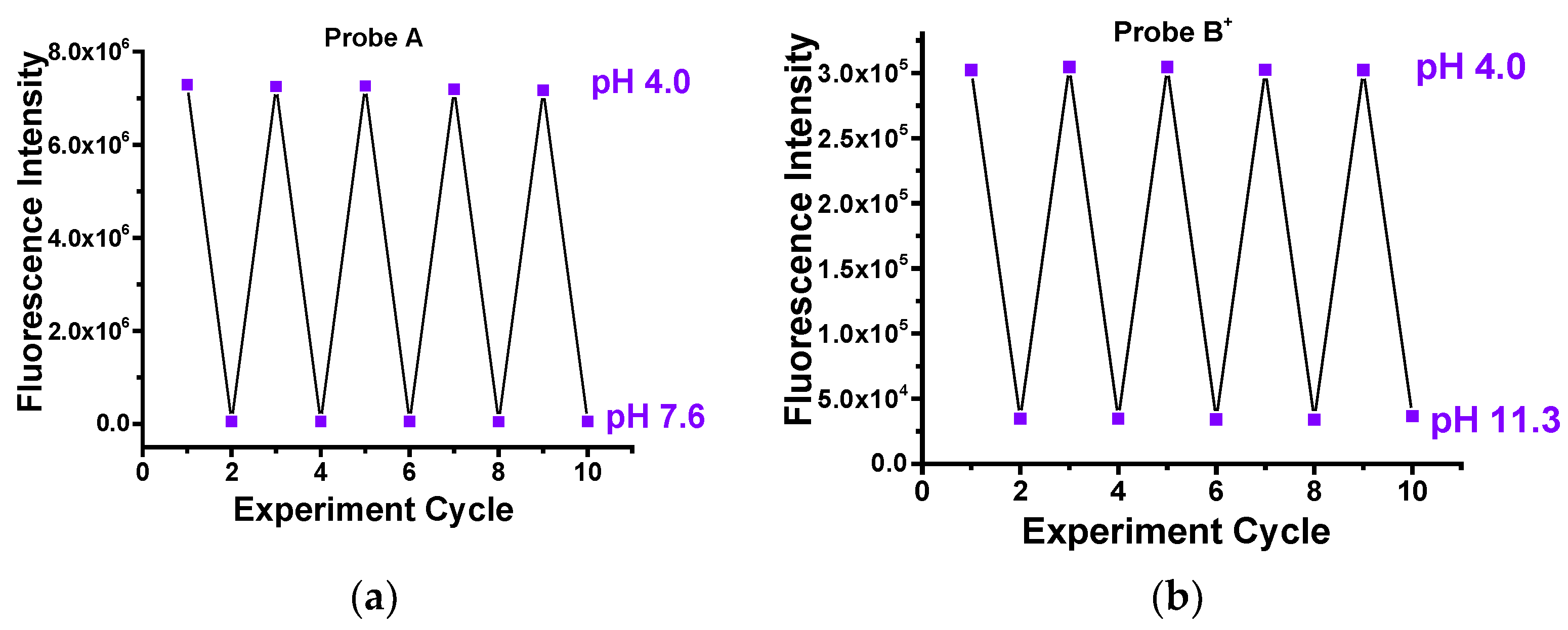
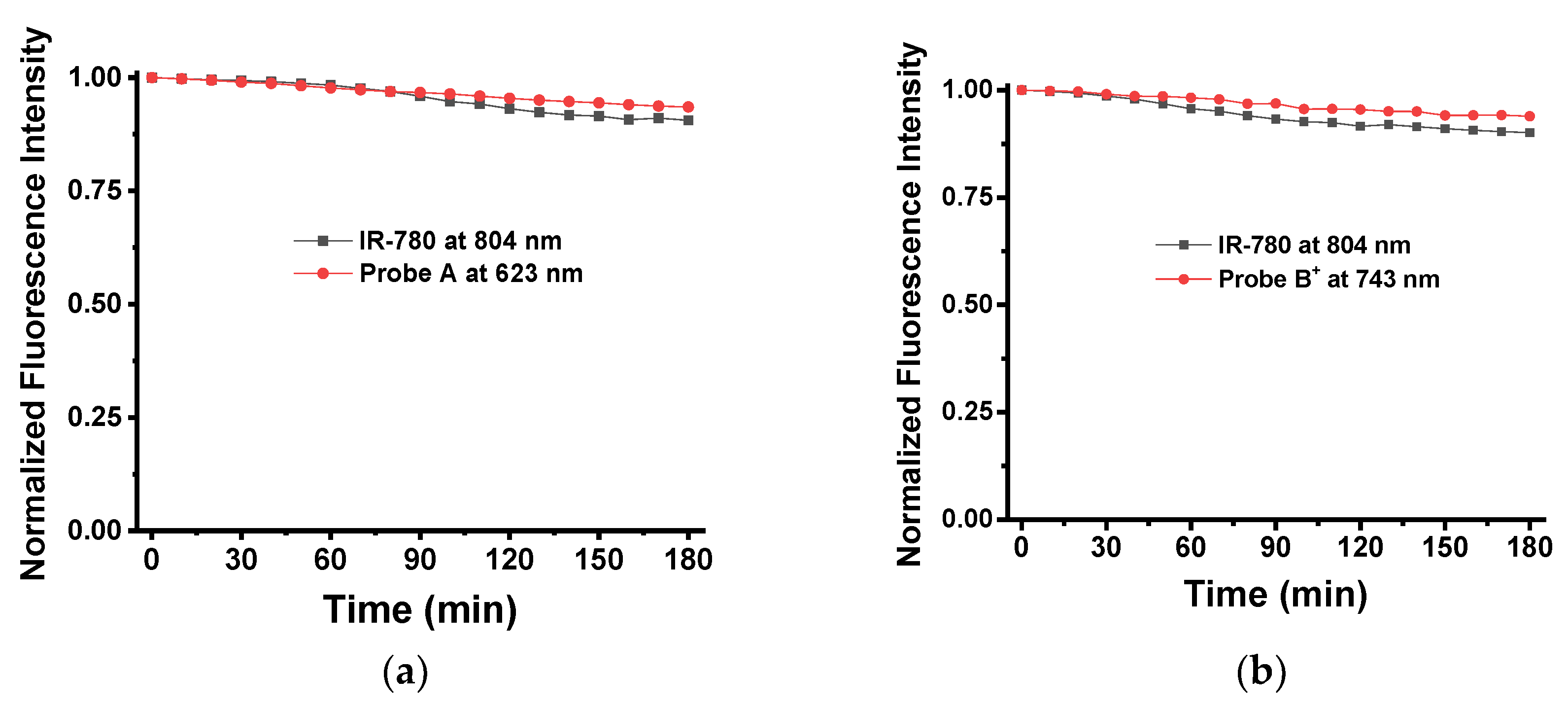
2.2. Optical Responeses of Fluorescent Probes to pH Changes
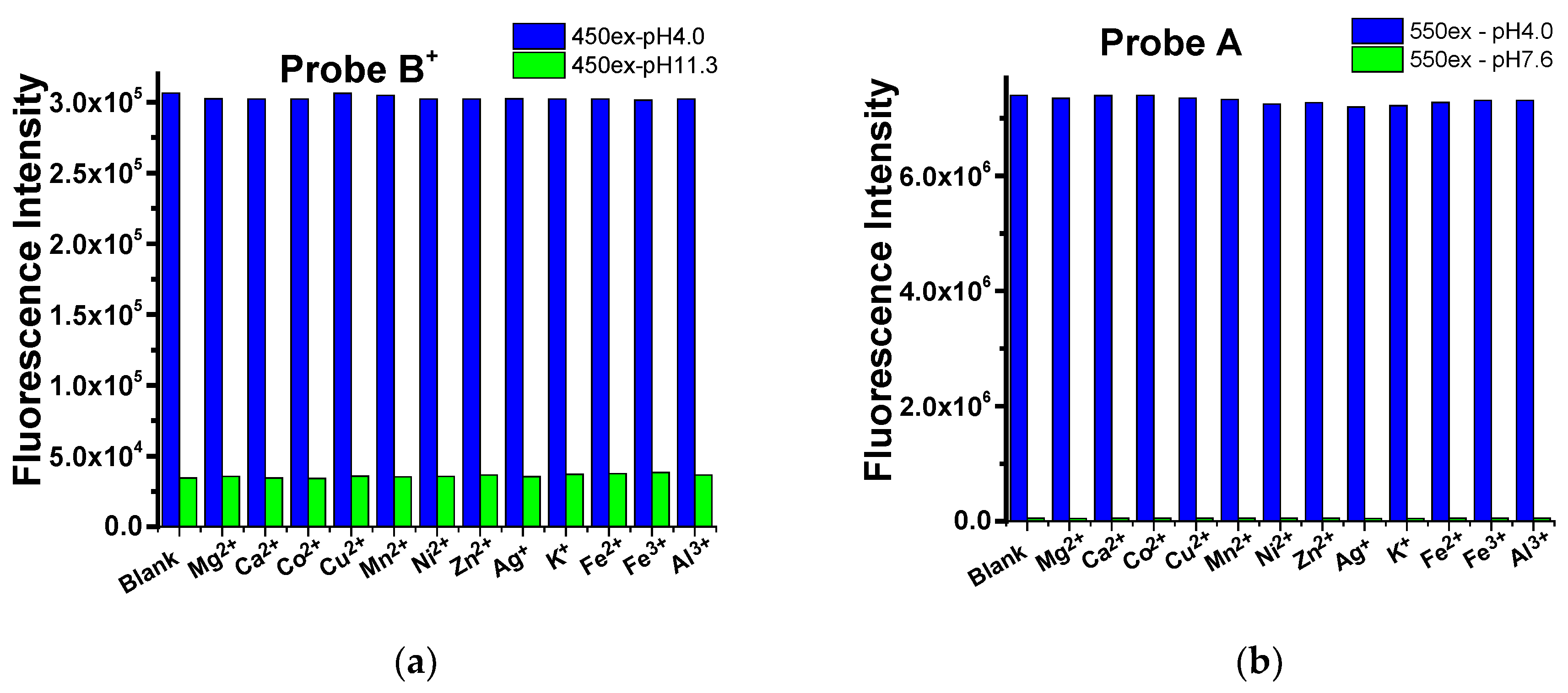
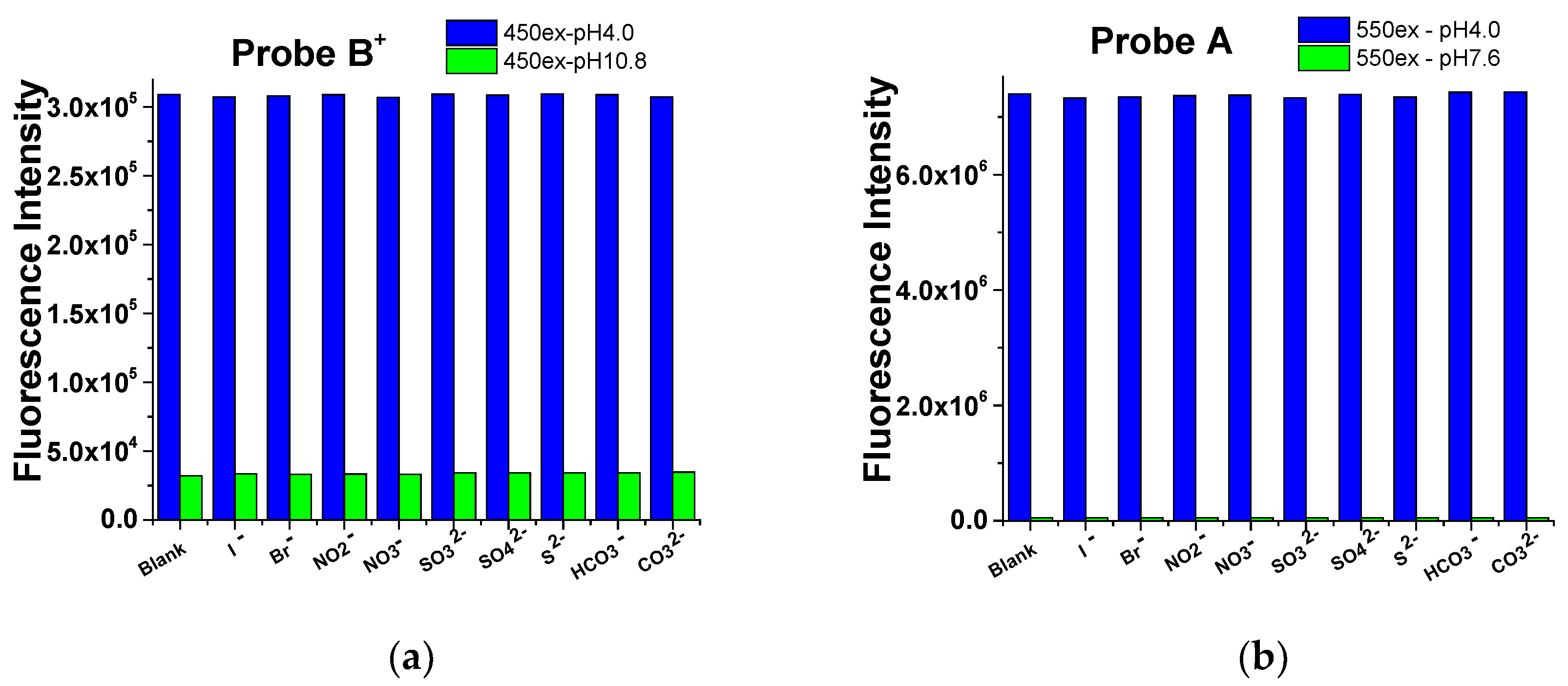
2.3. Selectivity the Probes to pH over Cations, Anions, and Amino Acids
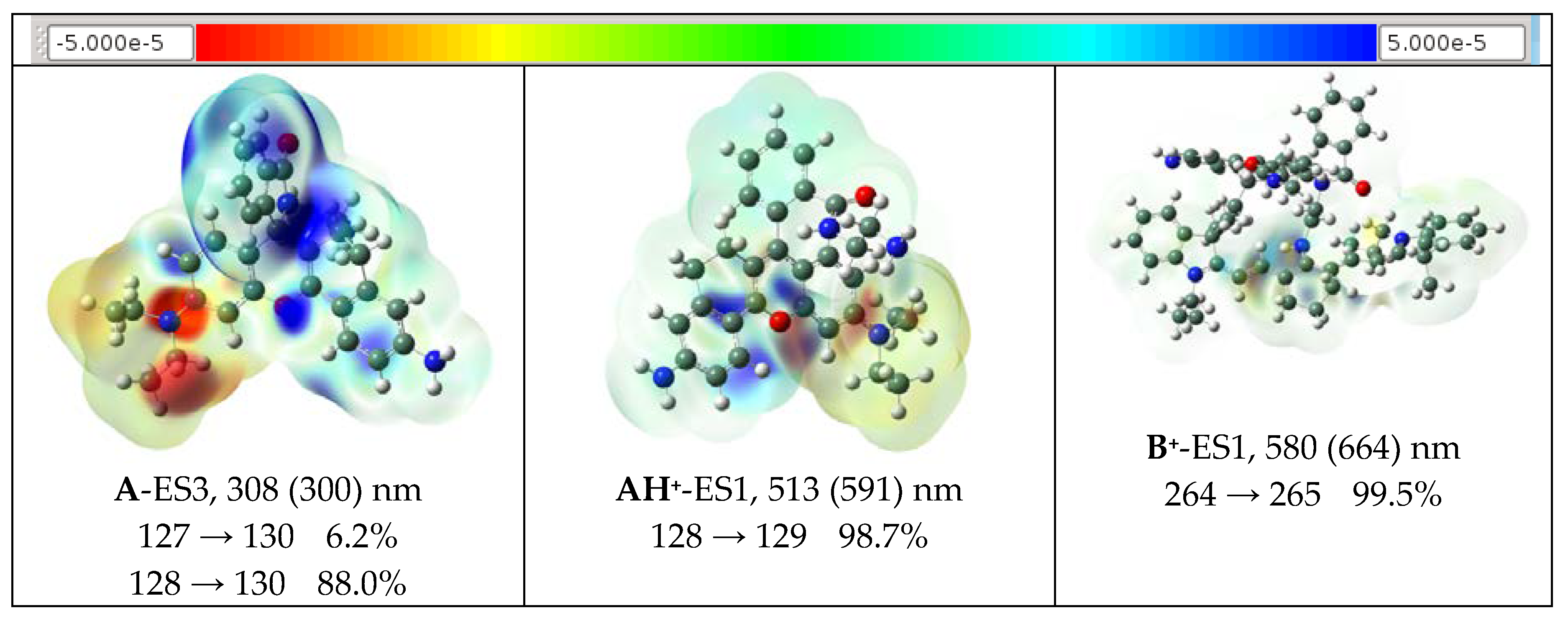
2.4. Theoretical Results
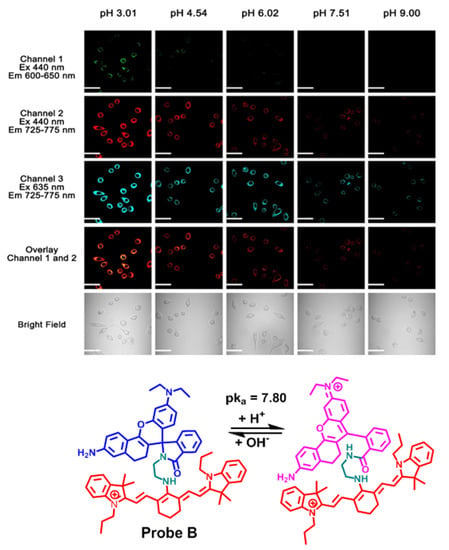
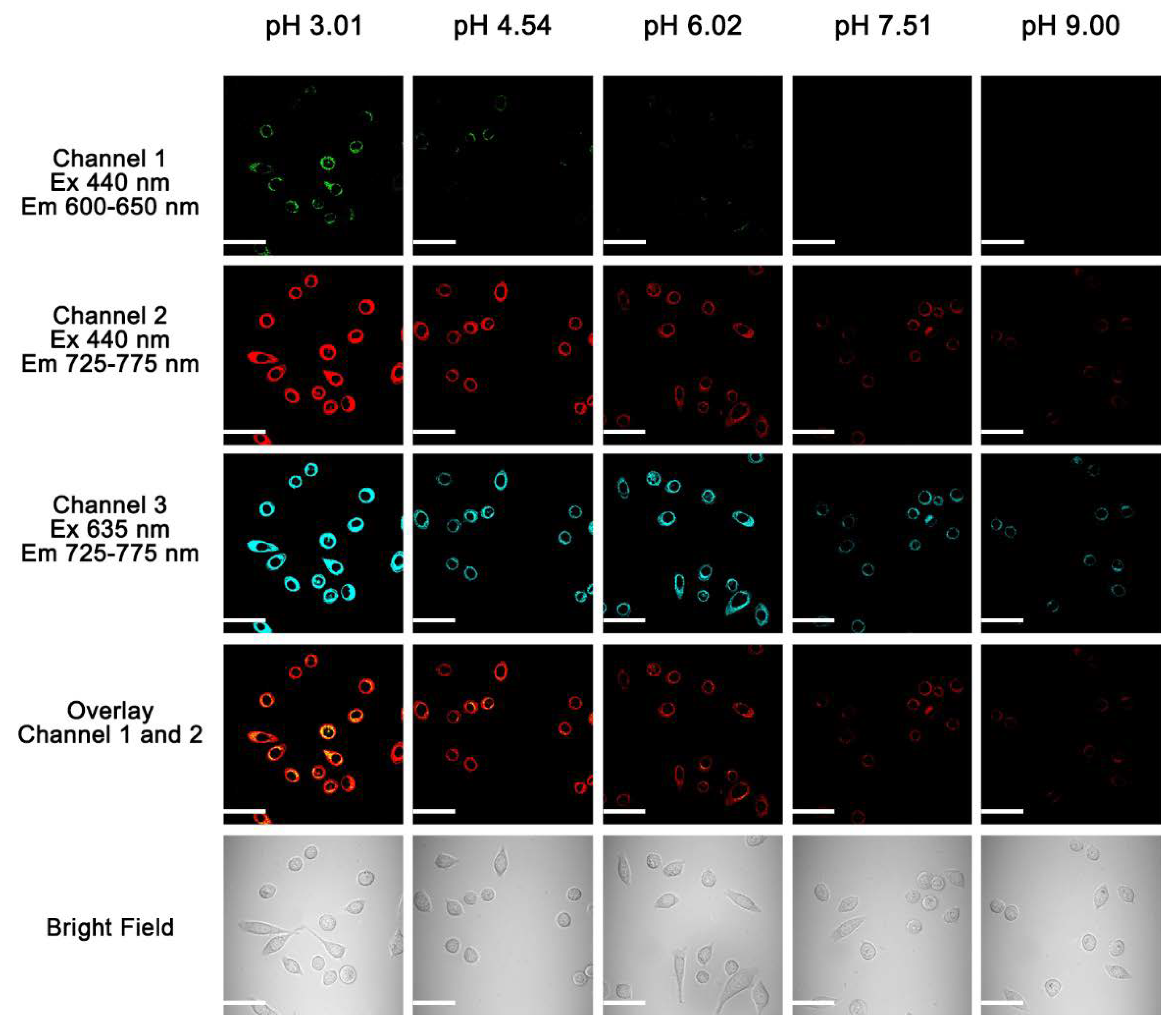
2.5. Cellular Fluorescence Imaging
3. Discussion
4. Materials and Methods
4.1. Synthesis of Probe A
4.2. Synthesis of Probe B+
4.3. Theoretical Calculations
4.4. Cell Culture and Cell Imaging Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johansson, A.C.; Appelqvist, H.; Nilsson, C.; Kagedal, K.; Roberg, K.; Ollinger, K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis 2010, 15, 527–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turk, B.; Turk, V. Lysosomes as “Suicide Bags” in Cell Death: Myth or Reality? J. Biol. Chem. 2009, 284, 21783–21787. [Google Scholar] [CrossRef] [PubMed]
- Stinchcombe, J.; Bossi, G.; Griffiths, G.M. Linking albinism and immunity: The secrets of secretory lysosomes. Science 2004, 305, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.T.; Ren, W.X.; Li, K.; Seo, J.; Sharma, A.; Yu, X.Q.; Kim, J.S. Fluorescent bioimaging of pH: From design to applications. Chem. Soc. Rev. 2017, 46, 2076–2090. