Rosmarinic Acid Derivatives’ Inhibition of Glycogen Synthase Kinase-3β Is the Pharmacological Basis of Kangen-Karyu in Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
2.1. Glycogen Synthase Kinase-3β (GSK-3β) Inhibition
2.2. GSK-3β Enzyme Kinetics
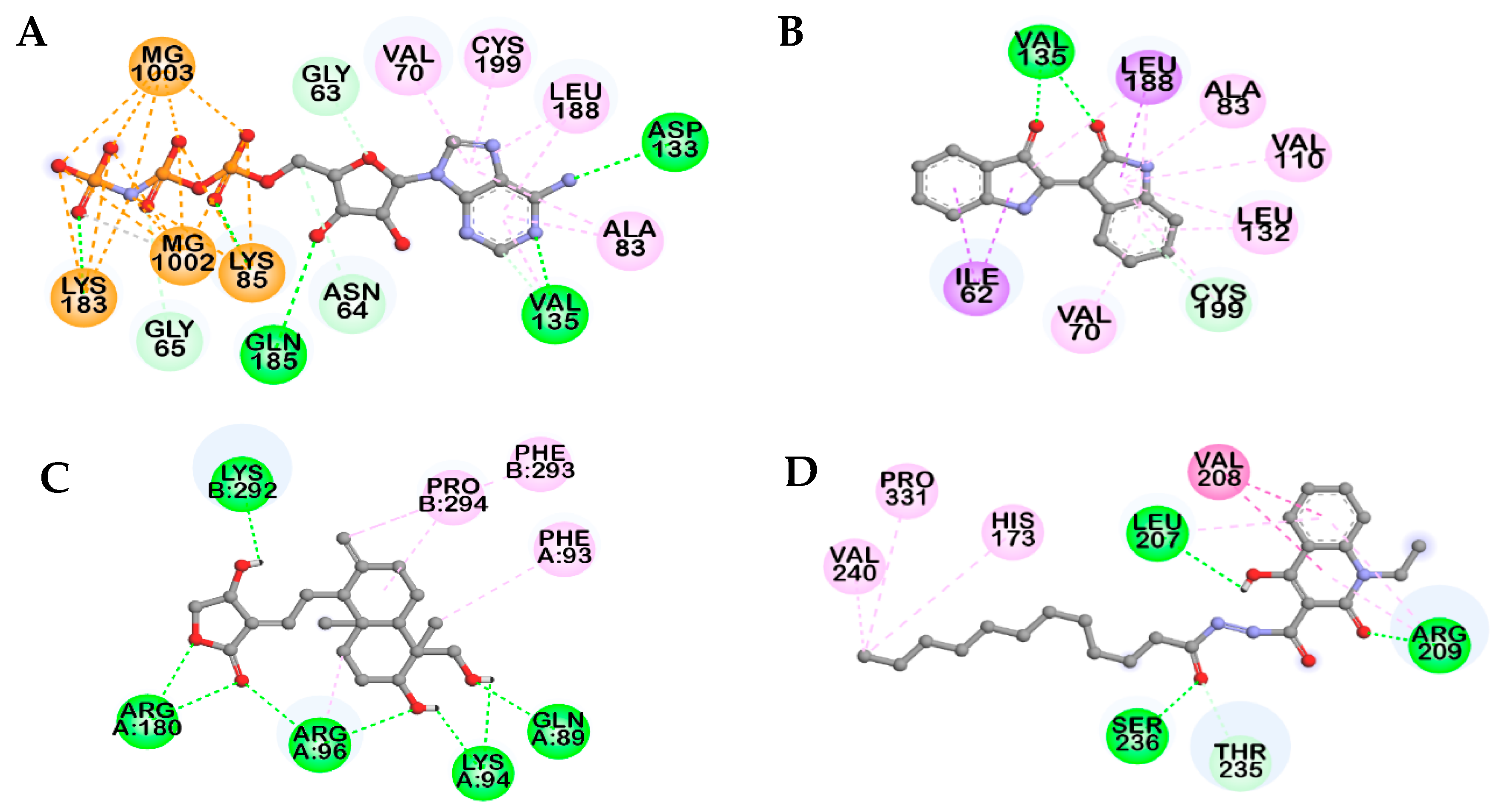
2.3. Docking Studies for GSK-3β Inhibition
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals and Reagents
4.3. Extract Preparation
4.4. GSK-3β Enzyme Inhibition Assay
4.5. GSK-3β Enzyme Kinetics
4.6. Docking Studies for GSK-3β Inhibition
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ADT | AutoDockTools |
| GSK-3β | Glycogen synthase kinase-3β |
| KK | Kangen-karyu |
| TCM | Traditional Chinese medicine |
References
- Hooper, C.; Killick, R.; Lovestone, S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008, 104, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Johnson, G.V.W. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004, 29, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front. Mol. Neurosci. 2011, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Sachs, B.D.; Rodriguiz, R.M.; Siesser, W.B.; Kenan, A.; Royer, E.L.; Jacobsen, J.P.; Wetsel, W.C.; Caron, M.G. The effects of brain serotonin deficiency on behavioural disinhibition and anxiety-like behaviour following mild early life stress. Int. J. Neuropsychopharmacol. 2013, 16, 2081–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. Akt/GSK3 signaling in the action of psychotropic drugs. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Freyberg, Z.; Ferrando, S.J.; Javitch, J.A. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am. J. Psychiatry. 2010, 167, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K. An emerging role for Wnt and GSK3 signaling pathways in schizophrenia. Clin. Genet. 2013, 83, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Mines, M.A.; Beurel, E.; Jope, R.S. Regulation of cell survival mechanisms in Alzheimer’s disease by glycogen synthase kinase-3. Int. J. Alzheimers Dis. 2011, 2011, 861072. [Google Scholar] [CrossRef] [PubMed]
- Phiel, C.J.; Wilson, C.A.; Lee, V.M.Y.; Klein, P.S. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature 2003, 423, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen synthase kinase-3 (GSK-3): Inflammation, diseases, and therapeutics. Neurochem. Res. 2007, 32, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, A.S.; Woodgett, J.R. Role of glycogen synthase kinase-3 in cancer: Regulation by Wnts and other signaling pathways. Adv. Cancer Res. 2002, 84, 203–229. [Google Scholar] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; Maestro, R.; et al. Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: Opportunities for therapeutic intervention. Leukemia 2014, 28, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Amar, S.; Belmaker, R.H.; Agam, G. The possible involvement of glycogen synthase kinase-3 (GSK-3) in diabetes, cancer and central nervous system diseases. Curr. Pharm. Des. 2011, 17, 2264–2277. [Google Scholar] [PubMed]
