Reaction between Indazole and Pd-Bound Isocyanides—A Theoretical Mechanistic Study
Abstract
:1. Introduction
2. Computational Details
2.1. Calculations of the Reaction Mechanism
2.2. Bond Analysis
3. Results and Discussion
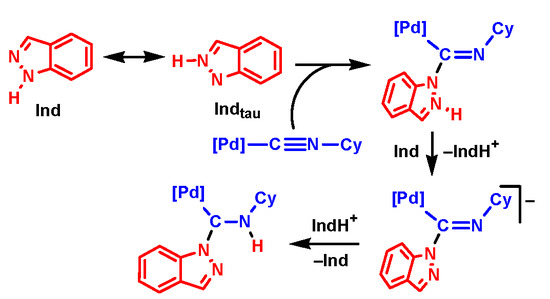
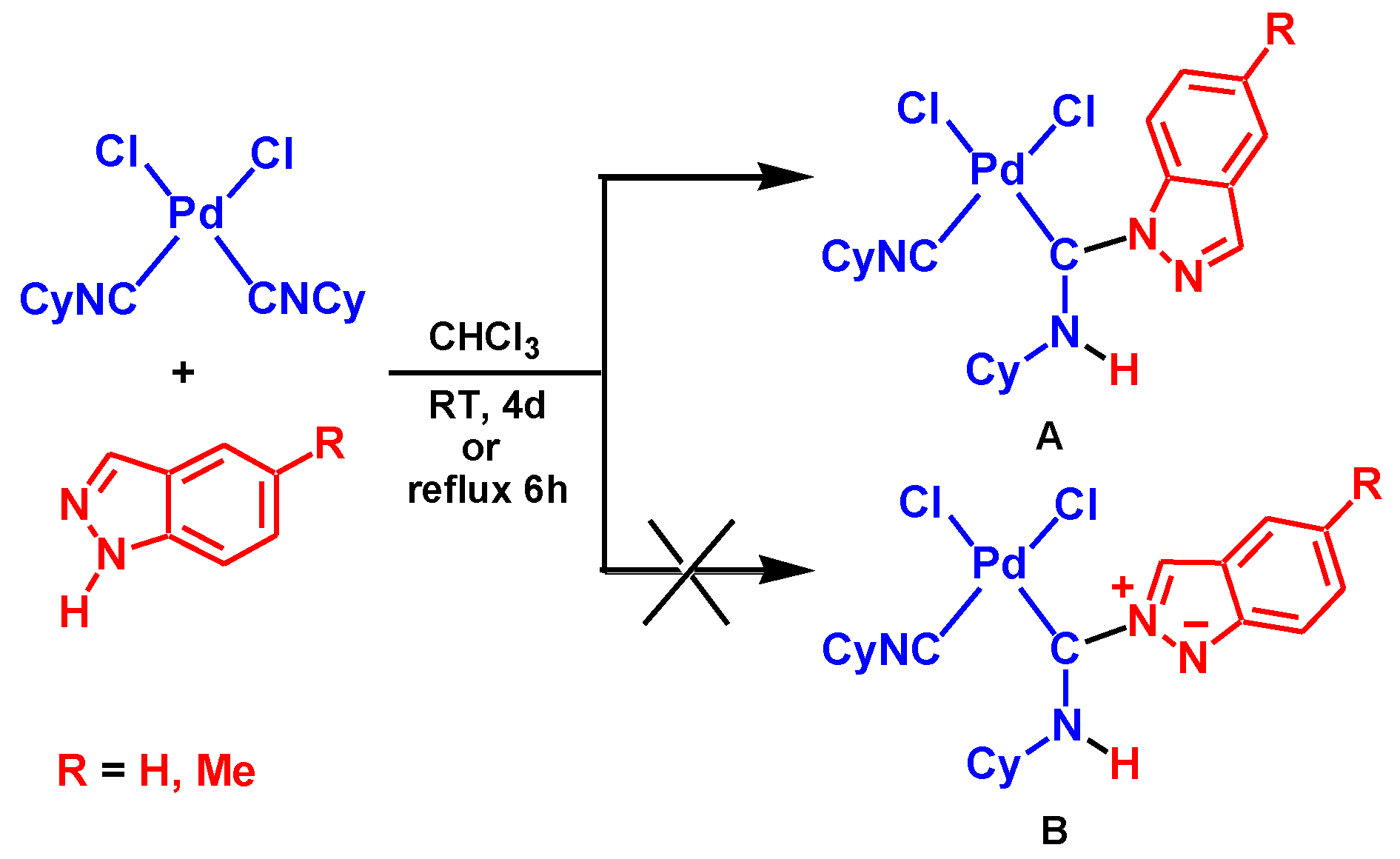
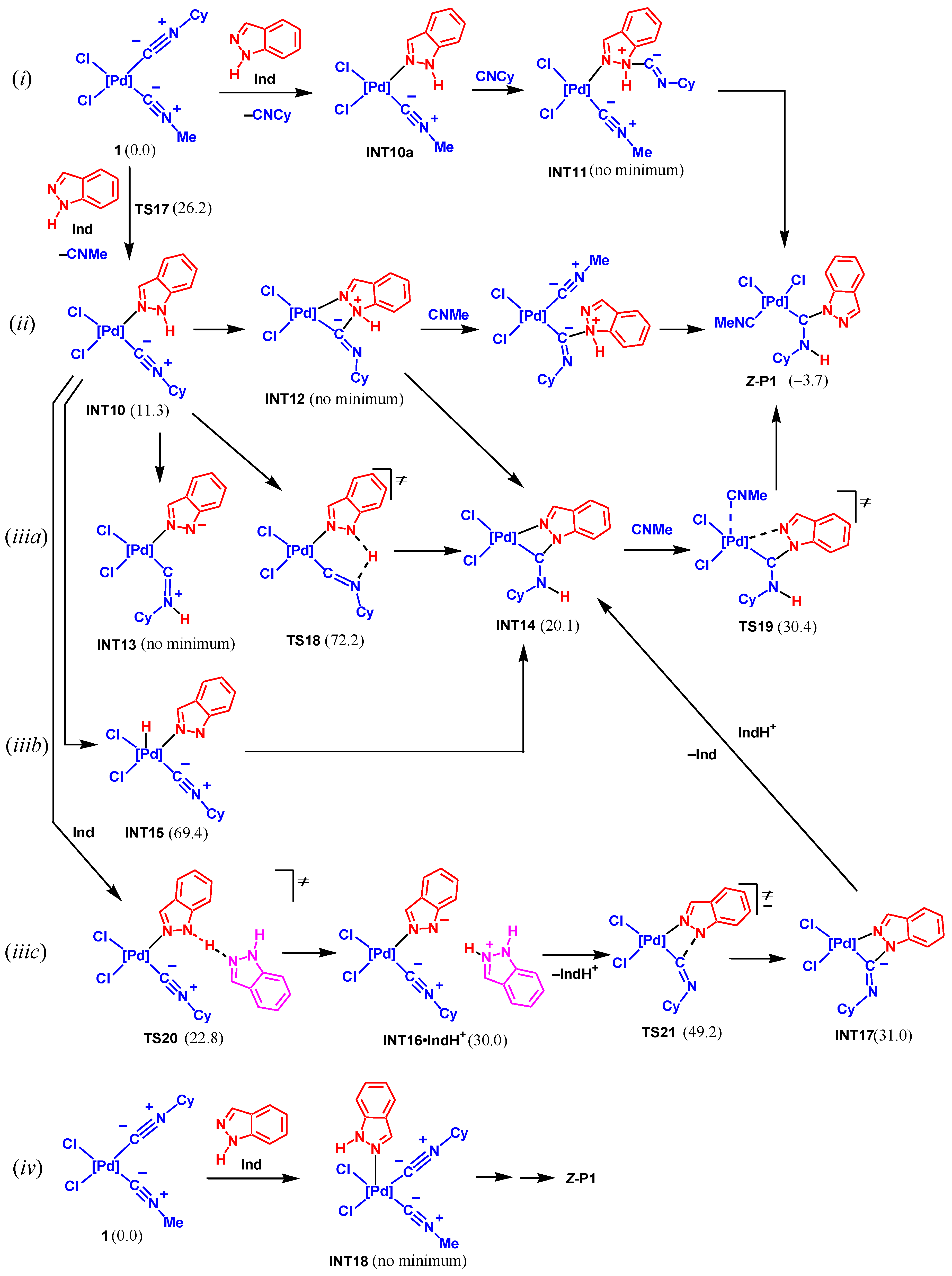
3.1. Reaction Mechanisms
3.2. Dissociative Mechanism
 ΔGad
ΔGad
 ΔG2
ΔG2
 ΔG3
ΔG3
3.3. Concerted Mechanism
3.4. Associative Mechanisms
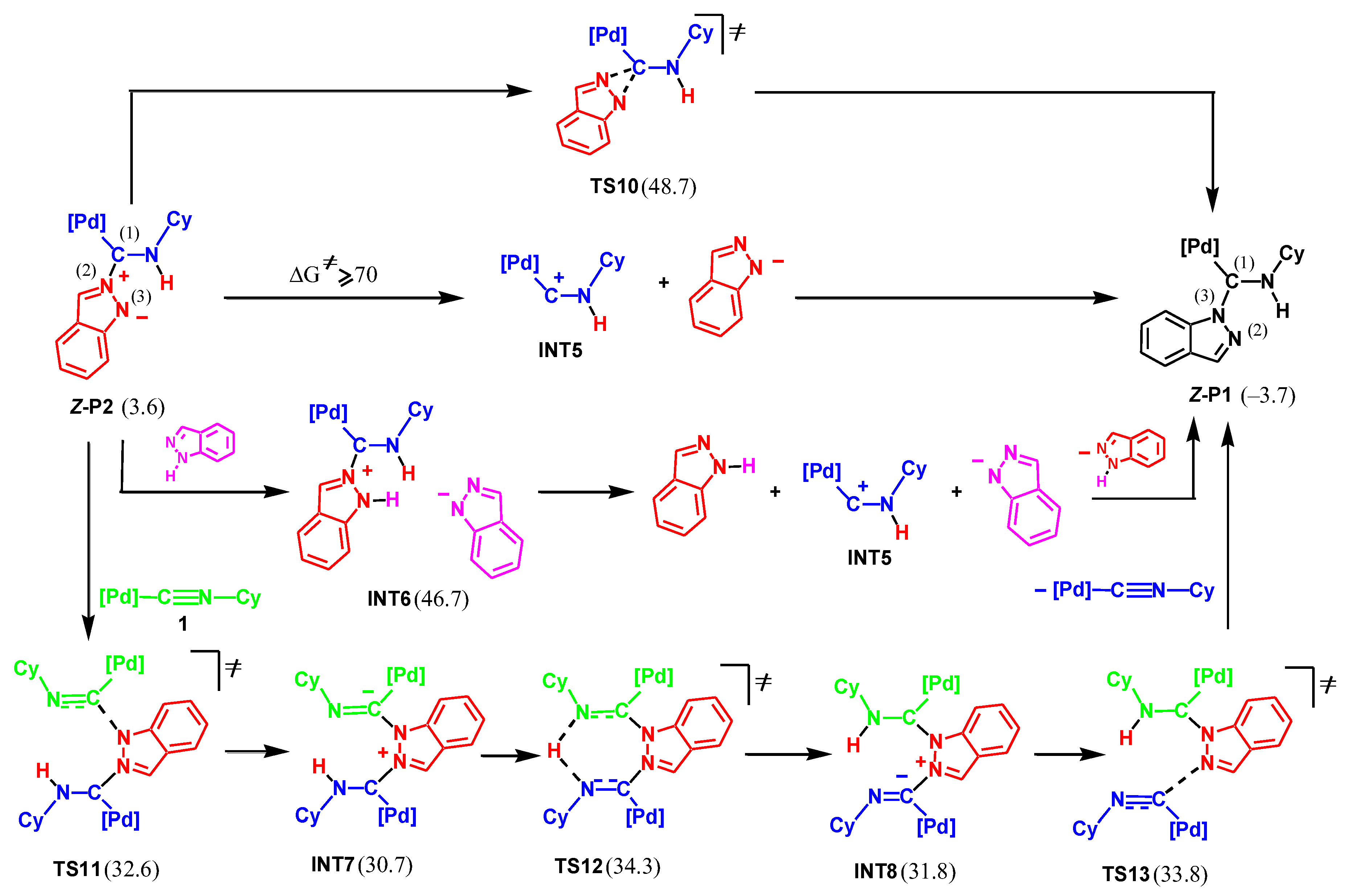
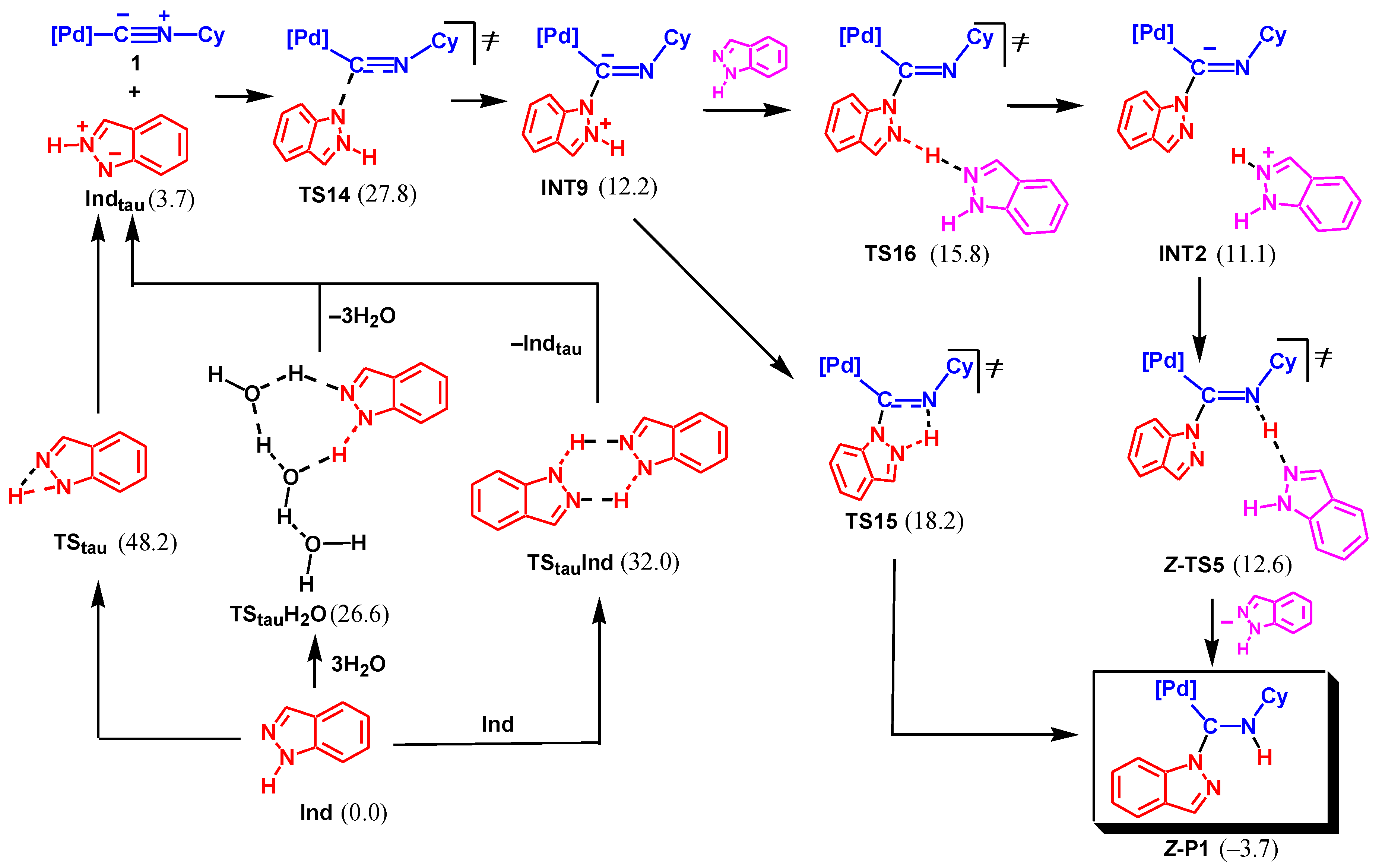
3.4.1. Nucleophilic Addition by the Amino Nitrogen Atom (Mechanism I)
3.4.2. Nucleophilic Addition by the Imino Nitrogen Atom (Mechanism II)
3.4.3. Isomerization of P2 into P1
3.4.4. Mechanism Involving the Less Stable Tautomeric Form of Indazole (Mechanism III)
3.4.5. Mechanisms based on the coordinated indazole
3.5. Bond Analysis
4. Final Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyarskiy, V.P.; Bokach, N.A.; Luzyanin, K.V.; Kukushkin, V.Y. Metal-mediated and metal-catalyzed reactions of isocyanides. Chem. Rev. 2015, 115, 2698–2779. [Google Scholar] [CrossRef] [PubMed]
- Hulme, C.; Lee, Y.-S. Emerging approaches for the syntheses of bicyclic imidazo[1,2-x]-heterocycles. Mol. Divers. 2008, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, S.; Heravi, M.M. Recent Application of Isocyanides in Synthesis of Heterocycles. Tetrahedron 2011, 67, 2707–2752. [Google Scholar] [CrossRef]
- Michelin, R.A.; Pombeiro, A.J.L.; Guedes da Silva, M.F.C. Aminocarbene complexes derived from nucleophilic addition to isocyanide ligands. Coord. Chem. Rev. 2001, 218, 75–112. [Google Scholar] [CrossRef]
- Hahn, F.E.; Jahnke, M.C. Heterocyclic Carbenes: Synthesis and Coordination Chemistry. Angew. Chem. Int. Ed. 2008, 47, 3122–3172. [Google Scholar] [CrossRef] [PubMed]
- Boyarskiy, V.P.; Luzyanin, K.V.; Kukushkin, V.Y. Acyclic diaminocarbenes (ADCs) as a promising alternative to N-heterocyclic carbenes (NHCs) in transition metal catalyzed organic transformations. Coord. Chem. Rev. 2012, 256, 2029–2056. [Google Scholar] [CrossRef]
- Nolan, S.P.; Navarro, O. C–C Bond Formation by Cross-coupling, in Comprehensive Organometallic Chemistry III; Canty, A., Ed.; Elsevier: Oxford, England, UK, 2007; Volume 11, pp. 1–38. [Google Scholar]
