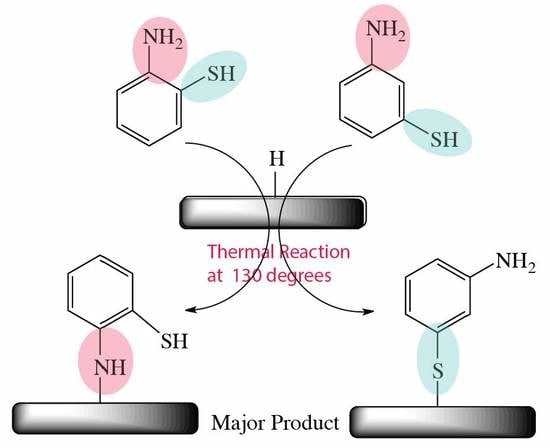
XPS Analysis of 2- and 3-Aminothiophenol Grafted on Silicon (111) Hydride Surfaces
Abstract
:1. Introduction
2. Results
3. Discussion
4. Methods and Materials
4.1. Thermal Reaction Protocol
4.2. Atomic Force Microscopy
4.3. X-ray Photoelectron Spectroscopy (XPS)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cao, X.P.; Hamers, R.J. Silicon surfaces as electron acceptors: Dative bonding of amines with si(001) and si(111) surfaces. J. Am. Chem. Soc. 2001, 123, 10988–10996. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.P.; Hamers, R.J. Interactions of alkylamines with the silicon (001) surface. J. Vac. Sci. Tech. B 2002, 20, 1614–1619. [Google Scholar] [CrossRef]
- Mui, C.; Wang, G.T.; Bent, S.F.; Musgrave, C.B. Reactions of methylamines at the si(100)-2x1 surface. J. Chem. Phys. 2001, 114, 10170–10180. [Google Scholar] [CrossRef]
- Tung, J.; Ching, J.Y.; Ng, Y.M.; Tew, L.S.; Khung, Y.L. Grafting of ring-opened cyclopropylamine thin films on silicon (100) hydride via uv photoionization. ACS Appl. Mater. Interfaces 2017, 9, 31083–31094. [Google Scholar] [CrossRef] [PubMed]
- Boukherroub, R.; Morin, S.; Bensebaa, F.; Wayner, D.D.M. New synthetic routes to alkyl monolayers on the si(111) surface. Langmuir 1999, 15, 3831–3835. [Google Scholar] [CrossRef]
- Buriak, J.M. Silicon-carbon bonds on porous silicon surfaces. Adv. Mater. 1999, 11, 265–267. [Google Scholar] [CrossRef]
- Buriak, J.M. Illuminating silicon surface hydrosilylation: An unexpected plurality of mechanisms. Chem. Mater. 2014, 26, 763–772. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Iqbal, M.; Dobbie, A.R.; Veinot, J.G.C. Surface-induced alkene oligomerization: Does thermal hydrosilylation really lead to monolayer protected silicon nanocrystals? J. Am. Chem. Soc. 2013, 135, 17595–17601. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.X.; Hessel, C.M.; Bogart, T.D.; Panthani, M.G.; Rasch, M.R.; Korgel, B.A. Room temperature hydrosilylation of silicon nanocrystals with bifunctional terminal alkenes. Langmuir 2013, 29, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Ruther, R.E.; Streifer, J.A.; Hamers, R.J. Uv-induced grafting of alkenes to silicon surfaces: Photoemission versus excitons. J. Am. Chem. Soc. 2010, 132, 4048–4049. [Google Scholar] [CrossRef] [PubMed]
- Buriak, J.M. Organometallic chemistry on silicon and germanium surfaces. Chem. Rev. 2002, 102, 1271–1308. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, T.; Alkunshalie, T.; Richardson, N.V. An hreels investigation of the adsorption of benzoic acid and aniline on si(100)-2x1. Surf. Sci. 1996, 368, 202–207. [Google Scholar] [CrossRef]
- Rummel, R.M.; Ziegler, C. Room temperature adsorption of aniline (c6h5nh2) on si(100)(2x1) observed with scanning tunneling microscopy. Surf. Sci. 1998, 418, 303–313. [Google Scholar] [CrossRef]
- Sieval, A.B.; Linke, R.; Heij, G.; Meijer, G.; Zuilhof, H.; Sudholter, E.J.R. Amino-terminated organic monolayers on hydrogen-terminated silicon surfaces. Langmuir 2001, 17, 7554–7559. [Google Scholar] [CrossRef]
- Bocking, T.; Kilian, K.A.; Hanley, T.; Ilyas, S.; Gaus, K.; Gal, M.; Gooding, J.J. Formation of tetra(ethylene oxide) terminated si-c linked monolayers and their derivatization with glycine: An example of a generic strategy for the immobilization of biomolecules on silicon. Langmuir 2005, 21, 10522–10529. [Google Scholar] [CrossRef] [PubMed]