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.K.; Huo, F.J.; Lee, S.; Yin, C.X.; Yoon, J. A review: The trend of progress about pH probes in cell application in recent years. Analyst 2017, 142, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Hu, Y.; Yoon, J. Fluorescent probes and bioimaging: Alkali metals, alkaline earth metals and pH. Chem. Soc. Rev. 2015, 44, 4619–4644. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Wang, J.B.; Bi, J.H.; Wang, X.; Fang, M.X.; Phillips, T.; May, A.; Conner, N.; Tanasova, M.; Luo, F.T.; et al. Fluorescent probes based on pi-conjugation modulation between hemicyanine and coumarin moieties for ratiometric detection of pH changes in live cells with visible and near-infrared channels. Sens. Actuators B Chem. 2018, 265, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Huang, Y.H.; Tian, H. Progress and Trends in AIE-Based Bioprobes: A Brief Overview. ACS Appl. Mater. Interfaces 2018, 10, 12217–12261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Chen, T.H.; Lee, H.M.; Bi, J.H.; Ghosh, A.; Fang, M.X.; Qian, Z.C.; Xie, F.; Ainsley, J.; Christov, C.; et al. Luminescent Probes for Sensitive Detection of pH Changes in Live Cells through Two Near-Infrared Luminescence Channels. ACS Sens. 2017, 2, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.L.; Zhang, P.P.; Liu, W.M.; Wang, M.Q.; Zhang, H.Y.; Wu, J.S.; Zhang, L.P.; Wang, P.F. Near-Infrared Probe Based on Rhodamine Derivative for Highly Sensitive and Selective Lysosomal pH Tracking. Anal. Chem. 2017, 89, 922–1929. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.X.; Adhikari, R.; Bi, J.H.; Mazi, W.; Dorh, N.; Wang, J.B.; Conner, N.; Ainsley, J.; Karabencheva-Christova, T.G.; Luo, F.T.; et al. Fluorescent probes for sensitive and selective detection of pH changes in live cells in visible and near-infrared channels. J. Mater. Chem. B 2017, 5, 9579–9590. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, J.L.; Du, J.J.; Peng, X.J. Fluorescent Probes for Sensing and Imaging within Specific Cellular Organelles. Acc. Chem. Res. 2016, 49, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Song, X.B.; Hu, M.Y.; Wang, C.; Xiao, Y. Near-infrared fluorescent probes with higher quantum yields and neutral pK(a) values for the evaluation of intracellular pH. RSC Adv. 2016, 6, 69641–69646. [Google Scholar] [CrossRef]
- Zhang, J.T.; Yang, M.; Li, C.; Dorh, N.; Xie, F.; Luo, F.T.; Tiwari, A.; Liu, H.Y. Near-infrared fluorescent probes based on piperazine-functionalized BODIPY dyes for sensitive detection of lysosomal pH. J. Mater. Chem. B 2015, 3, 2173–2184. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, Y.J.; Yang, S.; Zhao, Y.R.; Yuan, L.; Zheng, J.; Yang, R.H. Hemicyanine-based High Resolution Ratiometric near-Infrared Fluorescent Probe for Monitoring pH Changes in Vivo. Anal. Chem. 2015, 87, 2495–2503. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.Q.; Chen, S.M.; Shi, W.; Li, L.H.; Ma, H.M. Lysosomal pH Rise during Heat Shock Monitored by a Lysosome-Targeting Near-Infrared Ratiometric Fluorescent Probe. Angew. Chem. Int. Ed. 2014, 53, 10916–10920. [Google Scholar] [CrossRef] [PubMed]