- MacAulay, K.; Woodgett, J.R. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of Type 2 diabetes. Expert Opin. Ther. Targets 2008, 12, 1265–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewhurst, S.; Maggirwar, S.B.; Schifitto, G.; Gendelman, H.E.; Gelbard, H.A. Glycogen synthase kinase 3β (GSK-3β) as a therapeutic target in neuroAIDS. J. Neuroimmune Pharmacol. 2007, 2, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.V.; Bailey, C.D. Tau, where are we now? J. Alzheimers Dis. 2002, 4, 375–398. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, T.; Dewachter, I.; Lechat, B.; Gees, M.; Kremer, A.; Demedts, D.; Borghgraef, P.; Devijver, H.; Kügler, S.; Patel, S.; et al. GSK-3α/β kinases and amyloid production in vivo. Nature 2011, 480, E4–E5. [Google Scholar] [CrossRef] [PubMed]
- Hohman, T.J.; Koran, M.E.; Thornton-Wells, T.A. Interactions between GSK3β and amyloid genes explain variance in amyloid burden. Neurobiol. Aging 2014, 35, 460–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; Kim, Y.H.; Hebisch, M.; Sliwinski, C.; Lee, S.; D’Avanzo, C.; Chen, H.; Hooli, B.; Asselin, C.; Muffat, J.; et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 2014, 515, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.L.; et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Li, Q.X. Discovery of selective, substrate-competitive, and passive membrane permeable glycogen synthase kinase-3β inhibitors: Synthesis, biological evaluation, and molecular modeling of new C-glycosylflavones. ACS Chem. Neurosci. 2018, 9, 1166–1183. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Ishiguro, K.; Uchida, T.; Takashima, A.; Lemere, C.A.; Imahori, K. Preferential labeling of Alzheimer neurofibrillary tangles with antisera for tau protein kinase (TPK) I/glycogen synthase kinase-3β and cyclin-dependent kinase 5, a component of TPK II. Acta Neuropathol. 1996, 92, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Malagon, S.G.; Lopez Muñoz, A.M.; Doro, D.; Bolger, T.G.; Poon, E.; Tucker, E.R.; Adel Al-Lami, H.; Krause, M.; Phiel, C.J.; Chesler, L.; et al. Glycogen synthase kinase 3 controls migration of the neural crest lineage in mouse and Xenopus. Nat. Commun. 2018, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Park, C.H.; Matsumoto, K. Scientific evidence for therapeutic effects of Chinese prescription Kangen-karyu from pre-clinical animal experiments. Drug Discov. Ther. 2017, 11, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yokozawa, T.; Yamabe, N.; Tsuneyama, K.; Li, X.; Matsumoto, K. Kangen-karyu improves memory deficit caused by aging through normalization of neuro-plasticity-related signaling system and VEGF system in the brain. J. Ethnopharmacol. 2010, 131, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Yokozawa, T.; Cho, E.J.; Okamoto, T.; Sei, Y. Antioxidative effects related to the potential anti-aging properties of the Chinese prescription Kangen-karyu and Carthami Flos in senescence-accelerated mice. Arch. Gerontol. Geriatr. 