Effects of Water Content and Particle Size on Yield and Reactivity of Lignite Chars Derived from Pyrolysis and Gasification
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Water Content on Char Yield
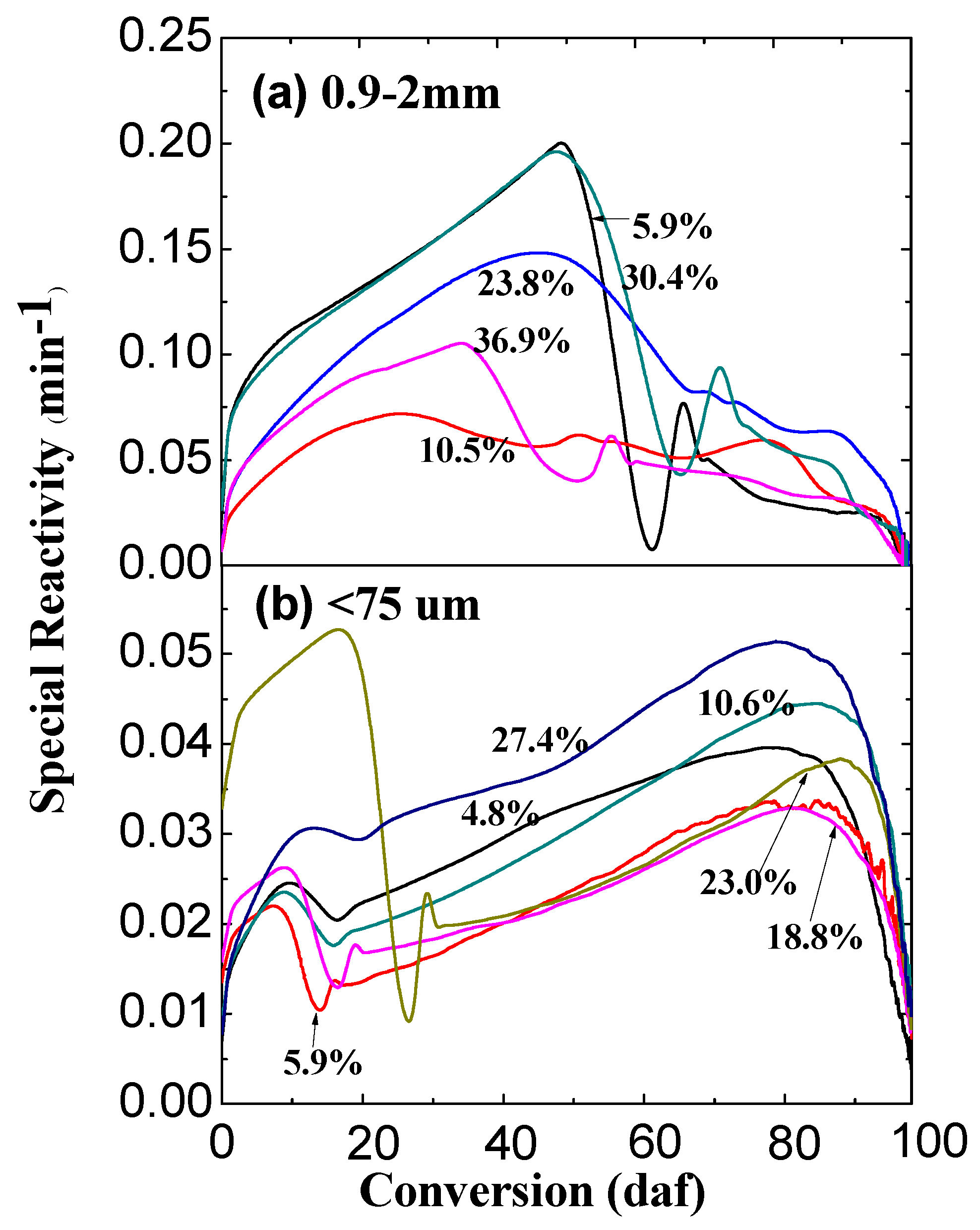
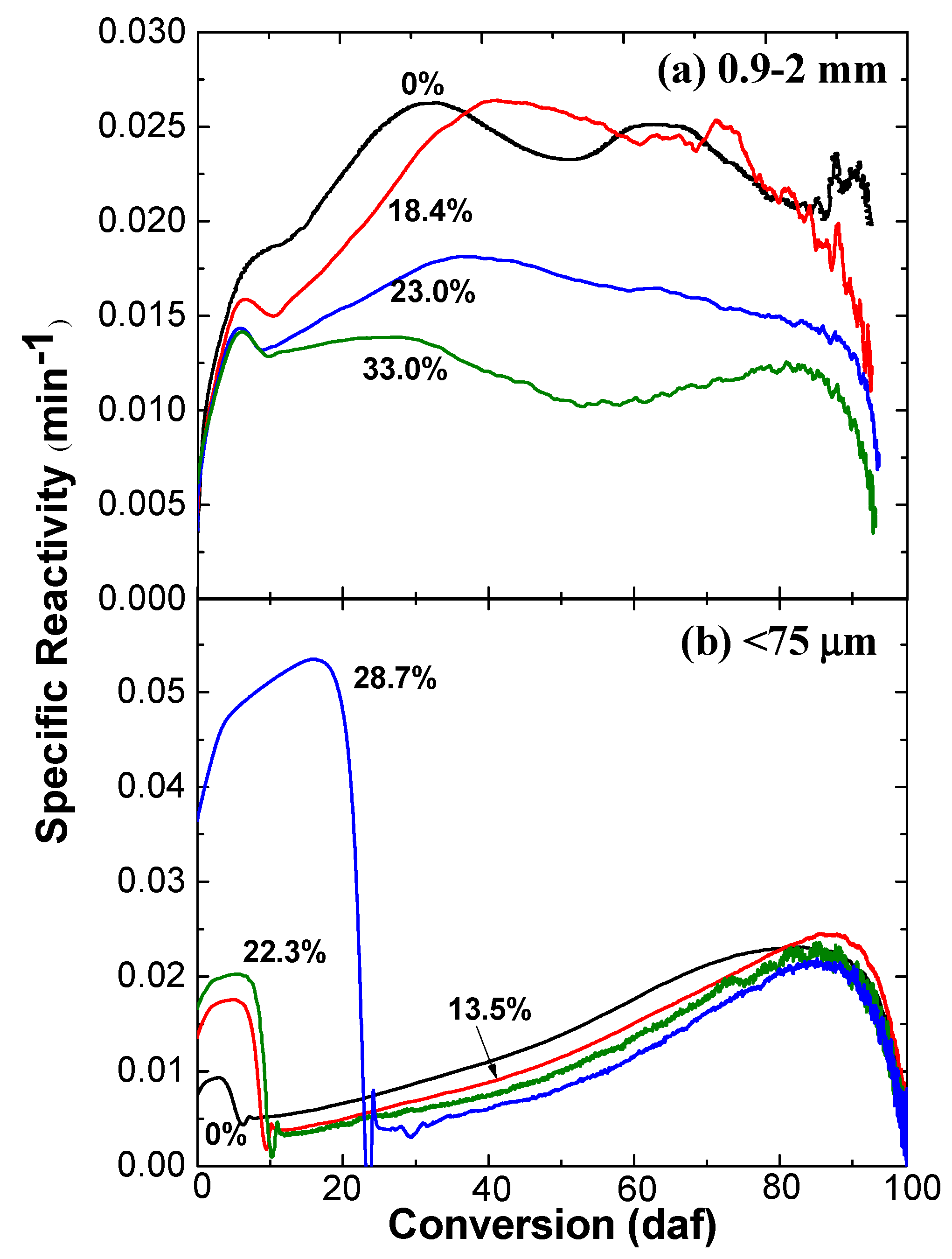
2.2. Effect of Water Content on Ex-Situ Char Reactivity
3. Materials and Methods
3.1. Coal Sample
3.2. Pyrolysis and Gasification
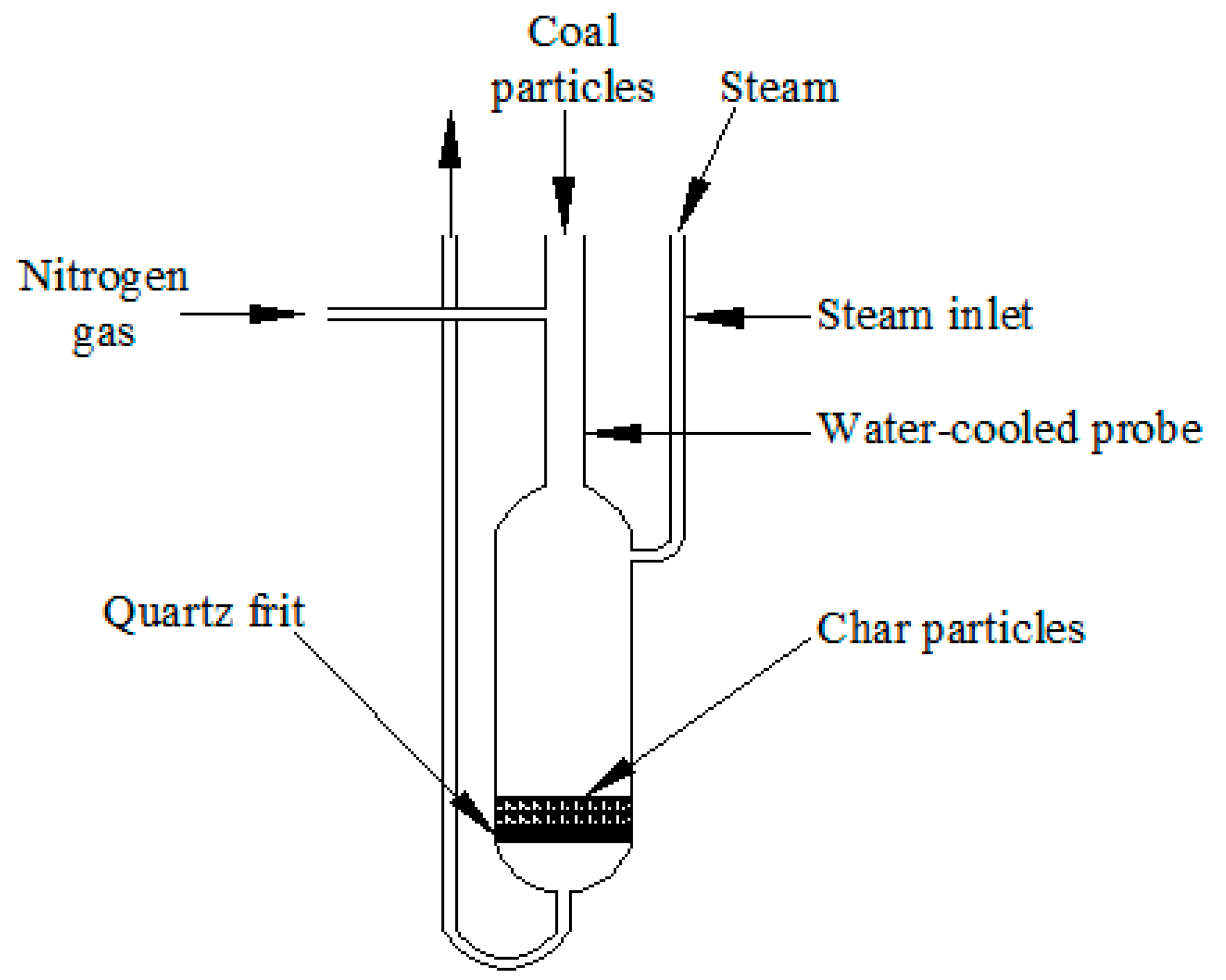
3.3. Reactivity Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, C.Z. Advances in the Science of Victorian Brown Coal; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Tian, B.; Qiao, Y.; Bai, L.; Feng, W.; Jiang, Y.; Tian, Y. Pyrolysis behavior and kinetics of the trapped small molecular phase in a lignite. Energy Convers. Manag. 2017, 140, 109–120. [Google Scholar] [CrossRef]
- Byambajav, E.; Hachiyama, Y.; Kudo, S.; Norinaga, K.; Hayashi, J. Kinetics and Mechanism of CO2 Gasification of Chars from 11 Mongolian Lignites. Energy Fuels 2016, 30, 1636–1646. [Google Scholar] [CrossRef]
- Fan, X.; Wang, C.F.; You, C.Y.; Wei, X.Y.; Chen, L.; Cao, J.P.; Zhao, Y.P.; Zhao, W.; Wang, Y.G.; Lu, J.L. Characterization of a Chinese lignite and the corresponding derivatives using direct analysis in real time quadrupole time-of-flight mass spectrometry. RSC Adv. 2016, 6, 105780–105785. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, S.; Ren, H.; Wang, N.; Zhu, H. Research of the drying characteristics of Tongliao-origin lignite in a fluidized bed dryer. Coal Prep. Technol. 2007, 4, 43–47. [Google Scholar]