- Vegesna, G.K.; Janjanam, J.; Bi, J.H.; Luo, F.T.; Zhang, J.T.; Olds, C.; Tiwari, A.; Liu, H.Y. pH-activatable near-infrared fluorescent probes for detection of lysosomal pH inside living cells. J. Mater. Chem. B 2014, 2, 4500–4508. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, J.S. Far-red and near infrared BODIPY dyes: Synthesis and applications for fluorescent pH probes and bio-imaging. Org. Biomol. Chem. 2014, 12, 3774–3791. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xiao, H.B.; Cheng, Y.F.; Zhang, W.; Huang, F.; Zhang, W.; Wang, H.; Tang, B. A near-infrared-emitting fluorescent probe for monitoring mitochondrial pH. Chem. Commun. 2014, 50, 7184–7187. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, R.; Ge, J.F.; Xu, Y.J.; Xu, Y.; Lu, J.M.; Itoh, I.; Ihara, M. Reversible Near-Infrared pH Probes Based on Benzo a phenoxazine. Anal. Chem. 2013, 85, 7419–7425. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Akers, W.; Bhushan, K.; Bloch, S.; Sudlow, G.; Tang, R.; Achilefu, S. Near-Infrared pH-Activatable Fluorescent Probes for Imaging Primary and Metastatic Breast Tumors. Bioconjug. Chem. 2011, 22, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.B.; Xia, S.; Fang, M.X.; Mazi, W.; Zeng, Y.B.; Johnston, T.; Pap, A.; Luck, R.L.; Liu, H.Y. New near-infrared rhodamine dyes with large Stokes shifts for sensitive sensing of intracellular pH changes and fluctuations. Chem. Commun. 2018; Advance article. [Google Scholar] [CrossRef]
- Shen, S.L.; Zhang, X.F.; Ge, Y.Q.; Zhu, Y.; Lang, X.Q.; Cao, X.Q. A near-infrared lysosomal pH probe based on rhodamine derivative. Sens. Actuators B Chem. 2018, 256, 261–267. [Google Scholar] [CrossRef]