2004, 39, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Okamoto, T.; Yokozawa, T. Beneficial effects of Chinese prescription Kangen-karyu on diabetes associated with hyperlipidemia, advanced glycation endproducts, and oxidative stress in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2009, 124, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Motohashi, K.; Kaneko, T.; Tanaka, Y.; Manome, N.; Irie, K.; Takata, J.; Egashira, N.; Oishi, R.; Okamoto, T.; et al. Neuroprotective effects of kangen-karyu on spatial memory impairment in an 8-arm radial maze and neuronal death in the hippocampal CA1 region induced by repeated cerebral ischemia in rats. J. Pharmacol. Sci. 2009, 109, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Noh, J.S.; Yamabe, N.; Okamoto, T.; Kang, K.S.; Zhao, Q.; Matsumoto, K.; Shibahara, N.; Yokozawa, T. Renoprotective effect of Kangen-karyu on the development of diabetic nephropathy in Type 2 diabetic db/db mice. J. Tradit. Med. 2010, 27, 192–203. [Google Scholar]
- Bhat, R.; Xue, Y.; Berg, S.; Hellberg, S.; Ormö, M.; Nilsson, Y.; Radesäter, A.C.; Jerning, E.; Markgren, P.O.; Borgegård, T. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J. Biol. Chem. 2003, 278, 45937–45945. [Google Scholar] [CrossRef] [PubMed]
- Leost, M.; Schultz, C.; Link, A.; Wu, Y.Z.; Biernat, J.; Mandelkow, E.M.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Zaharevitz, D.W.; et al. Paullones are potent inhibitors of glycogen synthase kinase-3β and cyclin-dependent kinase 5/p25. Eur. J. Biochem. 2000, 267, 5983–5994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclerc, S.; Garnier, M.; Hoessel, R.; Marko, D.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Biernat, J.; Wu, Y.Z.; Mandelkow, E.M.; et al. Indirubins inhibit glycogen synthase kinase-3β and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J. Biol. Chem. 2001, 276, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.G.; Danysz, W.; Dekundy, A.; Pulte, I. Memantine and cholinesterase inhibitors: Complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox. Res. 2013, 24, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Andersen, H.F. Analysis of the effect of memantine in reducing the worsening of clinical symptoms in patients with moderate to severe Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2007, 24, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Krishnankutty, A.; Kimura, T.; Saito, T.; Aoyagi, K.; Asada, A.; Takahashi, S.I.; Ando, K.; Ohara-Imaizumi, M.; Ishiguro, K.; Hisanaga, S.I. In vivo regulation of glycogen synthase kinase 3β activity in neurons and brains. Sci. Rep. 2017, 7, 8602. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Paudel, P.; Seong, S.H.; Kim, J.A.; Jung, H.A.; Choi, J.S. Computational insights into β-site amyloid precursor protein enzyme 1 (BACE1) inhibition by tanshinones and salvianolic acids from Salvia miltiorrhiza via molecular docking simulations. Comput. Biol. Chem. 2018, 74, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, K.; Sun, C.; Zheng, M. Analysis of active components in Salvia miltiorrhiza injection based on vascular endothelial cells protection. Acta Pharm. 2014, 64, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Wan, P.; Niu, M.; Liu, Q.; Zhang, Y.-Q.; Zhang, Z.-Y. Autotoxins Screening from aqueous extracts of Salvia miltiorrhiza Bge. based on spectrum-effect relationship between HPLC fingerprints and autotoxicity. Pak. J. Bot 2016, 48, 1467–1471. [Google Scholar]
- Chen, J.; Lee, F.S.C.; Li, L.; Yang, B.; Wang, X. Standardized extracts of Chinese medicinal herbs: Case study of Danshen (Salvia miltiorrhiza Bunge). J. Food Drug Anal. 2007, 15, 347–364. [Google Scholar]