- Kakaras, E.; Ahladas, P.; Syrmopoulos, S. Computer simulation studies for the integration of an external dryer into a Greek lignite-fired power plant. Fuel 2002, 81, 583–593. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Laursen, K.; Nakagawa, H.; Miura, K. Hydrothermal upgrading of Loy Yang Brown coal—Effect of upgrading conditions on the characteristics of the products. Fuel Process. Technol. 2008, 89, 391–396. [Google Scholar] [CrossRef]
- Bai, L.; Kudo, S.; Norinaga, K.; Wang, Y.G.; Hayashi, J.I. Kinetics and Mechanism of Steam Gasification of Char from Hydrothermally Treated Woody Biomass. Energy Fuels 2014, 28, 7133–7139. [Google Scholar] [CrossRef]
- Mursito, A.T.; Hirajima, T.; Sasaki, K. Upgrading and dewatering of raw tropical peat by hydrothermal treatment. Fuel 2010, 89, 635–641. [Google Scholar] [CrossRef]
- Bergins, C. Kinetics and mechanism during mechanical/thermal dewatering of lignite. Fuel 2003, 82, 355–364. [Google Scholar] [CrossRef]
- Bergins, C. Mechanical/thermal dewatering of lignite. Part 2: A rheological model for consolidation and creep process. Fuel 2004, 83, 267–276. [Google Scholar] [CrossRef]