- Marion, N.; Nolan, S.P. Well-Defined N-Heterocyclic Carbenes−Palladium(II) Precatalysts for Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Díez-González, S.; Marion, N.; Nolan, S.P. N-Heterocyclic carbenes in late transition metal catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, L.M. Acyclic aminocarbenes in catalysis. ACS Catal. 2012, 2, 1802–1816. [Google Scholar] [CrossRef]
- Herrmann, W.A. N-heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Kremzow, D.; Seidel, G.; Lehmann, C.W.; Furstner, A. Diaminocarbene- and Fischer-carbene complexes of palladium and nickel by oxidative insertion: Preparation, structure, and catalytic activity. Chem. Eur. J. 2005, 11, 1833–1853. [Google Scholar] [CrossRef] [PubMed]
- Dhudshia, B.; Thadani, A.N. Acyclic diaminocarbenes: simple, versatile ligands for cross-coupling reactions. Chem. Commun. 2006, 6, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Miltsov, S.; Karavan, V.; Boyarsky, V.; Gómez-de Pedro, S.; Alonso-Chamarro, J.; Puyol, M. New acyclic Pd–Diaminocarbene catalyst for suzuki arylation of meso-chlorosubstituted tricarboindocyanine dyes. Tetrahedron Lett. 2013, 54, 1202–1204. [Google Scholar] [CrossRef]
- Luzyanin, K.V.; Tskhovrebov, A.G.; Carias, M.C.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L.; Kukushkin, V.Y. Novel metal–mediated (M = Pd, Pt) coupling between isonitriles and benzophenone hydrazone as a route to aminocarbene complexes exhibiting high catalytic activity (M = Pd) in the suzuki–miyaura reaction. Organometallics 2009, 28, 6559–6566. [Google Scholar] [CrossRef]
- Slaughter, L.M. Covalent self-assembly of acyclic diaminocarbene ligands at metal centers. Comments Inorg. Chem. 2008, 29, 46–72. [Google Scholar] [CrossRef]
- Moncada, A.I.; Manne, S.; Tanski, J.M.; Slaughter, L.M. Modular chelated palladium diaminocarbene complexes: synthesis, characterization, and optimization of catalytic suzuki−miyaura cross–coupling activity by ligand modification. Organometallics 2006, 25, 491–505. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Luzyanin, K.V.; Kuznetsov, M.L.; Sorokoumov, V.N.; Balova, I.A.; Haukka, M.; Kukushkin, V.Y. Substituent R-dependent regioselectivity switch in nucleophilic addition of N-phenylbenzamidine to PdII– and PtII–complexed isonitrile RN≡C giving aminocarbene-like species. Organometallics 2011, 30, 863–874. [Google Scholar] [CrossRef]