- Chopra, T.P.; Longo, R.C.; Cho, K.; Halls, M.D.; Thissen, P.; Chabal, Y.J. Ethylenediamine grafting on oxide-free h-, 1/3 ml f-, and cl-terminated si(111) surfaces. Chem. Mater. 2015, 27, 6268–6281. [Google Scholar] [CrossRef]
- Khung, Y.L.; Ngalim, S.H.; Scaccabarozi, A.; Narducci, D. Thermal and uv hydrosilylation of alcohol-based bifunctional alkynes on si (111) surfaces: How surface radicals influence surface bond formation. Sci. Rep. 2015, 5, 11299. [Google Scholar] [CrossRef] [PubMed]
- Khung, Y.L.; Ngalim, S.H.; Scaccabarozzi, A.; Narducci, D. Formation of stable si-o-c submonolayers on hydrogen-terminated silicon(111) under low-temperature conditions. Beilstein J. Nanotechnol. 2015, 6, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.; Khung, Y.L. Influences of doping and crystal orientation on surface roughening upon alcohol grafting onto silicon hydride. Appl. Sci. 2017, 7, 859. [Google Scholar] [CrossRef]
- Tung, J.; Tew, L.S.; Coluccini, C.; Lin, Y.-D.; Khung, Y.L. Grafting behavior for the resonating variants of ethynylaniline on hydrogenated silicon (100) surfaces under thermal hydrosilylation. Chem. Eur. J. 2018, 24, 13270–13277. [Google Scholar] [CrossRef] [PubMed]
- Khung, Y.L.; Narducci, D. Surface modification strategies on mesoporous silica nanoparticles for anti-biofouling zwitterionic film grafting. Adv. Colloid Interf. Sci. 2015, 226, 166–186. [Google Scholar] [CrossRef] [PubMed]
- Khung, Y.L.; Graney, S.D.; Voelcker, N.H. Micropatterning of porous silicon films by direct laser writing. Biotech. Prog. 2006, 22, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Khung, Y.L.; Barritt, G.; Voelcker, N.H. Using continuous porous silicon gradients to study the influence of surface topography on the behaviour of neuroblastoma cells. Exp. Cell. Res. 2008, 314, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Wiggins, M.D.; Baird, R.J.; Wynblatt, P. Thermal nitridation of si(111) by nitric oxide. J. Vac. Sci. Tech. 1981, 18, 965–970. [Google Scholar] [CrossRef]
- Thissen, P.; Peixoto, T.; Longo, R.C.; Peng, W.; Schmidt, W.G.; Cho, K.; Chabal, Y.J. Activation of surface hydroxyl groups by modification of h- terminated si(111) surfaces. J. Am. Chem. Soc. 2012, 134, 8869–8874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Liao, L.S.; Chan, W.H.; Lee, S.T.; Sammynaiken, R.; Sham, T.K. Electronic structure of silicon nanowires: A photoemission and x-ray absorption study. Phys. Rev. B 2000, 61, 8298–8305. [Google Scholar] [CrossRef]
- Kamineni, H.S.; Kamineni, V.K.; Moore, R.L., II; Gallis, S.; Diebold, A.C.; Huang, M.; Kaloyeros, A.E. Optical and structural characterization of thermal oxidation effects of erbium thin films deposited by electron beam on silicon. J. Appl. Phys. 2012, 111, 013104. [Google Scholar] [CrossRef] [Green Version]
- Mannella, N.; Gabetta, G.; Parmigiani, F. Plasmon energy shift in porous silicon measured by x-ray photoelectron spectroscopy. Appl. Phys. Lett. 2001, 79, 4432–4434. [Google Scholar] [CrossRef]
- Senkevich, J.J.; Yang, G.R.; Tang, F.; Wang, G.C.; Lu, T.M.; Cale, T.S.; Jezewski, C.; Lanford, W.A. Substrate-independent sulfur-activated dielectric and barrier-layer surfaces to promote the chemisorption of highly polarizable metallorganics. Appl. Phys. A Mater. Sci. 2004, 79, 1789–1796. [Google Scholar] [CrossRef]