- Zhu, W.J.; Niu, J.Y.; He, D.D.; Sun, R.; Xu, Y.J.; Ge, J.F. Near-infrared pH probes based on phenoxazinium connecting with nitrophenyl and pyridinyl groups. Dyes Pigm. 2018, 149, 481–490. [Google Scholar] [CrossRef]
- Yu, H.; Sun, M.T.; Zhang, K.; Zhu, H.J.; Liu, Z.J.; Zhang, Y.J.; Zhao, J.; Wu, L.J.; Zhang, Z.P.; Wang, S.H. A reversible near-infrared pH probes for optical measurements of pH in complete water system and living cells. Sens. Actuators B Chem. 2015, 219, 294–300. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Liu, Z.N.; Lian, P.; Qian, J.; Li, X.W.; Wang, L.; Fu, W.; Chen, L.; Wei, X.B.; Li, C. Selective imaging and cancer cell death via pH switchable near-infrared fluorescence and photothermal effects. Chem. Sci. 2016, 7, 5995–6005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.L.; Jiang, C.; Li, K.; Liu, Y.H.; Xie, Y.M.; Yu, X.Q. Molecular engineering of a dual emission near-infrared ratiometric fluorophore for the detection of pH at the organism level. Analyst 2015, 140, 4608–4615. [Google Scholar] [CrossRef] [PubMed]
- Strobl, M.; Rappitsch, T.; Borisov, S.M.; Mayr, T.; Klimant, I. NIR-emitting aza-BODIPY dyes–new building blocks for broad-range optical pH sensors. Analyst 2015, 140, 7150–7153. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Xia, S.; Bi, J.H.; Fang, M.X.; Mazi, W.F.; Zhang, Y.B.; Conner, N.; Luo, F.T.; Lu, H.P.; Liu, H.Y. Ratiometric Near-Infrared Fluorescent Probes Based On Through Bond Energy Transfer and pi-Conjugation Modulation between Tetraphenylethene and Hemicyanine Moieties