- Ryabukhin, D.S.; Sorokoumov, V.N.; Savicheva, E.A.; Boyarskiy, V.P.; Balova, I.A.; Vasilyev, A.V. Catalytic activity of palladium acyclic diaminocarbene complexes in the synthesis of 1,3–diarylpropynones via sonogashira reaction: cross– versus homo–coupling. Tetrahedron Lett. 2013, 54, 2369–2372. [Google Scholar] [CrossRef]
- Bartolomé, C.; Ramiro, Z.; García-Cuadrado, D.; Pérez-Galán, P.; Raducan, M.; Bour, C.; Echavarren, A.M.; Espinet, P. Nitrogen acyclic gold(I) carbenes: excellent and easily accessible catalysts in reactions of 1,6-enynes. Organometallics 2010, 29, 951–956. [Google Scholar] [CrossRef]
- Bartolomé, C.; García-Cuadrado, D.; Ramiro, Z.; Espinet, P. Synthesis and catalytic activity of gold chiral nitrogen acyclic carbenes and gold hydrogen bonded heterocyclic carbenes in cyclopropanation of vinyl arenes and in intramolecular hydroalkoxylation of allenes. Inorg. Chem. 2010, 49, 9758–9764. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K.; Hengst, T.; Lothschütz, C.; Rominger, F. New and easily accessible nitrogen acyclic gold(I) carbenes: structure and application in the gold–catalyzed phenol synthesis as well as the hydration of alkynes. Adv. Synth. Catal. 2010, 352, 1315–1337. [Google Scholar] [CrossRef]
- Seo, H.; Snead, D.R.; Abboud, K.A.; Hong, S. Bulky acyclic aminooxycarbene ligands. Organometallics 2011, 30, 5725–5730. [Google Scholar] [CrossRef]
- Cavarzan, A.; Scarso, A.; Sgarbossa, P.; Strukul, G.; Reek, J.N.H. Supramolecular control on chemo- and regioselectivity via encapsulation of (NHC)-Au catalyst within a hexameric self-assembled host. J. Am. Chem. Soc. 2011, 133, 2848–2851. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, C.; Carrasco-Rando, M.; Coco, S.; Cordovilla, C.; Martín-Alvarez, J.M.; Espinet, P. Luminescent gold(I) carbenes from 2-pyridylisocyanide complexes: structural consequences of intramolecular versus intermolecular hydrogen-bonding interactions. Inorg. Chem. 2008, 47, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-W.; Chan, M.C.-W.; Cheung, K.-K.; Che, C.-M. Carbene and isocyanide ligation at luminescent cyclometalated 6-phenyl-2,2‘-bipyridyl platinum(II) complexes: Structural and spectroscopic studies. Organometallics 1999, 18, 3327–3336. [Google Scholar] [CrossRef]
- Crociani, B.; Boschi, T.; Belluco, U. Synthesis and reactivity of novel palladium(II)–isocyanide complexes. Inorg. Chem. 1970, 9, 2021–2025. [Google Scholar] [CrossRef]