- Bergins, C.; Hulston, J.; Strauss, K.; Chaffee, A.L. Mechanical/thermal dewatering of lignite. Part 3: Physical properties and pore structure of MTE product coals. Fuel 2007, 86, 3–16. [Google Scholar] [CrossRef]
- Vogt, C.; Wild, T.; Bergins, C.; Strauß, K.; Hulston, J.; Chaffee, A.L. Mechanical/thermal dewatering of lignite. Part 4: Physico-chemical properties and pore structure during an acid treatment within the MTE process. Fuel 2012, 93, 433–442. [Google Scholar] [CrossRef]
- Seepana, S.; Jayanti, S. Steam-moderated oxy-fuel combustion. Energy Convers. Manag. 2010, 51, 1981–1988. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Zhang, W.D.; Liu, P.; Zeng, G.; Sun, S.Z.; Wang, P.X.; Zhang, S. Kinetic characteristics of in-situ char gasification following the pyrolysis of a demineralized coal. Int. J. Hydrog. Energy 2018, 43, 10991–11001. [Google Scholar] [CrossRef]
- Yu, J.; Tahmasebi, A.; Han, Y.; Yin, F.; Li, X. A review on water in low rank coals: The existence, interaction with coal structure and effects on coal utilization. Fuel Process. Technol. 2013, 106, 9–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, X.; Jing, K.; Chang, L.; Bao, W. Study on the pore structure and oxygen-containing functional groups devoting to the hydrophilic force of dewatered lignite. Appl. Surf. Sci. 2015, 324, 90–98. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Bai, L.; Chen, Y.; Zhang, S.; Lin, X. Impacts of Inherent O-Containing Functional Groups on the Surface Properties of Shengli Lignite. Energy Fuels 2014, 28, 862–867. [Google Scholar] [CrossRef]