for Sensitive Detection of pH Changes in Live Cells. Bioconjug. Chem. 2018, 29, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Jokic, T.; Borisov, S.M.; Saf, R.; Nielsen, D.A.; Kuhl, M.; Klimant, I. Highly Photostable Near-Infrared Fluorescent pH Indicators and Sensors Based on BF2-Chelated Tetraarylazadipyrromethene Dyes. Anal. Chem. 2012, 84, 6723–6730. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, X.; Xie, C.; Ding, N.; Weng, X.F.; Lu, W.Y.; Wei, X.B.; Li, C. Imaging acidosis in tumors using a pH-activated near-infrared fluorescence probe. Chem. Commun. 2012, 48, 11677–11679. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Fu, Y.J.; Liu, Q.L.; Lu, D.T.; Dong, C.; Shuang, S.M. Novel far-visible and near-infrared pH probes based on styrylcyanine for imaging intracellular pH in live cells. Chem. Commun. 2012, 48, 11202–11204. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yu, F.; Li, P.; Tong, L.L.; Duan, X.; Xie, T.; Wang, X. A Near-Infrared Neutral pH Fluorescent Probe for Monitoring Minor pH Changes: Imaging in Living HepG2 and HL-7702 Cells. J. Am. Chem. Soc. 2009, 131, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- He, L.W.; Dong, B.L.; Liu, Y.; Lin, W.Y. Fluorescent chemosensors manipulated by dual/triple interplaying sensing mechanisms. Chem. Soc. Rev. 2016, 45, 6449–6461. [Google Scholar] [CrossRef] [PubMed]
- Rowland, C.E.; Brown, C.W.; Medintz, I.L.; Delehanty, J.B. Intracellular FRET-based probes: A review. Methods Appl. Fluoresc. 2015, 3, 042006. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A Density Functional with Spherical Atom Dispersion Terms. J. Chem. Theory Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef] [PubMed]