- Michelin, R.A.; Zanotto, L.; Braga, D.; Sabatino, P.; Angelici, R.J. Transition–metal–promoted cyclization reactions of isocyanide ligands. synthesis of cyclic diaminocarbenes from isocyanide complexes of palladium(II) and platinum(II) and X-ray structure of cis-Br2Pt[CN(C6H4-p-Me)CH2CH2N(H)](PPh3). Inorg. Chem. 1988, 27, 93–99. [Google Scholar] [CrossRef]
- Yu, I.; Wallis, C.J.; Patrick, B.O.; Diaconescu, P.L.; Mehrkhodavandi, P. Phosphine–tethered carbene ligands: template synthesis and reactivity of cyclic and acyclic functionalized carbenes. Organometallics 2010, 29, 6065–6076. [Google Scholar] [CrossRef]
- Arias, J.; Bardají, M.; Espinet, P. Luminescence and mesogenic properties in crown-ether-isocyanide or carbene gold(I) complexes: Luminescence in solution, in the solid, in the mesophase, and in the isotropic liquid state. Inorg. Chem. 2008, 47, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Crociani, B.; Uguagliati, P.; Belluco, U. Steric role of aromatic ring ortho-substituents in the mechanism of carbene formation from palladium(II) arylisocyanide complexes and anilines. J. Organomet. Chem. 1976, 117, 189–199. [Google Scholar] [CrossRef]
- Lazar, M.; Zhu, B.; Angelici, R.J. Non-nanogold catalysis of reactions of isocyanides, secondary amines, and oxygen to give ureas. J. Phys. Chem. C 2007, 111, 4074–4076. [Google Scholar] [CrossRef]
- Canovese, L.; Visentin, F.; Levi, C.; Bertolasi, V. Synthesis and mechanism of formation of novel NHC−NAC bis-carbene complexes of gold(I). Organometallics 2011, 30, 875–883. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.; García, L.; Mejuto, C.; Vivanco, M.; Díaz, M.R.; García-Granda, S. Strong electron-donating metalla-N-heterocyclic carbenes. Chem. Commun. 2014, 2129–2132. [Google Scholar] [CrossRef] [PubMed]
- Anding, B.J.; Ellern, A.; Woo, L.K. Comparative study of rhodium and iridium porphyrin diaminocarbene and N-heterocyclic carbene complexes. Organometallics 2014, 33, 2219–2229. [Google Scholar] [CrossRef]