- Dwivedi, N.; Rismani-Yazdi, E.; Yeo, R.J.; Goohpattader, P.S.; Satyanarayana, N.; Srinivasan, N.; Druz, B.; Tripathy, S.; Bhatia, C.S. Probing the role of an atomically thin sinx interlayer on the structure of ultrathin carbon films. Sci. Rep. 2014, 4, 5021. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.B.; Tay, B.K.; Chen, G.; Yang, S.R. Synthesis of silicon carbide nitride nanocomposite films by a simple electrochemical method. Electrochem. Commun. 2006, 8, 737–740. [Google Scholar] [CrossRef]
- Diller, K.; Klappenberger, F.; Marschall, M.; Hermann, K.; Nefedov, A.; Woll, C.; Barth, J.V. Self-metalation of 2h-tetraphenylporphyrin on cu(111): An x-ray spectroscopy study. J. Chem. Phys. 2012, 136, 014705. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.X.; Hu, X.X.; Ding, X.L.; Kong, H.C.; Sha, H.D.; Lin, H.; Wen, W.; Shen, G.X.; Guo, Z.; Ma, Z.F.; et al. Effects of cobalt precursor on pyrolyzed carbon-supported cobalt-polypyrrole as electrocatalyst toward oxygen reduction reaction. Nanoscale Res. Lett. 2013, 8, 478. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, C.Z.; Chan, K.Y. Structuring porous iron-nitrogen-doped carbon in a core/shell geometry for the oxygen reduction reaction. Adv. Eng. Mater. 2014, 4, 1400840. [Google Scholar] [CrossRef] [Green Version]
- Techane, S.D.; Gamble, L.J.; Castner, D.G. X-ray photoelectron spectroscopy characterization of gold nanoparticles functionalized with amine-terminated alkanethiols. Biointerphases 2011, 6, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.E.; Weidner, T.; Brison, J.; Graham, D.J.; Gamble, L.J.; Castner, D.G. Amine terminated sams: Investigating why oxygen is present in these films. J. Electron. Spectros. Relat. Phenomena. 2009, 172, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Thissen, P.; Seitz, O.; Chabal, Y.J. Wet chemical surface functionalization of oxide-free silicon. Prog. Surf. Sci. 2012, 87, 272–290. [Google Scholar] [CrossRef]
- Cleland, G.; Horrocks, B.R.; Houlton, A. Direct functionalization of silicon via the self-assembly of alcohols. J. Chem. Soc. Faraday Trans. 1995, 91, 4001–4003. [Google Scholar] [CrossRef]
- Khung, Y.L.; Ngalim, S.H.; Meda, L.; Narducci, D. Preferential formation of si-o-c over si-c linkage upon thermal grafting on hydrogen-terminated silicon (111). Chem. Eur. J. 2014, 20, 15151–15158. [Google Scholar] [CrossRef] [PubMed]
- Hacker, C.A.; Anderson, K.A.; Richter, L.J.; Richter, C.A. Comparison of si-o-c interfacial bonding of alcohols and aldehydes on si(111) formed from dilute solution with ultraviolet irradiation. Langmuir 2005, 21, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Ching, J.Y.; Lee, C.-H.; Khung, Y.L. Bioactivating silicon (100) surfaces with novel uv grafting of cyclopropylamine for promotion of cell adhesion. Materials 2018, 11, 713. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2- and 3- aminothiophenol and the surface grafts are available from the authors. |







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-H.; Chen, W.-C.; Khung, Y.L. XPS Analysis of 2- and 3-Aminothiophenol Grafted on Silicon (111) Hydride Surfaces. Molecules 2018, 23, 2712. https://doi.org/10.3390/molecules23102712
Lee C-H, Chen W-C, Khung YL. XPS Analysis of 2- and 3-Aminothiophenol Grafted on Silicon (111) Hydride Surfaces. Molecules. 2018; 23(10):2712. https://doi.org/10.3390/molecules23102712
Chicago/Turabian StyleLee, Chieh-Hua, Wan-Cian Chen, and Yit Lung Khung. 2018. "XPS Analysis of 2- and 3-Aminothiophenol Grafted on Silicon (111) Hydride Surfaces" Molecules 23, no. 10: 2712. https://doi.org/10.3390/molecules23102712