- Raghavachari, K.; Trucks, G.W. Highly correlated systems: Excitation energies of first row transition metals Sc-Cu. J. Chem. Phys. 1989, 91, 1062–1065. [Google Scholar] [CrossRef]
- Wachters, A.J.H. Gaussian basis set for molecular wavefunctions containing third-row atoms. J. Chem. Phys. 1970, 52, 1033–1036. [Google Scholar] [CrossRef]
- Hay, P.J. Gaussian basis sets for molecular calculations–representation of 3D orbitals in transition-metal atoms. J. Chem. Phys. 1977, 66, 4377–4384. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision, A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Fang, M.X.; Xia, S.; Bi, J.H.; Wigstrom, T.P.; Valenzano, L.; Wang, J.B.; Mazi, W.; Tanasova, M.; Luo, F.T.; Liu, H.Y. A cyanine-based fluorescent cassette with aggregation-induced emission for sensitive detection of pH changes in live cells. Chem. Comm. 2018, 54, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Zhang, S.W.; Jaishi, M.; Adhikari, R.; Bi, J.H.; Fang, M.X.; Xia, S.; Zhang, Y.B.; Luck, R.L.; Pati, R.; et al. New Near-Infrared Fluorescent Probes with Single-Photon Anti-Stokes-Shift Fluorescence for Sensitive Determination of pH Variances in Lysosomes with a Double-Checked Capability. ACS Appl. Bio Mater. 2018, 1, 549–560. [Google Scholar] [CrossRef]
- Ren, T.; Xu, W.; Jin, F.; Cheng, D.; Zhang, L.; Yuan, L.; Zhang, X. Rational Engineering of Bioinspired Anthocyanidin Fluorophores with Excellent Two-Photon Properties for Sensing and Imaging. Anal. Chem. 2017, 89, 11427–11434. [Google Scholar] [CrossRef] [PubMed]