- Vicente, J.; Chicote, M.T.; Huertas, S.; Jones, P.G. 1,1-Ethylenedithiolato complexes of palladium(II) and platinum(II) with isocyanide and carbene ligands. Inorg. Chem. 2003, 42, 4268–4274. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huynh, H.V. Mixed carbene–isocyanide Pd(II) complexes: Synthesis, structures and reactivity towards nucleophiles. Dalton Trans. 2009, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, A.-J.; Chicote, M.-T.; Bautista, D.; Vicente, J. Synthesis of palladium(II), -(III), and -(IV) complexes with acyclic diaminocarbene ligands. Organometallics 2012, 31, 3711–3719. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Böhling, C.; Lothschütz, C.; Rominger, F. From isonitriles to carbenes: Synthesis of new NAC− and NHC−palladium(II) compounds and their catalytic activity. Organometallics 2011, 30, 2411–2417. [Google Scholar] [CrossRef]
- Heathcote, R.; Howell, J.A.S.; Jennings, N.; Cartlidge, D.; Cobden, L.; Coles, S.; Hursthouse, M. Gold(I)–isocyanide and gold(I)–carbene complexes as substrates for the laser decoration of gold onto ceramic surfaces. Dalton Trans. 2007, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, C.; Carrasco-Rando, M.; Coco, S.; Cordovilla, C.; Espinet, P.; Martín-Alvarez, J.M. Gold(I)–carbenes derived from 4-pyridylisocyanidecomplexes: supramolecular macrocycles supported by hydrogen bonds, and luminescent behavior. Dalton Trans. 2007, 5339–5345. [Google Scholar] [CrossRef]
- Handa, S.; Slaughter, L.M. Enantioselective alkynylbenzaldehyde cyclizations catalyzed by chiral gold(I) acyclic diaminocarbene complexes containing weak Au–arene interactions. Angew. Chem. Int. Ed. 2012, 51, 2912–2915. [Google Scholar] [CrossRef] [PubMed]
- Uguagliati, P.; Crociani, B.; Belluco, U.; Calligaro, L. Solvent, ligand and temperature effects on the rates of reactions of cis-[PdCl2(CNR)(L)] with secondary amines. J. Organomet. Chem. 1976, 112, 111–121. [Google Scholar] [CrossRef]
- Spallek, M.J.; Riedel, D.; Rominger, F.A.; Hashmi, A.S.K.; Trapp, O. Six–membered, chiral NHCs derived from camphor: Structure–reactivity relationship in asymmetric oxindole synthesis. Organometallics 2012, 31, 1127–1132. [Google Scholar] [CrossRef]
- Ruiz, J.; Perandones, B.F.; García, G.; Mosquera, M.E.G. Synthesis of N-heterocyclic carbene complexes of manganese(I) by coupling isocyanide ligands with propargylamines and propargylic alcohols. Organometallics 2007, 26, 5687–5695. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Lothschütz, C.; Graf, K.; Häffner, T.; Schuster, A.; Rominger, F. A short way to switchable carbenes. Adv. Synth. Catal. 2011, 353, 1407–1412. [Google Scholar] [CrossRef]