- Fang, J.; Huang, D.; Zhao, W.; Ge, H.; Luo, H.B.; Xu, J. A new protocol for predicting novel GSK-3β ATP competitive inhibitors. J. Chem. Inf. Model. 2011, 51, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Gallivan, J.P.; Dougherty, D.A. Cation-π interactions in structural biology. Proc. Natl. Acad. Sci. USA 1999, 96, 9459–9464. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Liu, C.; Pang, M.; He, L.; Yang, B.; Fan, L.; Zhang, S.; Wang, X.; Liu, B.; Rong, L. Salvianolic acid B promotes neural differentiation of induced pluripotent stem cells via PI3K/AKT/GSK3β/β-catenin pathway. Neurosci. Lett. 2018, 671, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Huang, D.; Zhang, M.H.; Zhang, W.S.; Tang, Y.X.; Shi, Z.X.; Deng, L.; Zhou, D.H.; Lu, X.Y. Salvianolic acid B inhibits Aβ generation by modulating BACE1 activity in SH-SY5Y-APPsw cells. Nutrients 2016, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, S.J.; Kim, J.M.; Jeon, S.J.; Kim, D.H.; Cho, Y.W.; Son, K.H.; Lee, H.J.; Moon, J.H.; Cheong, J.H. Cognitive dysfunctions induced by a cholinergic blockade and Aβ25–35 peptide are attenuated by salvianolic acid B. Neuropharmacology 2011, 61, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Molecular pharmacology of rosmarinic and salvianolic acids: Potential seeds for Alzheimer’s and vascular dementia drugs. Int. J. Mol. Sci. 2018, 19, 458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, H.; Sheng, Y.; Li, L.; Ye, M.; Guo, D. HPLC determination and pharmacokinetic studies of salvianolic acid B in rat plasma after oral administration of radix Salviae Miltiorrhizae extract. Biomed. Chromatogr. 2005, 19, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Chen, Y.F.; Hsieh, Y.J.; Jaw, I.; Shiao, M.S.; Tsai, T.H. Bioavailability of salvianolic acid B in conscious and freely moving rats. Int. J. Pharm. 2006, 326, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, Y.; Tang, L.; Wang, J.; Guo, D.; Wang, M.; Zhang, X. Evaluation of brain targeting in rats of Salvianolic acid B nasal delivery by the microdialysis technique. Xenobiotica 2018, 48, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Sul, D.; Kim, H.S.; Lee, D.; Joo, S.S.; Hwang, K.W.; Park, S.Y. Protective effect of caffeic acid against β-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009, 84, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, L.; Song, W. Separation and quantitative determination of seven aqueous depsides in Salvia miltiorrhiza by HPTLC scanning. Acta Pharm. Sin. 1993, 28, 543–547. [Google Scholar]
- Eldar-Finkelman, H.; Martinez, A. GSK-3 inhibitors: Preclinical and clinical focus on CNS. Front. Mol. Neurosci. 2011, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Baki, A.; Bielik, A.; Molnár, L.; Szendrei, G.; Keserü, G.M. A high throughput luminescent assay for glycogen synthase kinase-3β inhibitors. Assay Drug Dev. Technol. 2007, 5, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S.; Morris, G.M.; Olson, A.J. Automated docking of flexible ligands: Applications of AutoDock. J. Mol. Recognit. 1996, 9, 1–5. [Google Scholar] [CrossRef]