- Avogadro an Open-Source Molecular Builder and Visualization Tool. Version 1.2.0. Available online: http://avogadro.cc/ (accessed on 12 October 2018).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1998, 108, 4439–4449. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anistropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
Sample Availability: Samples of the compounds are all available from the authors. |










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Bi, J.; Xia, S.; Mazi, W.; Wan, S.; Mikesell, L.; Luck, R.L.; Liu, H. A Near-Infrared Fluorescent Probe Based on a FRET Rhodamine Donor Linked to a Cyanine Acceptor for Sensitive Detection of Intracellular pH Alternations. Molecules 2018, 23, 2679. https://doi.org/10.3390/molecules23102679
Zhang Y, Bi J, Xia S, Mazi W, Wan S, Mikesell L, Luck RL, Liu H. A Near-Infrared Fluorescent Probe Based on a FRET Rhodamine Donor Linked to a Cyanine Acceptor for Sensitive Detection of Intracellular pH Alternations. Molecules. 2018; 23(10):2679. https://doi.org/10.3390/molecules23102679
Chicago/Turabian StyleZhang, Yibin, Jianheng Bi, Shuai Xia, Wafa Mazi, Shulin Wan, Logan Mikesell, Rudy L. Luck, and Haiying Liu. 2018. "A Near-Infrared Fluorescent Probe Based on a FRET Rhodamine Donor Linked to a Cyanine Acceptor for Sensitive Detection of Intracellular pH Alternations" Molecules 23, no. 10: 2679. https://doi.org/10.3390/molecules23102679