- Wanniarachchi, Y.A.; Slaughter, L.M. One-step assembly of a chiral palladium bis(acyclic diaminocarbene) complex and its unexpected oxidation to a bis(amidine) complex. Chem. Commun. 2007, 3294–3296. [Google Scholar] [CrossRef] [PubMed]
- Zanella, R.; Boschi, T.; Crociani, B.; Belluco, U. Reactions of palladium(II) isocyanide complexes with bifunctional amines. J. Organomet. Chem. 1974, 71, 135–143. [Google Scholar] [CrossRef]
- Zanella, R.; Boschi, T.; Nicolini, M.; Belluco, U. The reactions of bis-isocyanide derivatives of PdII with bidentate ligands. J. Organomet. Chem. 1973, 49, C91–C94. [Google Scholar] [CrossRef]
- Crociani, B.; Di Bianca, F.; Fontana, A.; Forsellini, E.; Bombieri, G. Nucleophilic attack at co-ordinated isocyanides promoted by the 2-pyridyl ligand. J. Chem. Soc. Dalton Trans. 1994, 407–414. [Google Scholar] [CrossRef]
- Belluco, U.; Michelin, R.A.; Ros, R.; Bertani, R.; Facchin, G.; Mozzon, M.; Zanotto, L. Synthesis of heterocyclic systems by activation of isocyanide, carbonyl, trifluoromethyl and nitrile ligands in platinum(II) and palladium(II) complexes. Inorg. Chim. Acta 1992, 198–200, 883–897. [Google Scholar] [CrossRef]
- Tschugajeff, L.; Skanawy-Grigorjewa, M. Reaction of K2PtCl4 with isonitriles and hydrazine. J. Russ. Chem. Soc. 1915, 47, 776. [Google Scholar]
- Tschugajeff, L.; Grigorjewa, M.; Posnjak, A. Über die hydrazine–carbilamin–komplexe des platins. Z. Anorg. Allg. Chem. 1925, 148, 37–42. [Google Scholar] [CrossRef]
- Rouschias, G.; Shaw, B.L. The chemistry and structure of Chugaev’s salt and related compounds containing a cyclic carbene ligand. J. Chem. Soc. A 1971, 2097–2104. [Google Scholar] [CrossRef]
- Burke, A.; Balch, A.L.; Enemark, J.H. Palladium and platinum complex resulting from the addition of hydrazine to coordinated isocyanide. J. Am. Chem. Soc. 1970, 92, 2555–2557. [Google Scholar] [CrossRef]
- Butler, W.M.; Enemark, J.H.; Parks, J.; Balch, A.L. Chelative addition of hydrazines to coordinated isocyanides. structure of Chugaev’s red salt. Inorg. Chem. 1973, 12, 451–457. [Google Scholar] [CrossRef]
- Badley, M.; Chatt, J.; Richards, R.L. Isonitrile complexes of platinum(II) and their reactions with alcohols and amines to give carbene complexes. J. Chem. Soc. A 1971, 21–25. [Google Scholar] [CrossRef]