- Bertrand, J.; Thieffine, S.; Vulpetti, A.; Cristiani, C.; Valsasina, B.; Knapp, S.; Kalisz, H.; Flocco, M. Structural characterization of the GSK-3β active site using selective and non-selective ATP-mimetic inhibitors. J. Mol. Biol. 2003, 333, 393–407. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds (caffeic acid, rosmarinic acid, magnesium lithospermate B, salvianolic acid A, salvianolic acid B and salvianolic acid C) are available from the authors and commercial sources. |




| Samples | IC50 Values (Mean ± SEM) a |
|---|---|
| Kangen-karyu | 17.05 ± 1.14 f |
| Aucklandiae Radix | 85.04 ± 6.32 d |
| Carthami Flos | 93.61 ± 3.99 c |
| Cnidii Rhizoma | 66.74 ± 2.05 e |
| Cyperi Rhizoma | 20.68 ± 2.50 f |
| Paeoniae Radix | 62.51 ± 1.89 e |
| Salviae Miltiorrhizae Radix | 7.77 ± 1.38 g |
| Luteolin b | 2.18 ± 0.13 h |
| Sample | IC50 (Mean ± SEM) a | Kic | Inhibition Mode |
|---|---|---|---|
| Salvianolic acid A | 30.21 ± 3.14 f | - | - |
| Salvianolic acid B | 6.97 ± 0.96 g | 5.44/6.32 | Competitive |
| Salvianolic acid C | 31.82 ± 2.08 f | - | - |
| Caffeic acid | 425.01 ± 7.61 d | - | - |
| Rosmarinic acid | 135.35 ± 4.69 e | - | - |
| Magnesium lithospermate B | 33.07 ± 3.88 f | - | - |
| Luteolin b | 2.18 ± 0.13 g | - | - |
| AR-A014418 b | 0.10 | 0.03 | ATP-competitive [30] |
| Alsterpaullone b | 0.004 | - | ATP-competitive [31] |
| Indirubin b | 0.60 | - | ATP-competitive [32] |
| Compounds | Binding Score (kcal/mol) a | No. of H-Bonds | H-bonds Interacting Residues b | Hydrophobic Interacting Residues b | Others |
|---|---|---|---|---|---|
| AMP-PNP a | −7.75 b | 11 | Lys85, Val135, Lys183, Gln185, Asp133, Gly63, Asn64 | π–Alkyl: Val70, Ala83, Leu188, Cys199, Ala83, Val135 | Electrostatic bond: MG1002, MG1003, Lys85, Lys183 |
| Indirubin a | −7.67 | 3 | Val135, Cys199 | π-Alkyl: Leu188, Ala83, Val110, Leu132, Cys199, Val70, Leu132, Leu188, Pi-Sigma: Ile62, Leu188 | |
| Andrographolide a | −8.17 | 8 | Gln89, Lys94, Arg96, Arg180, Lys292 | Alkyl: Pro294, Arg96; π–Alkyl: Phe93, Phe293 | |
| VP0.7 a | −6.75 | 4 | Arg209, Leu207, Ser236, Thr235 | Alkyl: Val240, Pro331; π–Alkyl: His173, Arg209, Leu207; amide–π stacked: Val208, Arg209 | |
| Salvianolic acid B | −6.18 | 4 | Thr138, Lys183, Gln185, Cys199, Asn186 | π–Alkyl: Leu188, Val70 | π–Cation: Lys85; metal–acceptor: MG1002 |
| −11.31 | 7 | Arg96, Lys292, Glu97, Leu88, Gly202 | π–Alkyl: Arg96, Val263, π–π T-shaped: Phe293, π–σ: Lys292 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudel, P.; Seong, S.H.; Zhou, Y.; Park, C.H.; Yokozawa, T.; Jung, H.A.; Choi, J.S. Rosmarinic Acid Derivatives’ Inhibition of Glycogen Synthase Kinase-3β Is the Pharmacological Basis of Kangen-Karyu in Alzheimer’s Disease. Molecules 2018, 23, 2919. https://doi.org/10.3390/molecules23112919
Paudel P, Seong SH, Zhou Y, Park CH, Yokozawa T, Jung HA, Choi JS. Rosmarinic Acid Derivatives’ Inhibition of Glycogen Synthase Kinase-3β Is the Pharmacological Basis of Kangen-Karyu in Alzheimer’s Disease. Molecules. 2018; 23(11):2919. https://doi.org/10.3390/molecules23112919
Chicago/Turabian StylePaudel, Pradeep, Su Hui Seong, Yajuan Zhou, Chan Hum Park, Takako Yokozawa, Hyun Ah Jung, and Jae Sue Choi. 2018. "Rosmarinic Acid Derivatives’ Inhibition of Glycogen Synthase Kinase-3β Is the Pharmacological Basis of Kangen-Karyu in Alzheimer’s Disease" Molecules 23, no. 11: 2919. https://doi.org/10.3390/molecules23112919