- Meng, X.; Gao, M.; Chu, R.; Miao, Z.; Wu, G.; Bai, L.; Liu, P.; Yan, Y.; Zhang, P. Construction of a macromolecular structural model of Chinese lignite and analysis of its low-temperature oxidation behavior. Chin. J. Chem. Eng. 2017, 25, 1314–1321. [Google Scholar] [CrossRef]
- Tian, B.; Qiao, Y.; Bai, L.; Liu, F.; Tian, Y.; Xie, K. Separation and structural characterization of groups from a high-volatile bituminous coal based on multiple techniques. Fuel Process. Technol. 2017, 159, 386–395. [Google Scholar] [CrossRef]
- Zhang, S.; Min, Z.; Tay, H.L.; Asadullah, M.; Li, C.Z. Effects of volatile–char interactions on the evolution of char structure during the gasification of Victorian brown coal in steam. Fuel 2011, 90, 1529–1535. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Chen, Z.; Chen, X.; Zhang, H.; Bai, L.; Zhang, S. Variations in char structure and reactivity due to the pyrolysis and in-situ gasification using Shengli brown coal. J. Anal. Appl. Pyrolysis 2015, 115, 233–241. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Dong, L.; Li, C.Z. Effects of gasification atmosphere and temperature on char structural evolution during the gasification of Collie sub-bituminous coal. Fuel 2014, 117, 1190–1195. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Chen, Z.; Bai, L.; Zhang, K.; Yang, S.; Zhang, S. Influence of cooling treatments on char microstructure and reactivity of Shengli brown coal. J. Fuel Chem. Technol. 2015, 43, 1–8. [Google Scholar] [CrossRef]