- Moncada, A.I.; Tanski, J.M.; Slaughter, L.M. Sterically controlled formation of monodentate versus chelating carbene ligands from phenylhydrazine. J. Organomet. Chem. 2005, 690, 6247–6251. [Google Scholar] [CrossRef]
- Wanniarachchi, Y.A.; Slaughter, L.M. Reversible chelate ring opening of a sterically crowded palladium bis(acyclic diaminocarbene) complex. Organometallics 2008, 27, 1055–1062. [Google Scholar] [CrossRef]
- Luzyanin, K.V.; Guedes da Silva, M.F.C.; Kukushkin, V.Y.; Pombeiro, A.J.L. First example of an imine addition to coordinated isonitrile. Inorg. Chim. Acta 2009, 362, 833–838. [Google Scholar] [CrossRef]
- Balch, A.L.; Parks, J.E. Platinum and palladium complexes formed by chelative addition of amines to isocyanides. J. Am. Chem. Soc. 1974, 96, 4114–4121. [Google Scholar] [CrossRef]
- Canovese, L.; Visentin, F.; Uguagliati, P.; Crociani, B.; Di Bianca, F. Mechanism of aminocarbene formation by nucleophilic attack on isocyanide ligands in platinum(II) 2-pyrazyl and 4-pyridyl complexes. J. Organomet. Chem. 1997, 535, 69–75. [Google Scholar] [CrossRef]
- Canovese, L.; Visentin, F.; Uguagliati, P.; Crociani, B. Mechanism of formation of (methoxy)(amino)–and bis(amino) carbene complexes by nucleophilic attack of methoxide ion and amines on platinum(II)-coordinated isocyanide in anhydrous methanol. J. Organomet. Chem. 1997, 543, 145–151. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Kukushkin, V.Y. Metal-mediated addition of N-nucleophiles to isocyanides: mechanistic aspects. Molecules 2017, 22, 1141. [Google Scholar] [CrossRef] [PubMed]
- Kinzhalov, M.A.; Boyarskiy, V.P.; Luzyanin, K.V.; Dolgushin, F.M.; Kukushkin, V.Y. Metal-mediated coupling of a coordinated isocyanide and indazoles. Dalton Trans. 2013, 42, 10394–10397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main–group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125, 194101–194118. [Google Scholar] [CrossRef] [PubMed]
- Frisch, G.W.; Trucks, H.B.; Schlegel, G.E.; Scuseria, M.A.; Robb, J.R.; Cheeseman, G.; Scalmani, V.; Barone, B.; Mennucci, G.A.; Petersson, H.; et al. Gaussian 09, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Andrae, D.; Haussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher-order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- ADF2013, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, Netherlands. Available online: http://www.scm.com (accessed on 30 October 2018).
- van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic regular two–component hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. [Google Scholar] [CrossRef]
- van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic total energy using regular approximations. J. Chem. Phys. 1994, 101, 9783–9792. [Google Scholar] [CrossRef]
- van Lenthe, E.; van Leeuwen, R.; Baerends, E.J.; Snijders, J.G. Relativistic regular two–component hamiltonians. Int. J. Quantum Chem. 1996, 57, 281–293. [Google Scholar] [CrossRef]
- van Lenthe, E.; Ehlers, A.; Baerends, E.-J. Geometry optimizations in the zero order regular approximation for relativistic effects. J. Chem. Phys. 1999, 110, 8943–8953. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density–functional exchange–energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density–functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Perdew, J.P. Erratum: Density–functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 34, 7406. [Google Scholar] [CrossRef]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H.A. Consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Mozzon, M.; Sgarbossa, P.; Tamburini, S.; Casarin, M.; Mangione, G.; Casella, G.; Venzo, A.; Rizzato, S.; Albinati, A. Pt(II) nitrile complexes: New insights on old complexes from a combined experimental and theoretical study. Inorg. Chim. Acta 2017, 455, 489–504. [Google Scholar] [CrossRef] [Green Version]
- Guerra, C.F.; Handgraaf, J.-W.; Baerends, E.J.; Bickelhaupt, F.M. Voronoi deformation density (VDD) charges: Assessment of the Mulliken, Bader, Hirshfeld, Weinhold, and VDD methods for charge analysis. J. Comput. Chem. 2004, 25, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Orenha, R.P.; Rocha, M.V.J.; Poater, J.; Galembeck, S.E.; Bickelhaupt, F.M. Nature of the Ru–NO coordination bond: Kohn–Sham molecular orbital and energy decomposition analysis. ChemistryOpen 2017, 6, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A.; Schuurmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 799–805. [Google Scholar] [CrossRef]
- Klamt, A. Conductor-like screening model for real solvents: A new approach to the quantitative calculation of solvation phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- Klamt, A.; Jonas, V. Treatment of the outlying charge in continuum solvation models. J. Chem. Phys. 1996, 105, 9972–9981. [Google Scholar] [CrossRef]
- Pye, C.C.; Ziegler, T. An implementation of the conductor–like screening model of solvation within the amsterdam density functional package. Theor. Chem. Acc. 1999, 101, 396–408. [Google Scholar] [CrossRef]
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles: Structure, Reactions, Synthesis and Applications; Wiley–VCH: Weinheim, Germany, 2012. [Google Scholar]
- Kuznetsov, M.L.; Kukushkin, V. Yu.; Pombeiro, A.J.L. Reactivity of Pt– and Pd–bound nitriles towards nitrile oxides and nitrones: Substitution vs. cycloaddition. Dalton Trans. 2008, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Menezes, F.M.C.; Kuznetsov, M.L.; Pombeiro, A.J.L. Isocyanide complexes with platinum and palladium and their reactivity toward cycloadditions with nitrones to form aminooxycarbenes: A theoretical study. Organometallics 2009, 28, 6593–6602. [Google Scholar] [CrossRef]
- Novikov, A.S.; Kuznetsov, M.L. Theoretical study of Re(IV) and Ru(II) bis–isocyanide complexes and their reactivity in cycloaddition reactions with nitrones. Inorg. Chim. Acta 2012, 380, 78–89. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |












© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casella, G.; Casarin, M.; Kukushkin, V.Y.; Kuznetsov, M.L. Reaction between Indazole and Pd-Bound Isocyanides—A Theoretical Mechanistic Study. Molecules 2018, 23, 2942. https://doi.org/10.3390/molecules23112942
Casella G, Casarin M, Kukushkin VY, Kuznetsov ML. Reaction between Indazole and Pd-Bound Isocyanides—A Theoretical Mechanistic Study. Molecules. 2018; 23(11):2942. https://doi.org/10.3390/molecules23112942
Chicago/Turabian StyleCasella, Girolamo, Maurizio Casarin, Vadim Yu. Kukushkin, and Maxim L. Kuznetsov. 2018. "Reaction between Indazole and Pd-Bound Isocyanides—A Theoretical Mechanistic Study" Molecules 23, no. 11: 2942. https://doi.org/10.3390/molecules23112942