Sample Availability: Samples of the char samples reported in this study are available from the authors. |

| Pyrolysis | Gasification | |||
|---|---|---|---|---|
| 0.9–2 mm | <75 µm | 0.9–2 mm | <75 µm | |
| Water Content (wt %) | 5.9 | 4.8 | 0 | 0 |
| 10.5 | 5.9 | 18.4 | 13.5 | |
| 23.8 | 10.6 | 23.0 | 22.3 | |
| 30.4 | 18.8 | 33.0 | 28.7 | |
| 36.9 | 23.0 | |||
| 27.4 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wang, Y.; Zhou, H.; Gao, Y.; Xu, D.; Bai, L.; Zhang, S. Effects of Water Content and Particle Size on Yield and Reactivity of Lignite Chars Derived from Pyrolysis and Gasification. Molecules 2018, 23, 2717. https://doi.org/10.3390/molecules23102717
Huang Y, Wang Y, Zhou H, Gao Y, Xu D, Bai L, Zhang S. Effects of Water Content and Particle Size on Yield and Reactivity of Lignite Chars Derived from Pyrolysis and Gasification. Molecules. 2018; 23(10):2717. https://doi.org/10.3390/molecules23102717
Chicago/Turabian StyleHuang, Yong, Yonggang Wang, Hao Zhou, Yaxuan Gao, Deliang Xu, Lei Bai, and Shu Zhang. 2018. "Effects of Water Content and Particle Size on Yield and Reactivity of Lignite Chars Derived from Pyrolysis and Gasification" Molecules 23, no. 10: 2717. https://doi.org/10.3390/molecules23102717






